Abstract
The earliest steps of embryonic neural development are orchestrated by sets of transcription factors that control at least three processes: the maintenance of proliferative, pluripotent precursors that expand the neural ectoderm; their transition to neurally committed stem cells comprising the neural plate; and the onset of differentiation of neural progenitors. The transition from one step to the next requires the sequential activation of each gene set and then its down-regulation at the correct developmental times. Herein, we review how these gene sets interact in a transcriptional network to regulate these early steps in neural development. A key gene in this regulatory network is FoxD4L1, a member of the forkhead box (Fox) family of transcription factors. Knock-down experiments in Xenopus embryos show that FoxD4L1 is required for the expression of the other neural transcription factors, whereas increased FoxD4L1 levels have three different effects on these genes: up-regulation of neural ectoderm precursor genes; transient down-regulation of neural plate stem cell genes; and down-regulation of neural progenitor differentiation genes. These different effects indicate that FoxD4L1 maintains neural ectodermal precursors in an immature, proliferative state, and counteracts premature neural stem cell and neural progenitor differentiation. Because it both up-regulates and down-regulates genes, we characterized the regions of the FoxD4L1 protein that are specifically involved in these transcriptional functions. We identified a transcriptional activation domain in the N-terminus and at least two domains in the C-terminus that are required for transcriptional repression. These functional domains are highly conserved in the mouse and human homologues. Preliminary studies of the related FoxD4 gene in cultured mouse embryonic stem cells indicate that it has a similar role in promoting immature neural ectodermal precursors and delaying neural progenitor differentiation. These studies in Xenopus embryos and mouse embryonic stem cells indicate that FoxD4L1/FoxD4 has the important function of regulating the balance between the genes that expand neural ectodermal precursors and those that promote neural stem/progenitor differentiation. Thus, regulating the level of expression of FoxD4 may be important in stem cell protocols designed to create immature neural cells for therapeutic uses.
Keywords: Neural plate stem cells, neural gene regulatory network, FoxD5, FoxD4L1.1, FoxD4, neural induction, neural fate stabilization, neural ectodermal precursors, neural progenitors, embryoid bodies, retinoic acid induction
Introduction
The earliest steps of embryonic neural development are orchestrated by sets of transcription factors that control at least three processes: the maintenance of proliferative, pluripotent precursors that expand the neural ectoderm; their transition to neurally committed stem cells comprising the neural plate; and the onset of differentiation of neural progenitors. The transition from one step to the next requires the sequential activation of each gene set and then its down-regulation at the correct developmental times. Identifying these proteins and determining how they interact in a gene regulatory network has been the focus of developmental genetic studies for over two decades. It is now of practical, clinical significance as well because there is a great deal of interest in determining how pluripotent stem cells differentiate into neurons in culture to provide new therapies for neurodegenerative diseases. A major focus in the neural stem cell field has been on the applied aspects, i.e., developing new culturing approaches and protocols to obtain specific kinds of neurons and glia. For example, midbrain dopamine neurons are needed to treat Parkinson’s disease, striatal neurons are needed for Huntington’s disease, cortical neurons are needed for stroke and head trauma and motor neurons are needed for spinal atrophies. Perhaps because neural cells are among the easiest cell types to form in embryonic stem cell (ESC) cultures - and in fact it is very difficult to prevent them from differentiating into neurons and glia - understanding the underlying molecular mechanisms of the earliest events in neural development has not been a priority. However, we truly do not have an adequate understanding of the molecular interactions that occur during culture protocols that cause ESCs or induced pluripotent stem cells (iPSCs) to become neural precursor cells in the first place. Moreover, it is possible that if the appropriate first steps are not engaged, subsequent in vitro differentiation of specific neural cell classes will be aberrant, despite expression of some cell class specific markers. Such aberrations would complicate the eventual use of in vitro differentiated stem cells in clinical therapies for neurodegenerative disorders.
Understanding how the embryonic nervous system develops from the unspecified ectoderm has been one of the most intensely investigated developmental processes. The process of “neural induction” was discovered in the early 1920s when Spemann and Mangold observed that cells originally fated to give rise to epidermis (skin) form neural tube when they are exposed to dorsally specified mesoderm [1]. Over the past two decades signaling factors that mediate neural induction and transcription factors that control individual steps in early neural development have been identified. However, we still do not understand what regulates the balance between these factors so that they can promote the progressive, concerted transition from neural ectodermal precursors to regionally specified neural progenitors.
Nonetheless, our current, albeit incomplete, knowledge of embryonic neural development has provided some important clues about how to drive stem cells into the appropriate cell types for regenerative therapies. For example, adding neural inducing factors to ESC or iPSC cultures directs these cells to become neural ectodermal precursors (also called primitive neural stem cells) [2,3]. Information from the embryo about how later patterning occurs also can be applied to stem cell cultures to cause them to form specific kinds of neurons. For example, neurally induced ESCs that are posteriorized with retinoic acid and ventralized with Sonic hedgehog (Shh) preferentially form spinal motor neurons [4], as in the embryo. Likewise, neurally induced iPSC cultures treated with factors known in the embryo to induce ventral midbrain cells (Shh, Wnt, BDNF) are enriched in the midbrain dopamine neurons needed to ameliorate Parkinsonian symptoms [3]. Thus, understanding the several steps in early neural development that occur in the embryo can lead to more productive in vitro approaches to yield transplantable neural precursors for regenerative applications.
To date, most emphasis has been put on forcing stem cells to a neural fate and then differentiating them into specific cell types. However, a common problem with these protocols is the difficulty in maintaining neural precursors in an immature, undifferentiated state. For many therapeutic applications this would be advantageous because it would expand neural precursor cells without the spontaneous and premature differentiation that commonly occurs in these cultures. In the embryo, proliferative neural ectodermal precursors first form from embryonic ectoderm in response to neural inductive signaling. Next, as the neural plate forms, these cells transition into neural plate stem cells which subsequently differentiate into regionally-specified neural progenitor cells. Whereas in the embryo, proliferative, undifferentiated neural precursors can be maintained, it is rare to see this kind of cell in ESC cultures. However, similar primitive neural precursors do form in mouse ESC cultures when neural inductive signaling is maintained either by adding anti-BMP factors or growing cells at a low enough density to dilute endogenously produced BMPs [2]. The molecular mechanisms by which this primitive state is acquired and maintained, and by which these cells transition to the better characterized neural stem cell state are not well understood in either the embryo or in stem cell cultures. In this review, we present what is known about the factors involved in transitioning neural ectodermal precursors to neural plate stem cells and differentiating neural progenitors in embryos. We summarize what we have discovered about the transcriptional regulation of this process by studying transcriptional interactions in the intact Xenopus embryo, and we discuss whether this information is likely to be similar in mouse ESCs that are cultured according to protocols designed to produce neurons. In particular, we will describe the molecular mechanisms by which a forkhead box (Fox) transcription factor, FoxD4L1 (aka FoxD5 and FoxD4L1.1 in fish and frogs) regulates other neural genes during the early steps in the formation of the Xenopus nervous system, and discuss our findings regarding the role of the homologous mouse gene in an ESC system. The similarities and differences that we found provide important clues for maintaining neural precursors in an immature state that may be useful for regenerative therapeutic approaches.
Neural induction, expansion of the neural ectoderm and neural fate stabilization
The vertebrate neural ectoderm forms on the dorsal side of the embryo in response to signals from the adjacent dorsal mesoderm, which is commonly called the “Organizer” in frog and fish and the “Node” in chick and mouse (reviewed in [5-9]). The cells comprising the Organizer secrete small diffusible molecules that bind to either BMPs or Wnts in the extracellular space and prevent those ligands from activating their receptors in the adjacent ectoderm. Embryonic ectodermal cells exposed to BMPs and Wnts become epidermis, whereas they become neural in the absence of these two signals. The nascent neural ectodermal cells, which we refer to as neural ectodermal (NE) precursors, express a large number of neural transcription factors (nTFs), including members of the Fox, Sox, Zic and Irx families, that are co-expressed in broad overlapping domains (Figure 1). Based on changes in gene expression domains that occur after single genes are knocked down or over-expressed in the NE precursors, the nTFs appear to have several different activities. They can: 1) regulate the competence of the NE precursors to respond to neural inducing signals; 2) stabilize the newly induced neural fate so that these cells become refractory to local BMP and Wnt signals; 3) expand the size of the neural plate stem cell population; and 4) regulate the onset of neural progenitor differentiation (reviewed in [9,10]). Some of the nTFs appear to cause neural plate expansion by maintaining an immature NE precursor state, whereas others cause neural plate expansion by delaying the onset of bHLH neural differentiation factors, such as members of the Neurogenin (Ngn) and NeuroD families. An important gap in our knowledge, however, is how the nTFs interact with each other in a regulatory network to maintain NE precursors as neurogenic, establish the neural plate stem cells and initiate the differentiation of neural progenitors. Understanding how the nTFs regulate each other is fundamental for discovering the molecular mechanisms underlying this progression. As a starting point, we will consider each of the proposed activities of the nTFs during neural development: competence, stabilization, expansion and differentiation.
Figure 1.
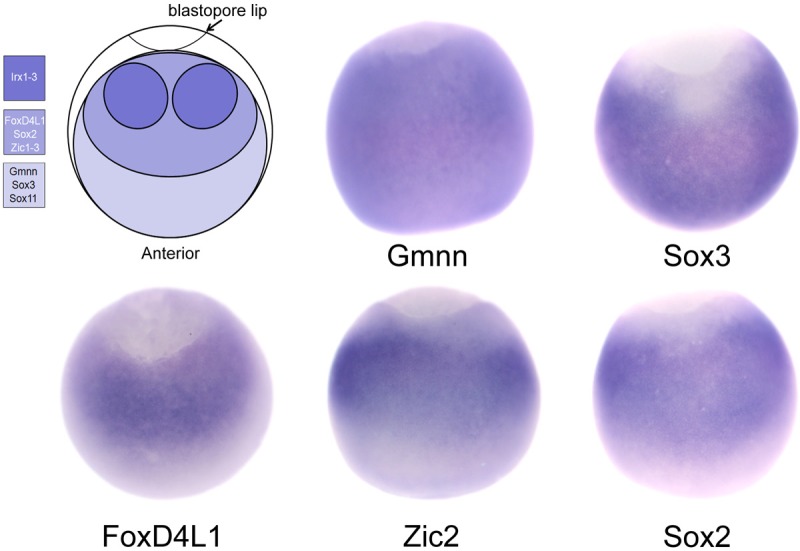
Several transcription factor expression domains overlap in the newly induced neural ectoderm. Upper left cartoon diagrams the overlapping expression domains of 12 neural transcription factors from a dorsal view in an early gastrulating Xenopus embryo. Similarly oriented embryos show the broad expression domains of Gmnn and Sox3, and the slightly smaller domains of FoxD4L1, Zic2 and Sox2. At this stage of development, the Irx genes are weakly expressed in the same domain as FoxD4L1/Zic/Sox2, with higher expression in lateral patches.
During development, cells acquire the ability to respond to an inductive signal, presumably by expressing the required receptors and/or intracellular effector proteins; this process is referred to as “competence”. For neural development, this means that embryonic ectoderm acquires the ability to ignore BMP and Wnt signals in their environment and thereafter begin to express neural specific genes. Of course, one aspect of not responding to BMP and Wnt ligands in the local environment is the availability of BMP and Wnt antagonists that have diffused from the Organizer. Additional “competence” factors have been described. For example, Zic1, a zinc-finger transcription factor, causes the embryonic ectoderm to be more sensitive to neural induction by Noggin [11], although the mechanism by which this occurs has not been defined. In contrast, there is quite a lot of evidence that response to FGF signaling is an important component of neural competence. FGFs are well known to be required for mesoderm induction. But, evidence from the chick embryo also showed that FGF signaling facilitates neural induction [12-14]. One of the downstream effectors of this FGF neural promoting activity is Churchill (ChCh), a zinc finger protein [15]. Because ectopic expression of ChCh up-regulates early neural genes and down-regulates early mesodermal genes, it is proposed that during gastrulation ChCh directs cells that are responding to FGF signaling from a mesodermal fate to a neural ectoderm fate. This activity is mediated, at least in part, by up-regulating the expression of Sip1 (Smad interacting protein 1). Sip1 was identified by its ability to bind to Smad proteins, which are the intracellular effectors of TGFb signaling including BMPs [16]. Sip1 is expressed in the dorsal ectoderm at the time that neural induction is occurring, converts naïve ectoderm to neural ectoderm, represses mesoderm genes and inhibits BMP signaling [17-19]. More recent work demonstrated that the POU91 transcription factor, which is the frog homologue of the mammalian pluripotency gene Oct4, acts upstream of Chch and Sip1 to regulate the FGF-mediated switch from mesodermal to neural fates [20]. Based on these studies in embryos, it would be very informative to determine whether the Oct/ChCh/Sip1 pathway is active in stem cell cultures that are prone to spontaneously differentiate into neural tissue. Although under some conditions attenuation of FGF signaling allows human ESCs to form neural tissues [21], it could be argued that it is the mesoderm-promoting pathway that is being blocked in these cultures. In fact, it was recently shown that SIP1 plays a key role in the decision between neural ectoderm and mesendoderm in human ESCs and mouse epiblast stem cells [22]. An in depth study of the timing of effectiveness of these neural-competence factors in both embryos and ESC cultures could show how to drive cells towards a neural fate and prevent mesoderm and endoderm formation.
Once the neural ectoderm is induced and the nTFs are activated, the tissue continues to be exposed to both BMP and Wnt signals from the surrounding mesoderm and ectoderm (Figure 2). It has been proposed that some of the earliest expressed nTFs somehow stabilize the neural fate program, thus preventing cells from reverting to a non-neural (i.e., epidermal) fate [10]. Since several of the nTF genes can be directly repressed by BMP-regulated transcription factors, such as members of the Vent family [23,24], stabilizing a cell’s commitment to a neural fate is important as the signaling environment changes in the embryo or as some of the cells in culture secrete BMPs and Wnts [2]. One possible mechanism by which neural fate stabilization may occur is by the nTFs attenuating BMP signaling. In fact, Gmnn and Irx1/Xiro1 each reduce Bmp4 mRNA levels when ectopically expressed in epidermal domains [25-27], and Sox3 down-regulates the expression of the BMP target, Vent2, which is required for epidermis formation [28]. We showed that FoxD4L1 similarly down-regulates the expression of genes that comprise the BMP pathway, including their epidermal gene targets, and also reduces the nuclear localization of phosphorylated SMADs1/5/8, which are the effectors of BMP signaling [29,30]. Hyodo-Miura et al. [31] showed that Sox11 interacts with the MAP kinase NLK to antagonize Wnt signaling by phosphorylating the TCF/βcatenin complex. Another assay that indirectly indicates that the BMP and/or Wnt pathway has been repressed is the ectopic expression of nTF mRNA in the epidermal lineage, which is a field of high BMP/Wnt expression. We found in Xenopus embryos that FoxD4L1 ectopically induced several nTFs in presumptive epidermal cells [29]. Together, these studies demonstrate that the combined anti-BMP and anti-Wnt activities of several of the earliest expressed nTFs maintain a permissive neural ectoderm environment by dampening the effects of inhibitory BMP and Wnt signals that persist in the embryo after neural induction. Maintenance of a BMP/Wnt-free environment effectively stabilizes the neural fate of the NE precursors, which allows them to transition into neural plate stem cells and prevents them from converting back to a non-neural state. To our knowledge, the roles of these early nTFs in stabilizing neural fates in ESC cultures have not been explored. But, maintaining neural ectodermal precursors does depend on inhibiting endogenously produced BMP signaling [2], and maintenance of SOX1 (another nTF) expression in human ESC and iPSC cultures requires continuous down-regulation of BMP signaling [32,33]. Thus, the maintenance of the NE precursor state in ESC and iPSC cultures requires as yet undefined transcriptional regulation.
Figure 2.
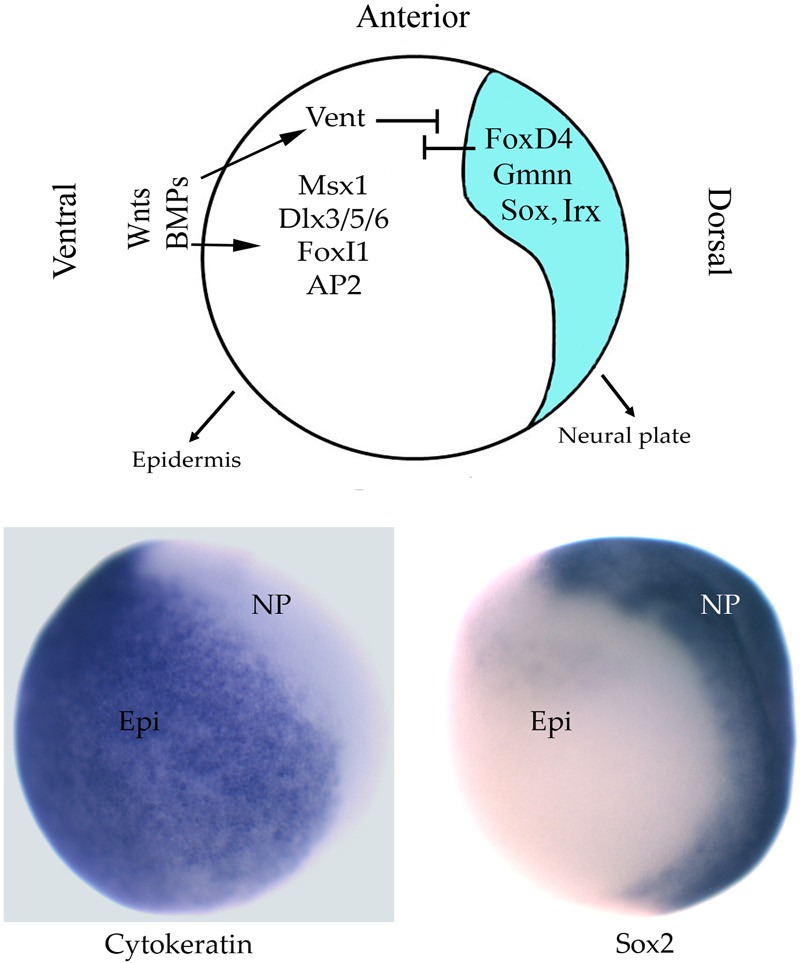
After the neural ectoderm is induced, its fate is stabilized by transcription factors that oppose BMP and Wnt signals. Top: Side view of a late gastrula Xenopus embryo after neural induction has occurred. The ectoderm is divided into two domains: neural (blue), which will give rise to the neural plate, and non-neural (white), which will give rise to the epidermis. Ventrally, BMP and Wnt signals activate epidermis specific transcription factors (e.g., Msx, Dlx, FoxI, AP2) and Vent transcription factors that repress neural ectoderm. Dorsally, neural induction activates transcription factors (e.g., FoxD4, Gmnn, Sox, Irx) that stabilize neural fate by opposing BMP and Wnt activities. Bottom: The two transcriptionally different ectodermal domains are illustrated by expression of epidermis-specific Cytokeratin in the epidermis (Epi) and Sox2 expression in the neural plate (NP).
Expansion of the neural plate stem cells is a common phenotype observed in embryos when the level of each nTF is experimentally increased in NE precursors. This phenotype may occur because the nTFs have expanded the domain in which BMP signaling is repressed, as described above. In addition, neural plate expansion also results from the ability of some nTFs to promote the proliferation of neural plate stem cells, and/or delay their differentiation into neural progenitors. For example, in Xenopus embryos, Gmnn maintains NE precursors in a proliferative state by modulating interactions between the SWI/SNF complex and the bHLH neural differentiation genes [34,35]. FoxD4L1 expands the expression domain of a marker of immature neural ectoderm (Otx2), increases the number of proliferating cells (Figure 3), and down-regulates the expression of bHLH neural differentiation genes [36]. Both Gmnn and Foxd4l1 are down-regulated as neural plate stem cells exit the cell cycle and differentiate into neural progenitors [25,36]. Zic2 also represses bHLH neural differentiation genes and counteracts the formation of extra neurons when bHLH genes are ectopically expressed in the epidermis [37]. These studies indicate that Gmnn, FoxD4L1 and Zic2 cause neural plate expansion by promoting NE precursor and neural stem cell proliferation and delaying the bHLH-mediated establishment of differentiating neural progenitors.
Figure 3.
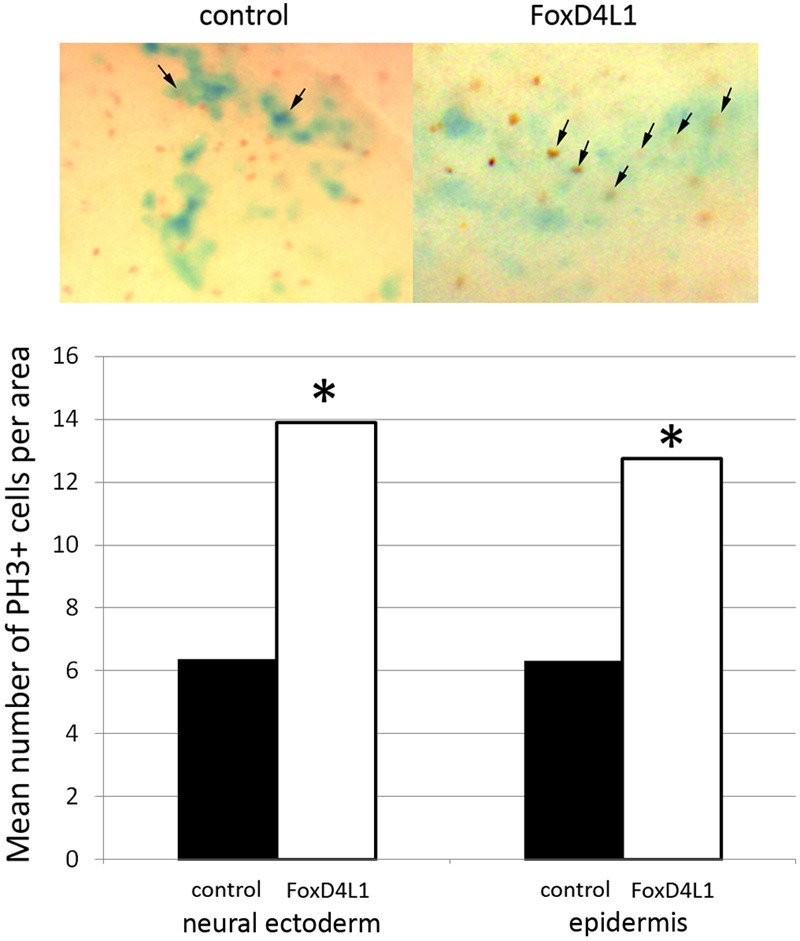
FoxD4L1 increases the number of proliferative cells when expressed in either neural ectoderm or epidermis. Clones of cells, identified by blue cytoplasmic expression of the beta-galactosidase lineage marker, were immunostained for the mitosis marker, phosphorylated histone H3 (PH3). The mean number of labeled cells in the clone (arrows in top panel) was counted. Expression of FoxD4L1 in the clone significantly increased the number of PH3-positive cells over controls (p<0.05; t-test).
The remaining nTFs appear to counteract this activity by transitioning cells to begin to differentiate, a process that is controlled by the well-studied bHLH neural differentiation genes and requires cells to exit the cell cycle. In several animals, SoxB1 family members (Sox1, Sox2, Sox3) maintain neural stem populations and must be down-regulated for neural progenitor differentiation to proceed [38-44]. When expressed at high levels, each maintains neural stem cells in a proliferative state upstream of neuronal differentiation genes [41,42,44-48]. Likewise, Sox11, a member of the SoxC subfamily, is up-regulated as neural stem cells transition to neural progenitor cells, and later maintains pan-neural gene expression in neuronal progenitors [43,49,50]. Thus, together these Sox genes may function downstream of FoxD4L1, Gmnn and Zic2 to promote the initial step from neural stem to neural progenitor cell. Several studies show that Zic and Irx genes function in neural progenitors downstream of the SoxB1 genes to promote the onset of bHLH neural differentiation gene expression. Zic1 is required for the expression of SoxD (a member of the SoxG group; [51], which causes ectopic neural masses that express bHLH neural differentiation genes [52], and Zic1 and Zic3 promote the expansion of neural progenitors in the spinal cord and forebrain [53-56]. In Drosophila, Iroquois genes are required for the activation of the proneural bHLH genes [57], and in Xenopus the homologous Irx/Xiro genes are expressed just prior to the earliest expressed bHLH neural differentiation genes, promote the onset of neural differentiation [58,59], but suppress terminal differentiation into neurons [60]. Thus, all of the nTFs that are expressed at the earliest stages of neural development have intertwining roles in maintaining a NE precursor state, regulating the size of the neural plate stem cell population and controlling the onset of differentiation of the neural progenitor cells (Figure 4).
Figure 4.
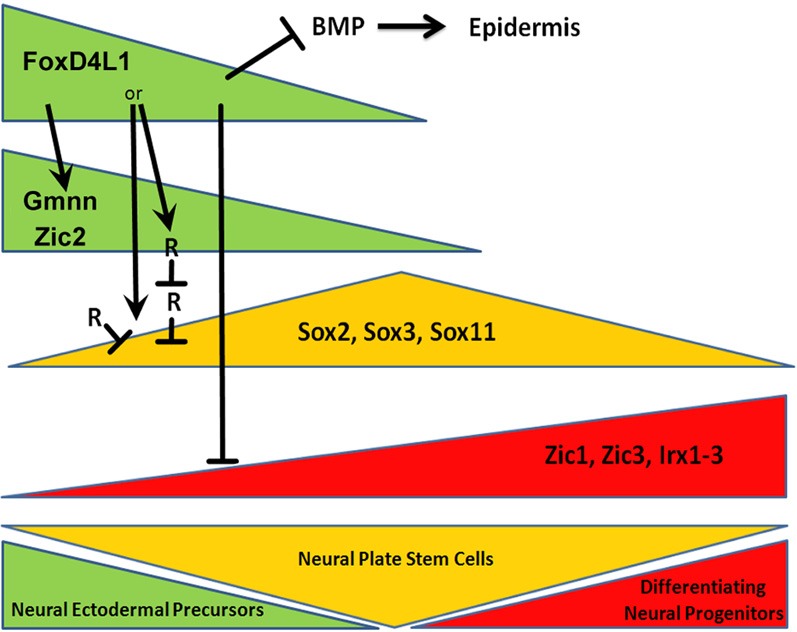
Neural transcription factors can be divided into three groups that act sequentially during the transition from a newly induced neural ectoderm (left side) to the onset of neuronal differentiation (right side). At early stages, neural ectodermal precursors (green) express high levels of FoxD4L1, which directly up-regulates Gmnn and Zic2, transiently down-regulates the Sox neural plate stem cell genes (yellow) directly and/or indirectly, and down-regulates the neural progenitor genes (red). FoxD4L1 also opposes epidermal fate by down-regulating the BMP pathway. As FoxD4L1, Gmnn and Zic2 levels decrease, the neural ectoderm transitions to the neural plate and high levels of Sox gene expression. Finally, as the neural plate stem cells begin to differentiate, neural precursor gene expression predominates.
A neural gene regulatory network
The studies summarized above took a gene-by-gene approach to understand the functions of each nTF during the progressive transition from neural induction to neuronal differentiation. Based on their sequential and overlapping activities it seems likely that the nTFs coordinately function in a gene regulatory network (Figure 4). An initial set of nTFs maintains cells in a neural ectoderm precursor state that expands the neural ectoderm to its appropriate size. These are then down-regulated and a different set of nTFs is activated to convert the neural ectoderm precursors to neural stem cell constituents of the neural plate. Finally, neural stem cell nTFs are down-regulated and a third set of nTFs are activated that initiate neural differentiation. Our understanding of the functional and transcriptional relationships between these proteins is woefully incomplete, yet this information is fundamental for understanding how the balance between neural precursor, neural stem and neural progenitors is molecularly regulated. Since developmental events often are controlled by regulatory networks of transcription factors that control temporal and region-specific gene expression [61], we sought to place a large number of these nTFs in a regulatory network to determine their distinct roles in the progression from NE precursors to differentiated neurons.
To accomplish this, we analyzed the epistatic position of FoxD4L1 amongst these other nTFs. First, we tested whether FoxD4L1 is required for their expression. We reduced the level of endogenous FoxD4L1 protein in NE precursors by introducing anti-sense morpholino oligonucleotides (MOs) directed against its mRNA into the major neural lineage of cleavage stage embryos. The expression of each of the other nTFs was significantly reduced in the absence of FoxD4L1 [29], thus placing it upstream in the network (Figure 5). Second, we elevated FoxD4L1 levels in NE precursors by introducing its mRNA into the major neural lineage. We observed three effects: 1) Gmnn and Zic2 expression levels were up-regulated; 2) Sox2, Sox3 and Sox11 expression was transiently down-regulated in NE precursors, which resulted in an expanded neural plate; and 3) Zic1, Zic3, and Irx1-3 were down-regulated in both NE precursors and neural plate stem cells. These results indicate that FoxD4L1 up-regulates early-acting nTF genes that maintain immature NE precursors, delays the expression of intermediate-acting nTFs that maintain neuneural plate stem cells by a transient down-regulation, and down-regulates later-acting nTF genes that promote neural progenitor differentiation (Figures 4, 5).
Figure 5.
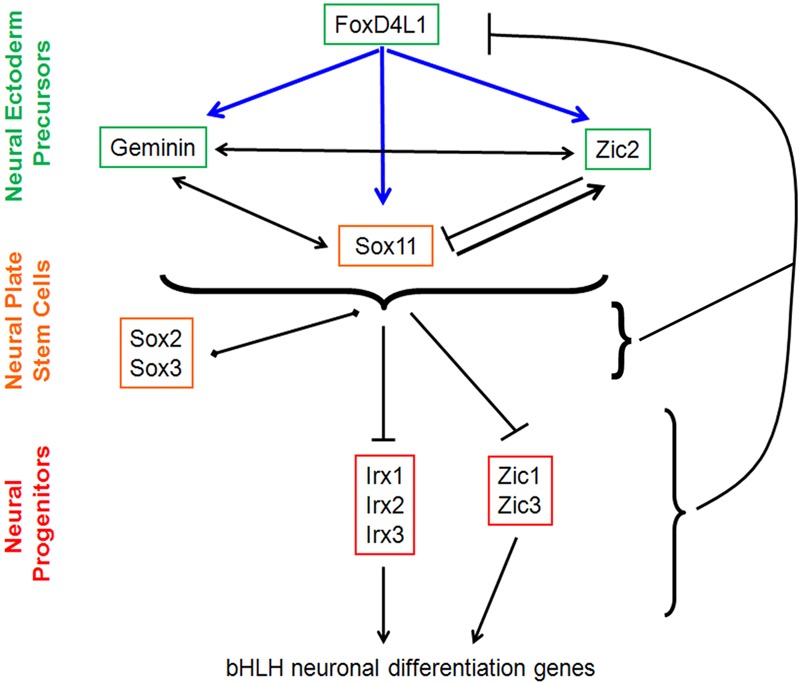
The neural ectoderm gene regulatory network that is active as cells transition from neural ectoderm precursors to neural plate stem cells and then to differentiating neural progenitors. Knock-down experiments demonstrate that FoxD4L1 is required for the expression of the other genes in this network. FoxD4L1 directly up-regulates Geminin, Zic2 and Sox11 (blue arrows). These three genes also regulate each other. Together, the NE precursor genes delay the expression of neural plate stem cell genes (Sox2, Sox3) and down-regulate neural progenitor genes (Zic, Irx), which are required for the expression of the bHLH neuronal differentiation genes. Zic and Irx also feedback to down-regulate FoxD4L1. Arrows depict up-regulation; bars depict down-regulation; diamonds depict delayed expression.
Interestingly, FoxD4L1 expression is down-regulated at early neural plate stages [36,62,63]. While it is initially expressed throughout the NE precursor domain, in the neural plate it first becomes confined to an anterior band and the midline, and eventually is maintained only as a stripe in the midbrain. We postulated that this regression results from negative feedback from some of the nTFs. None of the early-acting nTFs (Gmnn, Zic2, Sox11) significantly alters FoxD4L1 expression, consistent with our interpretation that these three genes act downstream of FoxD4L1 [29]. Conversely, intermediate-acting (Sox2, Sox3) and late-acting (Zic1, Zic3, Irx1-3) nTFs repress FoxD4L1, indicating that they feedback to down-regulate FoxD4L1 and thereby release its inhibition on neural differentiation (Figure 5). We speculate that this allows NE precursors to progress to neural plate stem cells and to neural progenitors.
Another aspect to the gene regulatory network was revealed by a method that is commonly used in Xenopus embryos to determine direct gene targets [64]. One can express the mRNA encoding a hormone-inducible form of a transcription factor at cleavage stages. The fusion protein is immediately synthesized, but it cannot be transported into the nucleus in the absence of hormone. When the embryos reach gastrulation stages, they are incubated in cycloheximide to prevent further protein synthesis, and then treated with hormone. If a target gene is directly regulated by the transcription factor, it will still be activated in the absence of protein synthesis, whereas if an intermediate protein is required no activation will occur. Using this method, we discovered that Gmnn, Zic2 and Sox11 each are direct transcriptional targets of FoxD4L1 [29]. An in silico analysis of the ~1600 bp upstream of the transcriptional start site of each gene revealed at least one Forkhead consensus binding site, supporting the conclusion that FoxD4L1 may directly regulate Gmnn, Zic2 and Sox11. In additional experiments we expressed Gmnn, Zic2 and Sox11 in the absence of FoxD4L1; the results demonstrated that together these three genes can mediate the FoxD4L1 effects on the remaining nTFs [29]. Consistent with this proposed network, in chick Gmnn interacts in a complex at a neural enhancer to promote Sox2 transcription [65]. From these results we can construct a gene regulatory network in which FoxD4 is a critical upstream component. It directly activates a transcriptional triad consisting of Gmnn, Zic2 and Sox11, which in turn regulates the more down-stream components that promote the transition to neural stem and neural progenitor cells (Figure 5).
The experiments described above demonstrate that FoxD4L1 is a critical element in the gene regulatory network that regulates the balance between NE precursors, neural plate stem cells and regionally specified neural progenitor cells. Elucidating how these different nTF genes interact to regulate this balance between expanding precursor populations and initiating differentiation should ultimately prove useful for preventing the formation of mesodermal and endodermal cells in neuronal culture protocols, expanding the number of neural precursors available and preventing their premature differentiation. Therefore, we embarked on a detailed structure-function analysis of the FoxD4L1 protein.
FoxD4L1 regulates nTF genes by both transcriptional activation and repression
Xenopus FoxD4L1 has the interesting property of up-regulating some nTF genes and down-regulating others. Determining how it has this dual effect is key to understanding its transcriptional role in the gene network that regulates early neural development. A fundamental question is: does FoxD4L1 function as a transcriptional activator or a transcriptional repressor? We addressed this by making constructs that fused the FoxD4L1 DNA-binding domain to either VP16-activating or EnR-repressing domains; which fusion protein mimics the wild-type phenotype when expressed in a neural lineage indicates the transcriptional activity required for that phenotype. We found that FoxD4L1 transcriptional repression mediates the: 1) expansion of early pan-neural markers (Sox3, Otx2); 2) down-regulation of neural patterning genes (En2, Krox20); 3) down-regulation of neural progenitor-promoting nTFs (Zic1, Zic3, Irx1-3); and 4) down-regulation of neural differentiation genes (Ngn, NeuroD) [29,36]. In contrast, the up-regulation of Gmnn and Zic2 is mediated via transcriptional activation [29]. However, the effects of these constructs on the initial down-regulation of Sox2, Sox3 and Sox11 in NE precursors implicated both activating and repressing activities, suggesting that the regulation of Sox genes by FoxD4L1 is accomplished by both direct and indirect interactions that are yet to be defined (Figure 4). Together, these experiments indicate that FoxD4L1 can function as both a transcriptional activator and a repressor, depending upon the target gene, to balance the switch from proliferative NE precursor to differentiating neural progenitor.
Members of the Fox (forkhead box) gene family of transcription factors all contain a highly conserved Forkhead DNA binding domain, variations in which classify the genes into 19 sub-families (“A” through “S”) [66-70]. However, the amino- (N-) and carboxyl- (C-) terminal trans-regulatory domains of the protein that flank the forkhead box are highly divergent. Some Fox proteins regulate transcription by activation, some by repression, and a few by both, depending upon the cell type, the developmental state or the availability of interacting proteins. In addition, some Fox proteins act as “pioneer” transcription factors during development [71-73]. Pioneer factors stably bind to their recognition sites in chromatin domains of nuclear DNA that other factors cannot access, causing a conformational change that allows other proteins to engage the DNA [74].
Some Fox proteins contain acidic domains, in either the N- or C-terminal regions, that are thought to be involved in target gene activation [75,76]. In Xenopus, only members of the FoxD class contain a specific kind of acidic region termed the acidic blob (AB) [67]. To identify this region in Xenopus FoxD4L1 we performed a CLUSTALW alignment of several vertebrate FoxD4/FoxD4L1 proteins [77]. There is a 14 amino acid AB in the N-terminal sequence of all species examined, within which there are several highly conserved residues (Figure 6A). To determine whether the AB region of Xenopus FoxD4L1 is required for target gene transcriptional activation, we made several deletion constructs, expressed them within the neural ectoderm of embryos, and analyzed whether Gmnn or Zic2 was up-regulated within the lineage-labeled clone [77]. For both genes, deleting the entire N-terminus of FoxD4L1 caused a significant reduction in the percentage of embryos showing up-regulated target genes, whereas deleting the entire C-terminus had no effect. These results indicate that an activation domain likely resides in the N-terminal part of the protein. Activation was pinpointed to the AB by the observation that the percentage of embryos showing up-regulated Gmnn or Zic2 expression was dramatically reduced when the AB domain was deleted; similar sized alterations of putative functional domains in the C-terminus of the protein did not affect its ability to up-regulate either Gmnn or Zic2 [77]. Further sequence analysis of the AB region indicated that it consists of two highly acidic regions separated by a predicted β-strand (Figure 6A); further mutational analyses indicated that the ability to activate transcription requires flexibility of the protein at a glycine residue downstream of the β-strand that we predict brings the two acidic regions in close proximity [78]. Whether this conformational change promotes transcriptional activation by changing interactions with the DNA or allows/prevents protein-protein interactions has yet to be determined. Interestingly, this protein structure is highly conserved across fish, frog and mammals, indicating it is likely to have functional importance in mammalian stem cells.
Figure 6.
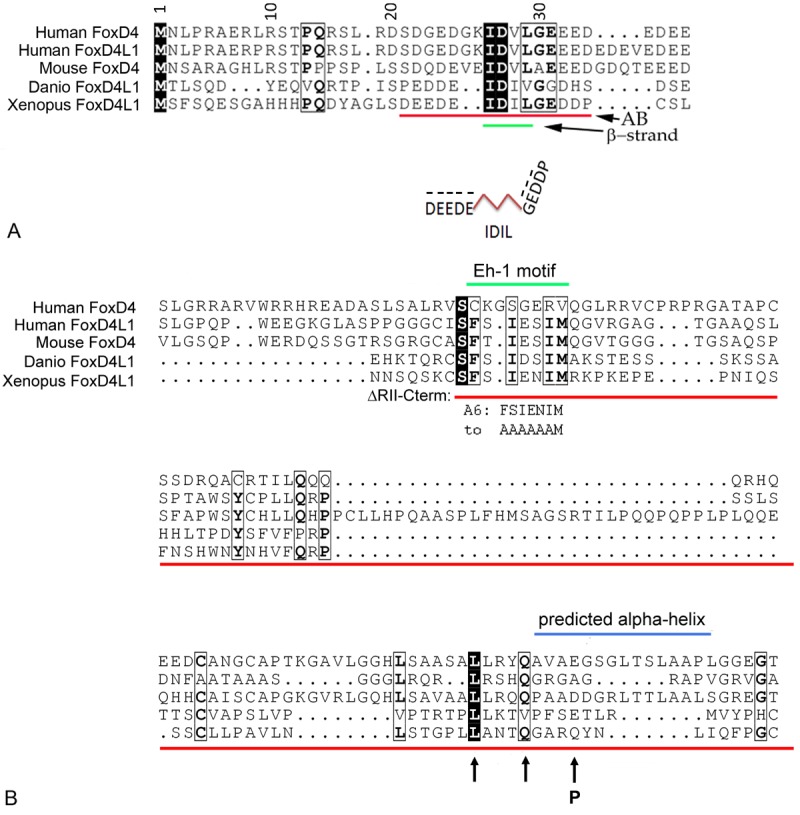
Functional domains of the FoxD4L1 protein are conserved across human, mouse, fish and frog. A: Within the first 40 amino acids in the N-terminus of FoxD4/FoxD4L1 proteins there is an acidic blob (AB) region (red line) that contains a short β-strand (IDIL/IDVL) flanked by negatively-charged (-) acidic amino acids (e.g., D, E). Structure-function studies carried out in Xenopus embryos show that this region is responsible for the transcriptional activation activity of FoxD4L1, and that a glycine residue (G) downstream of the β-strand is required for flexibility in the protein to bring the two acidic domains in proximity. B: Within the C-terminal region of the FoxD4/FoxD4L1 proteins is a conserved Eh-1 motif (green bar), which binds the Groucho/Grg transcriptional repressor co-factor, and a conserved region that is predicted to form an α-helical structure (blue bar). Deletion of the entire region (ΔRII-Cterm; red bar) eliminates the transcriptional repressive activity of FoxD4L1. Mutation of the Eh-1 motif (called A6) that changes the sequence from FSIENIM to AAAAAAM prevents Grg binding and reduces repression. In addition, mutation of a single amino acid predicted to break the α-helix (P to Q) reduces repression. These experiments indicate that both Grg binding and the α-helical structure are required for full repressive activity of the protein. Arrows at conserved L and Q indicate control mutations near the α-helix that had no effect on repressive function of the protein.
The C-terminal region of some Fox proteins contains domains implicated in transcriptional repression. These include a P/A/Q-rich region, a highly charged Region II (R-II) and an Engrailed homology region-1 (Eh-1) motif that can bind the well-known co-repressor protein, Groucho [Gro in fly; Grg in vertebrates; TLE in humans] (reviewed in [36,67,79]). The Eh-1 motif is conserved in several Fox proteins, including all FoxD proteins [79]. The interaction of this motif with Grg proteins facilitates the repression of the downstream targets of FoxD3, FoxA1 and FoxA2 [80,81]. The C-terminal portion of the Xenopus FoxD4L1 protein contains a P/A/Q rich region, an R-II domain, and within the R-II there is an Eh-1 motif that is highly conserved across vertebrate FoxD4/FoxD4L1-related proteins (Figure 6B). To determine whether any of these regions are specifically required for down-regulating FoxD4L1 targets, we made several deleted/mutated constructs, expressed them within the neural ectoderm, and analyzed whether Zic1, Zic3, Irx1, Irx2 or Irx3 expression within the lineage-labeled clone was down-regulated. First, we found that deleting the entire N-terminus or just the AB had no effect on the ability of FoxD4L1 to down-regulate each of these five nTF genes. This indicates that the portion of the protein that is involved in transcriptional activation has no role in transcriptional repression, and that the Zic and Irx genes can be repressed independent of the triad of activated target nTFs (Figure 5). In contrast, deletion of either the entire C-terminus or the RII-region plus the rest of the C-terminal domain (ΔRII-Cterm) results in a nearly complete loss of transcriptional repression of all five Zic and Irx genes. Since the P/A/Q-rich region is retained in the ΔRII-Cterm deleted mutants, we conclude that this domain plays a minor, if any role, in the protein’s repressive activity.
Since Grg can bind to the Eh-1 motif and thereby mediate the repressive effects of some other Fox proteins [79-81], we tested whether Grg is responsible for the ability of FoxD4L1 to repress these five nTFs. Co-immunoprecipitation assays showed that Grg4 can interact with FoxD4L1, and that this interaction is lost if the Eh-1 motif is deleted or mutated [77]. A mutant FoxD4L1 in which 6 of the 7 amino acids of the Eh-1 motif were changed to alanine (A6 mutant; Figure 6B) lost the ability to down-regulate the expression of Irx2 in the neural plate similar to the ΔRII-Cterm deletion, suggesting that the binding of Grg4 to the Eh-1 motif is sufficient for Irx2 repression. In contrast, repression of Zic1, Zic3, Irx1, and Irx3 was lost to a significantly greater extent with the ΔRII-Cterm construct compared to the A6 mutant. This indicates that there are additional regions in the RII-Cterm domain that are needed for full repression of these Zic and Irx genes. This conclusion was born out by two sets of experiments. First, by introducing a range of concentrations of FoxD4L1 and Grg4 mRNAs alone and together, we showed that FoxD4L1 and Grg4 cooperatively repress Zic1, Zic3, or Irx1 in a dose dependent manner. Surprisingly, however, in the absence of Grg4 high levels of FoxD4L1 still repress these three nTF genes. Because binding site affinity can affect transcription factor occupancy and activation versus repressive function [82], we speculate that Grg4 facilitates FoxD4L1 transcriptional repression when the concentration/occupancy of FoxD4L1 is low, but is not required when the concentration/occupancy of FoxD4L1 is high. Second, we performed several cross-species motif and secondary structure prediction analyses to predict conserved functional domains [78]. Two highly conserved motifs were identified downstream of the Eh-1 motif, suggesting that they could be involved in target repression. One of these overlapped with a predicted α-helix at the C-terminus (Figure 6B). Mutation of conserved amino acids predicted to destabilize the α-helix did not reduce FoxD4L1’s ability to repress Zic3 or Irx1. In contrast, substituting a proline residue within the predicted α-helical structure, which should break the helix, significantly reduced repression [78]. Together, these experiments show that FoxD4L1 can repress targets genes via two independent sites in the C-terminal region of the protein. The Eh-1 motif binds to Grg proteins, and based on work on FoxA proteins [74], likely interacts with histone modifying enzymes to close nucleosomes. How the second site in the C-terminal region mediates the molecular interaction of FoxD4L1 with either the DNA or unidentified co-factors remains to be determined. Because these predicted motifs/secondary structures are highly conserved across species, other members of the FoxD4/FoxD4L1 sub-family are likely to have similar functions.
FoxD4L1 and the Sox genes
Not every gene that is down-regulated by FoxD4L1 in embryo assays can be explained simply by domains within the C-terminus of the protein. In the course of these studies we investigated effects on four different members of the Sox family of nTFs: Sox2 and Sox3 (Sox B1 sub-family), Sox11 (SoxC sub-family) and SoxD (SoxG sub-family member that is unique to amphibians). All four of these Sox genes are expressed in NE precursors and neural plate stem cells, and all four require FoxD4L1 activity because their expression levels are significantly reduced when FoxD4L1 is knocked down in Xenopus embryos [29]. However, the effects of expressing either wild-type FoxD4L1, or the VP16- and EnR-FoxD4L1 fusion proteins have different effects on the different Sox genes. Increased expression of wild-type FoxD4L1 initially down-regulates Sox2, Sox3, and Sox11 in NE precursors [29], but this is a transient effect. When observed at neural plate stem cell stages, the domains of Sox2, Sox3, and Sox11 neural stem cells are expanded, and the level of expression of Sox11 is increased. In contrast, SoxD expression in NE precursors is unaffected by increases in wild-type FoxD4L1, but it is strongly repressed in neural plate stem cells. When we tested whether the down-regulation of each Sox gene was mediated by transcriptional activation (VP16-fusion protein) or transcriptional repression (EnR-fusion protein), the results were mixed [29]. Sox2 and Sox11 are initially repressed by both the activating and repressing forms of FoxD4L1, whereas Sox3 is initially repressed only by the repressing form and SoxD is weakly repressed only by the activating form. These results suggest that FoxD4L1 down-regulation of Sox genes involves intermediate proteins that are not yet identified (Figure 4).
Concordant with these results, we also found that the down-regulation of each Sox gene was altered by both N-terminal and C-terminal sequences [77]. While deletion of the entire C-terminus did not alter the percentage of embryos showing down-regulation of Sox2, it significantly reduced the down-regulation of Sox3, Sox11, and SoxD, as did the ΔRII-Cterm construct. In addition, the N-terminal mutants differentially affected the expression levels of the Sox genes. For Sox2 and Sox3, deleting the entire N-terminus or just the AB domain significantly increased the percentage of embryos showing down-regulation in NE precursors, indicating that the AB domain normally ameliorates the repression of Sox2 and Sox3 by FoxD4L1. We propose that either wild-type FoxD4L1 directly activates Sox2 and Sox3, which are secondarily repressed by other genes, or it activates genes that repress Sox gene repressors (Figure 4). In contrast, deleting either the entire N-terminus or just the AB domain caused Sox11 to be up-regulated, rather than down-regulated, in NE precursors. This suggests that FoxD4L1 normally activates a gene that represses Sox11. In fact, we showed that Sox11 expression is down-regulated by Zic2 [29] (Figure 5). Consequently, we propose that deletion of the AB domain, which causes a loss of Zic2 up-regulation, also leads to a de-repression of Sox11. Finally, although down-regulation of SoxD in neural plate stem cells is moderately affected by deletion of the AB domain, it is completely eliminated by deletion of the RII-Cterm domain; these results suggest that it is both activated and repressed by FoxD4L1. Together, these results indicate that FoxD4L1 affects Sox gene transcription by both activation and repression and most likely involves both direct regulation and indirect interactions involving intermediate genes (Figure 4). Unraveling the complex relationship between FoxD4L1 and the Sox proteins will be critical for understanding the transition of NE precursors to neural stem cells.
FoxD4L1 can convert non-neural ectoderm to neural ectoderm by combined transcriptional activation and transcriptional repression
A common method to demonstrate in embryos whether a protein is involved in the earliest steps of neural specification is to ectopically express a gene in the non-neural ectoderm that will give rise to epidermis and analyze whether neural genes are expressed in the foreign territory. Using this approach we showed that FoxD4L1 induces the ectopic expression of Gmnn, Zic2 and Sox11, and down-regulates BMP signaling and subsequent expression of epidermal genes [29,30]. We hypothesized that both gene repression and gene activation would be involved since ectodermal cells must turn off an epidermal program and turn on a neural program. In fact, deleting either the N-terminus or the C-terminus reduced, but did not eliminate ectopic induction of Gmnn, Zic2 or Sox11, suggesting that both domains and thereby both transcriptional activities, are required. This was confirmed by expressing either the AB domain deleted protein or the A6 mutant protein in the non-neural ectoderm; each significantly reduced, but did not eliminate, the frequency of ectopic expression of these three nTFs. Because both activation and repression are involved, we hypothesized that optimal ectopic induction requires that the nTFs are up-regulated via an intact AB domain and that a second set of genes are down-regulated via binding of Grg to the Eh-1 motif. Two experiments support this idea [77]. First, co-expression of the AB-deleted and A6 mutant constructs completely restored the frequency and intensity of ectopic induction in the epidermis. Since these constructs bind to DNA independently, they must be affecting at least two different targets, activating some (the A6 mutant contains an intact AB domain) and repressing others (the AB-deleted construct has an intact Eh-1 motif). Second, simultaneously preventing either activation or repression by co-expressing the AB-deleted construct with Grg MOs additively reduced ectopic induction. Together, these data demonstrate that the ability of FoxD4L1 to ectopically induce nTF genes in the non-neural ectoderm requires both activation of the neural genes, mediated via the AB domain, and repression of epidermal genes, mediated via the Eh-1 motif binding to Grg.
The experiments described above illuminate the functionality of the FoxD4L1 protein, which plays a pivotal role in the early steps of neural specification. First, they identify a specific domain in the N-terminal part of the protein that enables FoxD4L1 to activate a subset of target nTF genes. Second, they identify two domains in the C-terminal part of the protein that enables FoxD4L1 to repress a different subset of target genes. These findings illustrate how this single transcription factor can regulate the transition of immature, NE precursors to neurally-committed stem cells, and then to neural progenitors that are beginning to differentiate. Can this information help us understand how mammalian ESCs transition through a similar developmental program in culture?
Mammalian FoxD4L1/FoxD4
Despite the key role demonstrated for FoxD4L1 in early neural development in Xenopus embryos, little is known about its function in other vertebrates. All vertebrate genomes investigated to date contain a FoxD4L1 and/or FoxD4 gene, and where investigated these are expressed in the developing nervous system and occasionally in a few mesodermal structures [83-90]. The mouse has a single FoxD4 gene, whereas zebrafish and Xenopus have duplicated FoxD4L1 genes (FoxD4L1.1 and FoxD4L1.2, formerly called FoxD5a and FoxD5b). Interestingly, in primates a fusion event that led to the formation of chromosome 2 caused duplications in FoxD4. The protein encoded by human FOXD4L1 (2q13) is most similar to those encoded by fish and frog FoxD4L1 and by mouse FoxD4 [91]. The protein encoded by human FOXD4 (9p24.3) is most similar to five additional FoxD4L-related genes also located on chromosome 9 (FOXD4L2-L6) [83,91]. Only FoxD4L1 and FoxD4 are known to be transcriptionally active in the developing embryo or the nervous system, and only the proteins coded by these two genes contain an Eh-1 motif and a C-terminal α-helical domain similar to those functionally characterized in Xenopus embryos (Figure 6). The functions of the FoxD4 and FoxD4L1 genes in neural development have not yet been reported in other vertebrates. But in humans, a mutation in FOXD4 is associated with obsessive-compulsive disorder and suicidality [92], and a deletion on chromosome 9 that includes FOXD4 is associated with cerebral palsy and cerebral dysfunction [93]. Neurological disorders were also observed in a patient with partial monosomy of chromosome 9 involving the FOXD4 interval [94]. Because of the important role that FoxD4L1 plays in Xenopus neural development, and the scant, but consistent evidence that it may play a similar role in mammals, we have initiated studies to elucidate its role in regulating the formation of neurons in mouse ESC cultures.
nTFs and mouse ESC neural differentiation
One would predict from the embryo work reviewed above that during the differentiation of ESCs into neural stem cells (NSCs) FoxD4, Gmnn, Zic2 and Sox11 would act early to promote immature, proliferative NE precursors and impede neural differentiation (Figures 4, 5). To our knowledge, the role of Zic2 has not been examined in ESC culture, whereas Sox genes have been extensively studied. SoxB1 family proteins (Sox1, Sox2, Sox3) are expressed throughout mouse and chick embryo NE precursors and neural plate stem cells, and when expressed at high levels they prevent cells from differentiating into neurons [50,51]. In addition, Sox2 has an earlier function in ESCs: maintenance of pluripotency [95]. The Sox11 gene (SoxC family) appears to have different developmental roles in different vertebrates. In Xenopus, Sox11 is expressed throughout NE precursors and neural plate stem cells, overlapping completely with Sox2 and Sox3 expression [31]. However, in chick and mouse embryos, Sox11 is expressed later in development in neural progenitors and differentiating neurons, and does not overlap with Sox2 or Sox3 expression in the neural tube [50,51]; in mouse it also is expressed in several other tissues to support progenitor cell survival [96]. A recent study showed that Sox2, Sox3 and Sox11 bind to the promoters/enhancers of the same neural genes expressed in mouse NSCs, neural progenitors and differentiating neurons in a defined temporal sequence [97]. In ESCs, Sox2 binds to NSC genes to mark sites for later Sox3 binding. Subsequently in NSCs, Sox3 binds to neuronal differentiation genes to mark sites for later Sox11 binding. In each case, Sox protein binding results in bivalent chromatin marks, indicating that part of their effect is making the appropriate neural genes accessible to other transcriptional factors that are expressed later.
Based on work in the embryo, one also would expect Gmnn to be expressed at high levels in NE precursors during ESC neural differentiation protocols. Unlike the other nuclear proteins in the neural gene regulatory network (Figure 5), Gmnn does not act as a typical DNA binding transcription factor but instead works via several different protein-protein interactions to affect chromatin conformation. In the embryo, it promotes an uncommitted state by promoting Polycomb mediated repressive histone marks at differentiation-promoting genes [98]. It also prevents premature neurogenesis by antagonizing the interaction between Brg and bHLH neural differentiation factors [34]. Just like in the embryo, in mouse ESCs Gmnn is required for the acquisition of a neural fate, and can promote the formation of neural precursor cells even in the presence of high anti-neural BMP levels [99]. This study showed that Gmnn accomplishes this by maintaining the chromatin in a hyper-acetylated state and in an open conformation that allows other nTFs access to their binding sites on the DNA. Subsequent work showed that Gmnn promotes both activating and repressive histone modifications at neural differentiation genes, and that expressing Gmnn at high levels does not prevent neuronal differentiation [100]. These results suggest a model whereby Gmnn makes the chromatin accessible to both factors that promote NE precursors as well as to factors that will subsequently activate neural differentiation genes. This model helps explain the seemingly contradictory observations that Gmnn-deficient neural progenitors in the mouse cortex are defective in cell proliferation and differentiation [101], whereas Gmnn-deficient NSCs in neurospheres can still divide and differentiate into neurons and glia [102]. It is interesting to speculate that the preloading of chromatin-modifying nuclear proteins, such as Gmnn and Sox, on nTF genes may account for the ESC predisposition to differentiate into neurons.
Based on work from embryos, mouse FoxD4 should be expressed as an early response to neural inductive signaling and be one of the earliest nTFs expressed. It should precede expression of neural stem, neural progenitor and neuronal differentiation genes, and be down-regulated when neural differentiation begins. To test these predictions we have used a protocol in which mouse ESCs are cultured in rotating chambers to promote the formation of embryoid bodies (EBs). After 2 days in culture, EBs are treated with retinoic acid (RA) for 2 days to promote a neural program, and subsequently cultured in its absence for several days to promote neuronal differentiation (Figure 7A). EBs collected from each time point were analyzed by real-time qPCR, or fixed and immunostained for markers of the early steps of neural development (Figure 7B). In accord with the data from Xenopus embryos, FoxD4 expression is immediately up-regulated in EBs after neural induction (RA treatment), and at this time point markers of pluripotent stem cells drop. FoxD4 expression remains high for only two days in culture, i.e., just like in the embryo it is transient. At the time when FoxD4 expression begins to drop neural stem and neural progenitor markers appear and increase over time. Finally, when FoxD4 levels are minimal, bHLH neuronal differentiation markers and terminal neuronal markers are highly expressed. Thus, the time course of FoxD4 expression in an ESC neural differentiation culture system is in accord with the time course reported in the embryo (Figure 7). We are now manipulating the levels of FoxD4 at different stages of this neural differentiation protocol to test the hypothesis that FoxD4 is required for the production of immature, neurally-stabilized NE precursors, and that when it is expressed at high levels it reduces the production of differentiating neural progenitors. These experiments will determine whether in the mammalian ESC differentiation regimens, FoxD4 can be used to regulate the balance between neurally-committed, undifferentiated NE precursors and neuronal differentiation.
Figure 7.
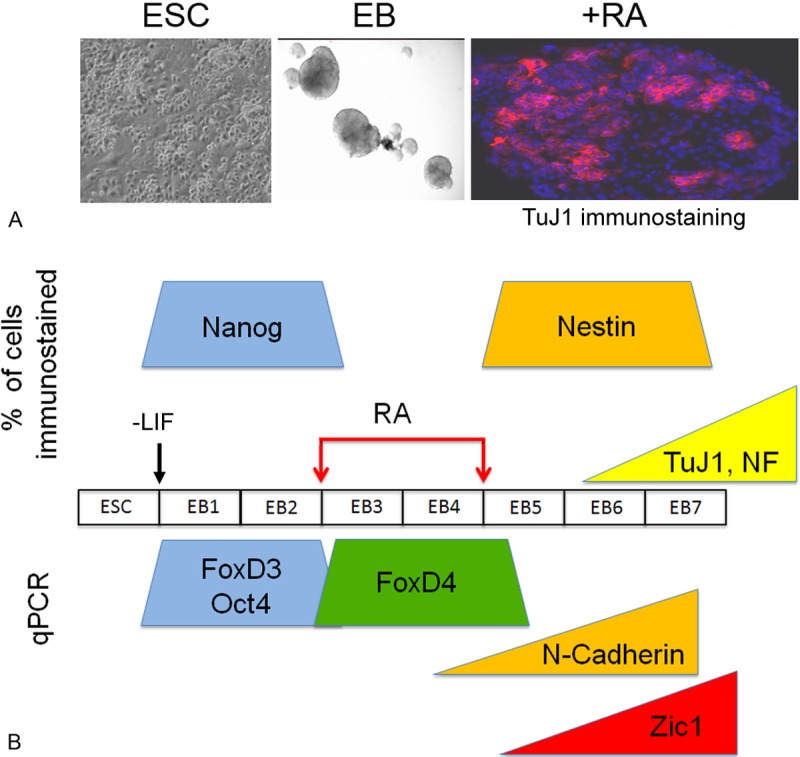
The timing of gene expression in a neuronal differentiation protocol of mouse embryonic stem cells is similar to that in a frog embryo. A: Mouse embryonic stem cells (ESC) are grown as colonies on a STO fibroblast feeder layer in a medium that contains 15% serum, LIF and β-mercaptoethanol. They form embryoid bodies (EB) when removed from the feeder layer, cultured in a medium that contains 10% serum and no LIF or β-mercaptoethanol, and shaken to keep them non-adherent. If EBs are treated for two days with retinoic acid (RA), and then cultured for an additional 3-5 days without RA they differentiate into neurons that express the neuron-specific Class III β-tubulin identified by the TuJ1 antibody, indicated by red immunofluorescent staining. Nuclei are stained blue with DAPI. B: The neuronal differentiation protocol used, as described above, indicating the number of days in culture (EB1, EB2, etc.) and demarcating the 2-day period of exposure to RA. Below the protocol is a summary of gene expression results from real-time quantitative PCR assays (qPCR) that indicate that mouse FoxD4 is activated immediately upon RA treatment, and it subsides shortly after RA removal from the culture. The up-regulation of FoxD4 coincides with the loss of expression of pluripotency genes (FoxD3, Oct4). The down-regulation of FoxD4 coincides with the expression of neural stem cell (N-Cadherin) and neural progenitor (Zic1) genes. Above the protocol is a summary of gene expression results using immunofluorescence assays that counted the percentage of cells in the EB that expressed various proteins. In agreement with the qPCR assays, the up-regulation of FoxD4 coincides with loss of expression of a pluripotency protein (Nanog); the down-regulation of FoxD4 coincides with expression of a neural stem cell protein (Nestin), ultimately resulting in the production of mature neurons, as indicated by antibodies against neuron-specific β-tubulin (TuJ1) and neurofilament (NF) proteins.
Conclusions
As work in the embryo reveals more details about the regulation of the earliest aspects of neural development, including competence to respond to neural induction, neural fate stabilization and the onset of neural differentiation, we need to test these details in cultured stem cell paradigms to facilitate their manipulation for therapeutic uses. We have dissected the role of FoxD4L1 in Xenopus neural development to reveal several important functions that we predict will be relevant to stem cell biology. In the embryo FoxD4L1 promotes the formation of immature, NE precursors whose neural state is stabilized against a changing signaling environment, and it delays the progression to neural stem and differentiating neural progenitor cells, resulting in an expanded neural plate. Perhaps in mouse ESC culture maintenance of high levels of FoxD4 will allow NE precursors to continue to divide and expand, and prevent premature neuronal differentiation. Because in the Xenopus embryo FoxD4L1 represses BMP expression and/or signaling, thus promoting an environment that favors neural development, in mouse ESC culture maintenance of FoxD4 expression may allow cells to maintain their neural fate and not be converted to other cell types - perhaps by antagonizing BMP signaling or its downstream targets. It is now important to test these possibilities experimentally, and elucidate the essential gene regulatory network in mammalian ESC- and iPSC-derived neural cell cultures. Using the information we have obtained from the embryo should greatly facilitate these efforts.
Acknowledgements
This work was supported by NIH grants NS23158 (SAM), DC011534 (ASL) and HD29178 (ASL), NSF grant MCB1121711 (SAM) and the George Washington University Medical Center Facilitating Funds (ASL and SAM). Dr. Klein’s efforts were supported by the National Science Foundation while working at the Foundation. Any opinion, finding, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Disclosure of conflict of interest
None.
References
- 1.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- 2.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2000;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 3.Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T. Parkinson’s disease induced pluripotent stem cells with triplication of the a-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichterle H, Lieberam I, Poter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 5.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K, Sokol SY. Early Development of Epidermis and Neural Tissue. In: Moody SA, editor. Principles of Developmental Genetics. New York: Elsevier; 2007. pp. 241–257. [Google Scholar]
- 8.Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–256. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers CD, Moody SA, Casey ES. Neural induction and factors that stabilize a neural fate. Birth Defects Res C Embryo Today. 2009;87:249–262. doi: 10.1002/bdrc.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasai Y. Identifying the missing links: genes that connect neural induction and primary neurogenesis in vertebrate embryos. Neuron. 1998;21:455–458. doi: 10.1016/s0896-6273(00)80554-1. [DOI] [PubMed] [Google Scholar]
- 11.Kuo JS, Patel M, Gamse J, Merzdorf C, Liu X, Apekin V, Sive H. Opl: a zinc finger protein that regulates neural determination and patterning in Xenopus . Development. 1998;125:2867–2882. doi: 10.1242/dev.125.15.2867. [DOI] [PubMed] [Google Scholar]
- 12.Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stren CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- 13.Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signaling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- 15.Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- 16.Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5’-CACCT sequences in candidate target genes. J Biol Chem. 1999;274:20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- 17.Eisaki A, Kuroda H, Fukui A, Asashima M. XSIP1, a member of two-handed zinc finger proteins, induced anterior neural markers in Xenopus laevis animal cap. BBRC. 2000;271:151–157. doi: 10.1006/bbrc.2000.2545. [DOI] [PubMed] [Google Scholar]
- 18.Nitta KR, Tanegashima K, Takahashi S, Asashima M. XSIP1 is essential for early neural gene expression and neural differentiation by suppression of BMP signaling. Dev Biol. 2004;274:258–267. doi: 10.1016/j.ydbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Nitta KR, Takahashi S, Haramoto Y, Fukuda M, Tanegashima K, Onuma Y, Asashima M. The N-terminus zinc finger domain of Xenopus SIP1 is important for neural induction, but not for suppression of Xbra expression. Int J Dev Biol. 2007;51:321–325. doi: 10.1387/ijdb.062252kn. [DOI] [PubMed] [Google Scholar]
- 20.Snir M, Ofir R, Elias S, Frank D. Xenopus laevis POU91 protein, an Oct3/4 homologue, regulates competence transitions from mesoderm to neural cell fates. EMBO J. 2006;25:3664–3674. doi: 10.1038/sj.emboj.7601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greber B, Coulon P, Zhang M, Moritz S, Frank S, Muller-Molina AJ, Arauzo-Bravo MJ, Han DW, Pape HC, Scholer HR. FGF signaling inhibits neural induction in human embryonic stem cells. EMBO J. 2012;30:4874–4884. doi: 10.1038/emboj.2011.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chng Z, Teo A, Pedersen RA, Vallier L. SIP1 mediates cell-fate decisions between neuroectoderm and mesendoderm in human pluripotent stem cells. Cell Stem Cell. 2010;6:59–70. doi: 10.1016/j.stem.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Taylor JJ, Wang T, Kroll KL. Tcf- and Vent-binding sites regulate neural-specific geminin expression in the gastrula embryo. Dev Biol. 2006;289:494–506. doi: 10.1016/j.ydbio.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Rogers C, Archer TC, Cunningham DD, Grammer TC, Silva Casey EM. Sox3 expression is maintained by FGF signaling and restricted to the neural plate by Vent proteins in the Xenopus embryo. Dev Biol. 2008;313:307–319. doi: 10.1016/j.ydbio.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroll KL, Salic AN, Evans LM, Kirschner MW. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development. 1998;125:3247–3258. doi: 10.1242/dev.125.16.3247. [DOI] [PubMed] [Google Scholar]
- 26.Glavic A, Gomez-Skarmeta JL, Mayor R. Xiro-1 controls mesoderm patterning by repressing bmp-4 expression in the Spemann organizer. Dev Dyn. 2001;222:368–376. doi: 10.1002/dvdy.1189. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Skarmeta J, de La Calle-Mustienes E, Modolell J. The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development. 2001;128:551–560. doi: 10.1242/dev.128.4.551. [DOI] [PubMed] [Google Scholar]
- 28.Rogers CD, Harafuji N, Archer T, Cunningham DD, Casey ES. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech Dev. 2009;126:42–55. doi: 10.1016/j.mod.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan B, Neilson KM, Moody SA. FoxD5 plays a critical upstream role in regulating neural fate and onset of differentiation. Dev Biol. 2009;329:80–95. doi: 10.1016/j.ydbio.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan B, Neilson KM, Moody SA. Microarray identification of novel downstream targets of FoxD5, a critical component of the neural ectodermal transcriptional network. Dev Dyn. 2010;239:3467–3480. doi: 10.1002/dvdy.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyodo-Miura J, Urushiyama S, Nagai S, Nishita M, Ueno N, Shibuya H. Involvement of NLK and Sox11 in neural induction in Xenopus development. Genes Cells. 2002;7:487–96. doi: 10.1046/j.1365-2443.2002.00536.x. [DOI] [PubMed] [Google Scholar]
- 32.Chaddah R, Arntfield M, Runciman S, Clarke L, van der Kooy D. Clonal neural stem cells from human embryonic stem cell colonies. J Neurosci. 2012;32:7771–7781. doi: 10.1523/JNEUROSCI.3286-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neely MD, Litt MJ, Tidball AM, Li GG, Aboud AA, Hopkins CR, Chamberlin R, Hong CC, Ess KC, Bowman AB. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and Sox1 expression during neural induction. ACS Chem Neurosci. 2012;3:482–491. doi: 10.1021/cn300029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo S, Herr A, Lim JW, Richardson GA, Richardson H, Kroll KL. Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 2005;19:1723–1734. doi: 10.1101/gad.1319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo S, Kroll KL. Geminin’s double life: chromatin connections that regulate transcription at the transition from proliferation to differentiation. Cell Cycle. 2006;5:374–379. doi: 10.4161/cc.5.4.2438. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan SA, Akers L, Moody SA. foxD5a, a Xenopus winged helix gene, maintains an immature neural ectoderm via transcriptional repression that is dependent on the C-terminal domain. Dev Biol. 2001;232:439–457. doi: 10.1006/dbio.2001.0191. [DOI] [PubMed] [Google Scholar]
- 37.Brewster R, Lee J, Ruiz i Altaba A. Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature. 1998;393:579–583. doi: 10.1038/31242. [DOI] [PubMed] [Google Scholar]
- 38.Penzel R, Oschwald R, Chen Y, Tacke L, Grunz H. Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis . Int J Dev Biol. 1997;41:667–677. [PubMed] [Google Scholar]
- 39.Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- 40.Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, Sasai Y. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development. 2000;127:791–800. doi: 10.1242/dev.127.4.791. [DOI] [PubMed] [Google Scholar]
- 41.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 42.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 43.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Wang TW, Sromberg GP, Whitney JT, Brower NW, Klymkowsky MW, Parent JM. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. J Comp Neurol. 2006;497:88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]
- 45.Li M, Pevny L, Lovell-Badge R, Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol. 1998;8:971–974. doi: 10.1016/s0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- 46.Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- 47.Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 48.Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006;20:3475–3486. doi: 10.1101/gad.403406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- 51.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizuseki K, Kishi M, Shiota K, Nakanishi S, Sasai Y. SoxD: an essential mediator of induction of anterior neural tissues in Xenopus embryos. Neuron. 1998;21:77–85. doi: 10.1016/s0896-6273(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 53.Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci USA. 1997;94:11980–11985. doi: 10.1073/pnas.94.22.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic family and its role in neural and neural crest development. Mech Dev. 1998;75:43–51. doi: 10.1016/s0925-4773(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 55.Aruga J, Tohmonda T, Homma S, Mikoshiba K. Zic1 promotes expansion of dorsal neural progenitors in spinal cord by inhibiting neuronal differentiation. Dev Biol. 2002;244:329–341. doi: 10.1006/dbio.2002.0598. [DOI] [PubMed] [Google Scholar]
- 56.Inoue T, Ota M, Ogawa M, Mikoshiba J, Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007;27:5461–5473. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez-Skarmeta JL, Diez del Corral R, de la Calle-Mustienes E, Ferré-Marcó D, Modolell J. Araucan and caupolican, two members of the novel iroquois complex, encode homeoproteins that control proneural and vein-forming genes. Cell. 1996;85:95–105. doi: 10.1016/s0092-8674(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 58.Bellefroid EJ, Kobbe A, Gruss P, Pieler T, Gurdon JB, Papalopulu N. Xiro3 encodes a Xenopus homolog of the Drosophila Iroquois genes and functions in neural specification. EMBO J. 1998;17:191–203. doi: 10.1093/emboj/17.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gómez-Skarmeta JL, Glavic A, de la Calle-Mustienes E, Modolell J, Mayor R. Xiro, a Xenopus homolog of the Drosophila Iroquois complex genes, controls development at the neural plate. EMBO J. 1998;17:181–190. doi: 10.1093/emboj/17.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de la Calle-Mustienes E, Glavic A, Modolell J, Gomez-Skarmeta JL. Xiro homeoproteins coordinate cell cycle exit and primary neuron formation by upregulating neuronal-fate repressors and downregulating the cell-cycle inhibitor XGadd45-γ. Mech Dev. 2002;119:69–80. doi: 10.1016/s0925-4773(02)00296-4. [DOI] [PubMed] [Google Scholar]
- 61.Levine M, Davidson EH. Gene regulatory networks for development. Proc Natl Acad Sci USA. 2005;102:4936–4942. doi: 10.1073/pnas.0408031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sölter M, Köster M, Hollemann T, Brey A, Pieler T, Knöchel W. Characterization of a subfamily of related winged helix genes, XFD-12/12’/12” (XFLIP), during Xenopus embryogenesis. Mech Dev. 1999;89:161–165. doi: 10.1016/s0925-4773(99)00195-1. [DOI] [PubMed] [Google Scholar]
- 63.Fetka I, Doederlein G, Bouwmeester T. Neuroectodermal specification and regionalization of the Spemann organizer in Xenopus . Mech Dev. 2000;93:49–58. doi: 10.1016/s0925-4773(00)00265-3. [DOI] [PubMed] [Google Scholar]
- 64.Shapira E, Marom K, Levy V, Yelin R, Fainsod A. The xvex-1 antimorph reveals the temporal competence for organizer formation and an early role for ventral homeobox genes. Mech Dev. 2000;90:77–87. doi: 10.1016/s0925-4773(99)00283-x. [DOI] [PubMed] [Google Scholar]
- 65.Papanayotou C, Mey A, Birot AM, Saka Y, Boast S, Smith JC, Samarut J, Stern CD. A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.0060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 67.Pohl BS, Knochel W. Of Fox and Frogs: Fox (fork head/winged helix) transcription factors in Xenopus development. Gene. 2005;344:21–32. doi: 10.1016/j.gene.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 68.Wijcher PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397:233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (Fox) gene families. Hum Genomics. 2010;4:345–352. doi: 10.1186/1479-7364-4-5-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaret KS. Regulatory phases of early liver development: Paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 72.Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol. 2008;73:119–126. doi: 10.1101/sqb.2008.73.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cirillo L, Lin FR, Cuesta I, Jarnik M, Friedman D, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FOXA) and GATA-4. Molec Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 75.Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988;335:683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- 76.Schuddekopf K, Schorpp M, Boehm T. The whn transcription factor encoded by the nude locus contains an evolutionarily conserved and functionally indispensable activation domain. Proc Natl Acad Sci USA. 1996;93:9661–9664. doi: 10.1073/pnas.93.18.9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neilson KM, Klein SL, Mhaske P, Mood K, Daar IO, Moody SA. Specific domains of FoxD4/5 activate and repress neural transcription factor genes to control the progression of immature neural ectoderm to differentiating neural plate. Dev Biol. 2012;365:363–375. doi: 10.1016/j.ydbio.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klein SL, Neilson KM, Orban J, Yaklichkin S, Hoffbauer J, Mood K, Daar IO, Moody SA. Conserved structural domains in FoxD4L1, a neural forkhead box transcription factor, are required to repress or activate target genes. PloS One. 2013;8:e61845. doi: 10.1371/journal.pone.0061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yaklichkin S, Vekker A, Stayrook S, Lewis M, Kessler DS. Prevalence of the EHI Groucho interaction motif in the metazoan Fox family of transcriptional regulators. BMC Genomics. 2007;8:201–218. doi: 10.1186/1471-2164-8-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sekiya T, Zaret KS. Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates a compacted chromatin structure in vitro and impairs activator binding in vivo. Mol Cell. 2007;28:291–303. doi: 10.1016/j.molcel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yaklichkin S, Steiner AB, Lu Q, Kessler DS. FoxD3 and Grg4 physically interact to repress transcription and induce mesoderm in Xenopus . J Biol Chem. 2007;282:2548–2557. doi: 10.1074/jbc.M607412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Essien K, Vigneau S, Apreleva S, Singh LN, Bartolomei MS, Hannenhalli S. CTCF binding site classes exhibit distinct evolutionary, genomic, epigenomic and transcriptomic features. Genome Biol. 2009;10:R131. doi: 10.1186/gb-2009-10-11-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan Y, Newman T, Linardopoulou E, Trask BJ. Gene content and function of the ancestral chromosome fusion site in human chromosome 2q13-2q14.1 and paralogous regions. Genome Res. 2002;12:1663–1672. doi: 10.1101/gr.338402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freyaldenhoven B, Fried C, Wielckens K. FOXD4a and FOXD4b, two new winged helix transcription factors, are expressed in human leukemia cell lines. Gene. 2004;294:131–140. doi: 10.1016/s0378-1119(02)00702-3. [DOI] [PubMed] [Google Scholar]
- 85.Kaestner KH, Monaghan AP, Kern H, Ang SL, Weitz S, Lichter P, Schütz G. The mouse fkh-2 gene. Implications for notochord, foregut, and midbrain regionalization. J Biol Chem. 1995;270:30029–30035. doi: 10.1074/jbc.270.50.30029. [DOI] [PubMed] [Google Scholar]
- 86.Katoh M, Katoh M. Human FOX gene family (Review) Int J Oncol. 2004;25:1495–1500. [PubMed] [Google Scholar]
- 87.Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- 88.Suda Y, Nakabayashi J, Matsuo I, Aizawa S. Functional equivalency between Otx2 and Otx1 in development of rostral head. Development. 1999;126:743–757. doi: 10.1242/dev.126.4.743. [DOI] [PubMed] [Google Scholar]
- 89.Tuteja G, Kaestner KH. SnapShot: forkhead transcription factors I. Cell. 2007;130:1160. doi: 10.1016/j.cell.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Lee HC, Tseng WA, Lo FY, Liu TM, Tsai HJ. FoxD5 mediates anterior-posterior polarity through upstream modulator Fgf signaling during zebrafish somitogenesis. Dev Biol. 2009;336:232–245. doi: 10.1016/j.ydbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Human Genomics. 2010;4:345–352. doi: 10.1186/1479-7364-4-5-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minoretti P, Arra M, Emanuele E, Olivieri V, Aldeghi A, Politi P, Martinelli V, Pesenti S, Falcone C. A W148R mutation in the human FOXD4 gene segregating with dilated cardiomyopathy, obsessive-compulsive disorder, and suicidality. Int J Mol Med. 2007;19:369–372. [PubMed] [Google Scholar]
- 93.Chen CP, Lin SP, Su YN, Su JW, Chern SR, Town DD, Wang W. Pure distal 9p deletion in a female infant with cerebral palsy. Genet Couns. 2012;23:215–221. [PubMed] [Google Scholar]
- 94.Freitas El, Gribble SM, Simoni M, Vieira TP, Silva-Grecco RL, Balarin MA, Prigmore E, Krepisschi-Santos AC, Rosenberg C, Szuhai K, van Haeringen A, Carter NP, Gil-da-Silva-Lopes VL. Maternally inherited partial monosomy 9p (pter ® p24.1) and partial trisomy 20p (pter ® p12.1) characterized by microarray comparative genomic hybridization. Am J Med Genet A. 2011;155A:2754–2761. doi: 10.1002/ajmg.a.34168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avilon AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhattaram P, Penzo-Méndez A, Sock E, Colmenares C, Kaneko KJ, Vassilev A, Depamphilis ML, Wegner M, Lefebvre V. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun. 2010;1:9. doi: 10.1038/ncomms1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bergsland M, Ramskold D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim JW, Hummert P, Mills JC, Kroll KL. Geminin cooperates with Polycomb to restrain multi-lineage commitment in the early embryo. Development. 2011;138:33–44. doi: 10.1242/dev.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yellajoshyula D, Patterson ES, Elitt MS, Kroll KL. Geminin promotes neural fate acquisition of embryonic stem cells by maintaining chromatin in an accessible and hyperacetylated state. Proc Natl Acad Sci USA. 2011;108:3294–3299. doi: 10.1073/pnas.1012053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yellajoshyula D, Lim JW, Thompson DM Jr, Witt JS, Patterson ES, Kroll KL. Geminin regulates the transcriptional and epigenetic status of neuronal fate-promoting genes during mammalian neurogenesis. Mol Cell Biol. 2012;32:4549–4560. doi: 10.1128/MCB.00737-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spella M, Kyrousi C, Kritikou E, Stathopoulou A, Guillemont F, Kioussis D, Pachnis V, Lygerou Z, Taraviras S. Geminin regulates cortical progenitor proliferation and differentiation. Stem Cells. 2011;8:1269–1282. doi: 10.1002/stem.678. [DOI] [PubMed] [Google Scholar]
- 102.Schultz KM, Banisadr G, Lastra RO, McGuire T, Kessler JA, Miller RJ, McGarry TJ. Geminin-deficient neural stem cells exhibit normal cell division and normal neurogenesis. PLoS One. 2011;6:e17736. doi: 10.1371/journal.pone.0017736. [DOI] [PMC free article] [PubMed] [Google Scholar]


