Abstract
Human cognitive aging has been too long neglected and underappreciated for its critical importance to quality of life in old age. The articles in this session present novel approaches to improving cognitive function in normal aging persons with drugs and interventions that are based on findings in epidemiology, studies in aged animals, and in vitro research. In addition, since aging is the primary risk factor for Alzheimer's disease, these studies also have implications as interventions for prevention and treatment. As a field of research, new knowledge regarding the causes and mechanisms of cognitive aging are ripe for translation into human studies, with the application of this knowledge leading the development of interventions and therapeutics for the prevention of cognitive decline in old age and Alzheimer's disease.
Keywords: Cognitive aging, Alzheimers, Clinical trial, Cognition
The articles in this final session of the Cognitive Aging Summit addressed novel approaches to improving the course of normal cognitive aging with drugs and interventions derived from epidemiological evidence, research in aged animals and in vitro studies. They are among the most innovative strategies currently being studied in critical tests of efficacy in humans. It is noteworthy that, at least thus far, the drugs and interventions being evaluated in these trials appear to have few “side effects.” This is especially important for treatments targeting “normal cognitive aging,” because they would need to be extremely safe (and effective) to receive the Food and Drug Administration (FDA) approval for long-term administration in a large population of essentially “normal” people. Additional constraints on drugs for this indication are likely to include ease of delivery (ie, oral availability) and low cost. We should also recognize the significant distinction between treatments aimed at “symptomatic” improvement for some of the mild deficits of cognitive aging, versus “disease modifying” drugs that slow the course of a neurodegenerative process.
In the first presentation, Gallagher's work illustrated how current advances in basic research can be translated in clinical drug studies. Her program employs new knowledge regarding hippocampal circuits, memory formation, and the application of brain imaging techniques, notably functional magnetic resonance imaging (fMRI), in testing drugs for cognitive aging. The fMRI enables the study of drug effects on neuronal network activity in response to cognitive task demands, offering significant insight beyond structural MRI and metabolic imaging (eg, FDG-PETs). Gallagher's novel hypothesis postulates “overactivity” of selective hippocampal circuits as a key contributor to memory impairment in aging. Her therapeutic strategy involves dampening this excess activity in humans with amnestic mild cognitive impairment (aMCI) using an FDA-approved antiepileptic found to benefit memory at low doses in aged rats. The treatment hypothesis under investigation is that lowering dentate gyrus/CA3 hyperactivity, as determined by fMRI, will improve memory performance. Thus, her work opens new avenues in the use of functional neuroimaging for developing drugs to treat cognitive aging.
Executive function is critical to daily life, and significant changes occur with aging that can affect independence and activities of daily living. There have been few, if any, randomized human clinical trials in which executive function has been the primary outcome measure in a “cognitively normal” aging population. In studies reviewed by van Dyck, guanfacine is being investigated for the treatment of prefrontal executive dysfunction in healthy aging. Although prefrontal cortical (PFC) cognitive dysfunction is a common feature of healthy aging, the challenges in developing interventions are significant, particularly given the complexity of PFC function. Aged monkeys have provided a valuable animal model for rational drug development, documenting that administration of the α2A-adrenoceptor agonist guanfacine can substantially reverse age-related working memory deficits dependent on the PFC. The cognitive effects of this drug are now being investigated in healthy elderly humans. The study design is rather unique and sets the stage for future studies of pharmacological agents aimed at preferentially affecting executive function in aged populations. Clinical meaningfulness will be measured as secondary outcomes including the Clinical Global Impression of Change (CGIC), and a quality of life measure, the SF-36. Assessing effects on these measures is extremely important because it could have a significant impact on whether the FDA is likely to approve drugs for indications such as PFC-mediated cognitive dysfunction in normal aging.
Behavioral and lifestyle interventions including diet, exercise, and cognitive training are emerging as potentially powerful strategies for promoting brain health. Studies in animal models offer the advantage over human research that dietary and behavioral conditions can be precisely controlled for prolonged periods, allowing the relative contributions of each intervention to be evaluated. The research program reviewed by Cotman takes advantage of a novel dog model (the beagle) for their studies of a behavioral intervention for cognitive aging. Noteworthy features of this model are that, unlike many more common laboratory animals, beagles develop significant brain amyloid deposition during aging, and they display a surprisingly rich repertoire of cognitive capacities. Cotman's group is examining the effectiveness of behavioral enrichment (social, physical, and cognitive exercise), dietary supplementation (antioxidants and mitochondrial cofactors), and combined intervention for ameliorating age-related cognitive decline. The initial findings underscore a number of key points in the study of lifestyle interventions. First, multifactorial interventions that address risk factors identified in laboratory and human epidemiological research are likely to be required. Second, demonstrating efficacy in humans will require the convergence of data from animal models, epidemiological studies to identify the most critical variables to be studied in clinical trials. Research design will be crucial, and we will need novel approaches in clinical investigation in order to establish proof of concept evidence that human cognitive aging can be slowed or prevented by specific behavioral interventions.
In the final presentation of the session, Chapman outlined work using functional neuroimaging to study the influence of cognitive and physical training on “gist reasoning.” As noted previously, executive function declines with aging, and older persons change the way they make decisions, increasing the use of gist reasoning, possibly as a consequence of underlying changes in processing speed and working memory capacities. This shift toward gist reasoning can be adaptive, and Chapman suggests that brain and physical training may provide differential benefits. In addition, their goal is to enhance cognitively meaningful outcomes, including skills critical for everyday life, functions such as reasoning, problem solving, goal management, and other executive capacities and not simply to improve performance on isolated tasks/skills within the laboratory setting. Their ongoing work reinforces the view that interventions for cognitive aging must show “clinical meaningfulness”—a critical, but difficult, hurdle to overcome in cognitive aging research to date.
THE CLINICAL STUDY as EXPERIMENT: TEST of a PIVOTAL HYPOTHESIS—Michela Gallagher
New tools are rapidly changing the landscape for discovery in translational research. In particular, studies of computational functions in large-scale networks in animals are making greater contact with the use of neuroimaging in humans to advance our understanding of both normal cognition and clinical conditions. Here, we focused on the hippocampal network, building on research in an animal model of age-related memory impairment to inform the use of fMRI in people with aMCI, a condition in which memory loss is greater than would be expected for a person's age. Parallels observed in this work, translating from animal to man, suggest new ways to test hypotheses about the functional significance of altered fMRI signals in neurocognitive aging.
As a background to the study of neurocognitive aging, great progress has been made in understanding the fundamental contribution of the medial temporal lobe to memory processes. Recordings from ensembles of single neurons in the brains of young laboratory rats have confirmed what computational models have predicted about the network properties of cortical input into the dentate gyrus and CA3 (DG/CA3) subregions of the hippocampal formation. These components of the medial temporal lobe system are especially critical for encoding distinctive representations of experiences that share overlapping elements with prior memories. The generality of this network's properties has been extended to humans with the application of advanced neuroimaging tools. When high-resolution methods in fMRI were used to study the function of hippocampal subregions, the first evidence was provided of a corresponding computation for DG/CA3 in the brains of young adults (1).
Our research has shown how this rapid and distinctive encoding of new information is lost in the aged hippocampus of rats with memory impairment. Rather than creating distinctive representations, such aged rats retrieve prior representations, a process known as “pattern completion,” distinguishing it from “pattern separation.” Tied to the computational shift just described, CA3 neurons in aged animals with poor memory also exhibit excessive activity, that is, higher firing rates (2). This is not surprising because pattern completion is mediated by excitatory autoassociative connections, whereby collaterals of the CA3 neurons synapse on many other CA3 neurons, forming the majority of CA3 inputs. A shift in the balance between extrinsic cortical inputs that drive pattern separation and autoassociative control that mediates pattern completion could contribute to both elevating CA3 activity and preventing the encoding of new information.
Directly based on the dysfunctional properties of the DG/CA3 network in studies of aged animals, fMRI was used to localize signals during performance of a task that taxes pattern separation in patients with aMCI compared with age-matched controls. Similar to aged rats with memory loss, greater activation within the hippocampal formation was restricted to the DG/CA3 in aMCI relative to the age-matched control group (3). Behavioral assessments using procedures like those in the fMRI task had earlier shown less pattern separation with a shift to pattern completion in elderly human subjects (3,4). In Stark et al. (3) that shift was further accentuated in aMCI as indicated by a worsening performance in the scanning task relative to age-matched controls.
The corresponding localization of greater activation in conditions of memory loss in both animals and humans is a striking finding. Changes in the underlying neural circuitry, which have been studied in great detail in aged animal models, suggest that overactivity in this network is a dysfunctional condition. This idea was directly tested and supported by targeting excess activity in the CA3 region of aged rats with impairment using methods that were found to benefit memory performance (5). By contrast, signals of greater fMRI activation in the aging brain have often been viewed as serving a compensatory function. In order to test the functional significance of increased hippocampal activation, research directly based on the animal model is now underway in aMCI using an FDA-approved antiepileptic that was efficacious in aged rats at low doses. If increased activation serves a compensatory function, then treatments that lower the fMRI signal would be expected to worsen performance. On the other hand, if the condition in aMCI has computational consequences similar to that in aged rodent brain, then lowering DG/CA3 activity may improve memory performance. Thus, this translational approach could yield valuable insights into the functional significance of neuroimaging signals in a clinical setting.
GUANFACINE for PREFRONTAL EXECUTIVE DYSFUNCTION in HEALTHY AGING—Christopher H. van Dyck
Although PFC cognitive dysfunction is a characteristic and disabling feature of healthy aging (6,7), no treatment has been developed to date for the amelioration of these symptoms. Studies of monkeys have revealed PFC cognitive dysfunction with advancing age, associated with neuropathological and neurochemical changes in the PFC (8). Working memory deficits in aged monkeys are improved by the α2A-adrenoceptor agonist guanfacine (9–12); however, the cognitive effects of this drug have not yet been investigated in healthy elderly humans. The overarching objective of this project is to evaluate guanfacine as a treatment for PFC cognitive dysfunction in elderly participants. This Pilot Clinical Trial aims to provide essential preliminary data that will advance the design of a subsequent full-scale clinical trial.
A working model of guanfacine's possible mechanism of action is shown in Figure 1. Guanfacine is thought to alter intracellular signaling events that regulate the strength of PFC working memory network connections. Working memory networks excite each other through connections on dendritic spines to keep information “in mind” (13). Recent research suggests that cAMP signaling weakens these network connections by opening K+ channels on dendritic spines (14). Stimulation of α2A receptors by guanfacine inhibits cAMP production (15), closing K+ channels and strengthening network connections (14). As cAMP signaling is elevated in the aged PFC (16), guanfacine may be especially helpful in strengthening PFC network connections in the aged. Recent physiological evidence shows that guanfacine can enhance PFC network firing in aged monkeys (Figure 2) (17). This work demonstrated a dramatic age-related decline in PFC neuronal firing during working memory. Iontophoretic application of guanfacine onto aged PFC neurons significantly increased neuronal firing to more youthful levels.
Figure 1.
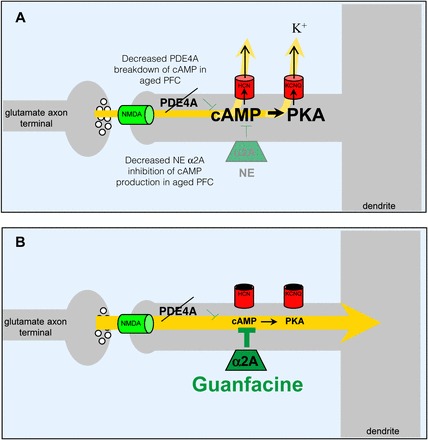
Working model of intracellular signaling events that regulate the strength of PFC network connections. A. In the aged PFC, increased cAMP opens K+ channels on spines, which weakens network connections. Examples of K+ channels: HCN= Hyperpolarization-activated Cyclic Nucleotide-gated channels; KCNQ=Kv.72/3/5. B. Guanfacine treatment inhibits cAMP production, which strengthens network connections. (Adapted from Reference 17.)
Figure 2.
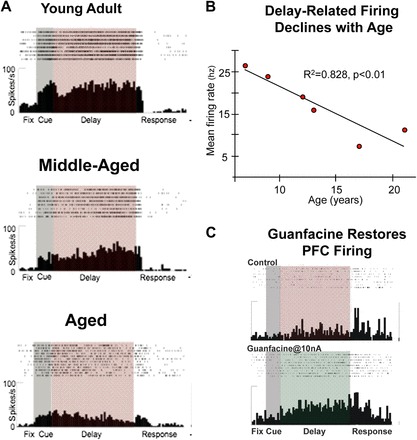
Electrophysiological studies of PFC network firing in monkeys. A. Working memory-related PFC neuronal firing is markedly reduced with advancing age. Examples show representative individual neurons from young adult, middle-aged, and aged monkeys. B. Delay-related PFC neuronal firing rate as a function of age in monkeys. There is a highly significant decline in firing rate with advancing age. C. Iontophoresis of guanfacine (bottom graph) onto PFC neurons restores memory-related firing in aged monkeys compared to control conditions (top graph). (Adapted from Reference 17.)
The present study is the first to test whether guanfacine might similarly enhance PFC executive function in cognitively normal elderly humans. In healthy young adults, single doses of guanfacine have been associated with beneficial effects on computerized executive function tasks in one of two studies (18–20). However, based on primate studies, guanfacine should have its greatest effects in healthy older adults (12). Given the evidence that the most robust deficits in executive function appear over age 75 (21,22), the major eligibility criteria include age ≥75 years; good general physical health; and absence of dementia or MCI.
This is a randomized, double-blind placebo-controlled trial of guanfacine in which a total of 123 participants are randomized to one of two dose levels of guanfacine (0.1 and 0.5 mg) or placebo once daily for 12 weeks. Outcome measures are administered at 0, 6, and 12 weeks. The primary outcome measure is a composite Z-score from an executive function neuropsychological test battery. It includes four computerized tests from the CANTAB (23), as well as Stroop Interference, and Trailmaking Test B. Tests in this battery were selected to be (a) sensitive to aging; (b) sensitive to naturally occurring PFC lesions; and (b) responsive to guanfacine or other α2A agonists in previous human trials. Secondary outcomes include the Clinical Global Impression of Change (CGIC) (24) and a quality of life measure, the SF-36 (25). This exploratory “proof of concept” study may provide the rationale for a subsequent full-scale clinical trial of guanfacine for age-associated PFC-mediated cognitive dysfunction.
BEHAVIORAL and DIETARY STRATEGIES SYNERGIZE to PROMOTE SUCCESSFUL AGING—Carl W. Cotman
Lifestyle interventions including diet, exercise, and cognitive training are emerging as powerful strategies to promote successful aging. However, the optimal interventions for enhancing cognition and maximizing the benefits to brain health are unknown. Studies in animal models have advantages over human studies, in that dietary and behavioral conditions can be precisely controlled over prolonged periods, allowing the relative contributions of each intervention to be evaluated. While some such studies exist using rodents, few have been undertaken in higher animal models. We have used the aged canine (dog) to examine the effectiveness of behavioral enrichment, dietary supplementation, or the combined intervention to counteract age-related cognitive decline. We have discovered that behavioral enrichment or dietary supplementation each can improve cognition and that the combined intervention has synergistic benefits on cognitive performance and counteracting age-related degenerative changes in the brain.
The aged canine is one of the best and most accessible animal models of brain aging that parallels many of the key features of human brain aging, including MCI and early Alzheimer's disease (26). Like the aged human brain, the canine brain shows increased oxidative damage, mitochondrial dysfunction, selective neuron loss, decreased hippocampal neurogenesis, and accumulation of beta-amyloid (Aβ) pathology with age. The aged canine is a natural model of Aβ accumulation, as the canine and human Aβ protein is 100% homologous, whereas the APP sequences share 98% homology. Indeed, the canine brain accumulates senile plaques with age and the accumulation of Aβ1 –42 and Aβ1 –40 progresses in a similar fashion to that occurring in the human brain. In parallel, oxidative damage and mitochondrial reactive oxygen species generation increase with age in the canine brain, similar to reports of oxidative damage in human brain in aging, MCI and most extensively in Alzheimer's disease. Rodent studies reveal that oxidative stress is one mechanism that increases Aβ deposition, which in turn, exacerbates oxidative stress.
Our research has pioneered the use of the canine to identify dietary or behavioral interventions to prevent or slow age-related cognitive decline. Notably, we have found that long-term dietary supplementation with cellular antioxidants (vitamins E, C, etc.) and mitochondrial cofactors (α-lipoic acid and carnitine) (AOX), or behavioral enrichment with social, cognitive, and exercise components (ENR), can effectively improve cognitive performance and reduce brain pathology of aged canines (26). Cognition is improved on several tasks of executive function as well as in tasks of spatial learning and memory (27). In general, the combined intervention (AOX + ENR) resulted in greater cognitive improvement than either treatment alone. For example, in tasks of spatial memory, performance was enhanced the most with the combined intervention, with final performance of the ENR/AOX group similar to that of all animals at the start of the intervention (2 + years prior). Thus, the intervention “reversed” age-related cognitive decline.
These behavioral data provided the framework to next examine how these interventions affect cellular and molecular processes in the brain associated with aging. We have demonstrated that these interventions counteract age-related neuron loss, declines in neurogenesis, oxidative damage, and Aβ deposition, with some of these end points being more responsive to the interventions and being more strongly correlated with cognitive function. In particular, the most robust effects of the interventions were on reducing mitochondrial reactive oxygen species generation, decreasing protein oxidation, and inducing key antioxidant enzymes to counteract reactive oxygen species production and preserve mitochondrial function. In addition, factors important for synaptic plasticity were upregulated with the interventions, including brain-derived neurotrophic factor (BDNF), a key molecule that modulates learning and that declines with age. We found that BDNF mRNA levels were reduced in the temporal cortex in old cognitively impaired dogs compared with young dogs. Intervention in the aged dogs with AOX + ENR counteracted this decline, elevating BDNF mRNA over levels in untreated aged dogs, and approaching levels measured in the young group. Animals receiving either intervention alone ENR displayed intermediate levels of BDNF mRNA (28). Overall, the combined AOX + ENR treatment appeared to have additive or synergistic effects on several neurobiological endpoints, including BDNF, oxidative damage, and Aβ load, and on preserving and improving cognitive function (26).
Why are the effects of diet and behavioral enrichment synergistic? Declining mitochondrial function is often cited as one of the basic cellular events contributing to neuronal dysfunction with age. We found that the AOX diet counteracted age-related increases in mitochondrial reactive oxygen species generation and increased NADH-linked respiration, leading to improved mitochondrial function in the aged canine brain (29). We hypothesize that improved mitochondrial function is a significant mechanism underlying improvements with the AOX diet. We further suggest that improved mitochondrial function with the AOX diet allows the aged brain to respond better to behavioral interventions than when mitochondria are partially dysfunctional. In other words, neurons with healthy mitochondria are more able to benefit from ENR, a novel concept in the field. Taken together, our findings suggest that the AOX and ENR interventions may engage molecular mechanisms that enhance “cognitive reserve”, allowing the aged brain to maintain intact cognitive abilities despite the presence of Aβ and other pathologies in the aged brain.
EXERCISING the MIND and BODY: AGING and BRAIN HEALTH—Sandra Bond Chapman, Raksha Mudar, Elizabeth Bartz, Molly Keebler, Hanzhang Lu and John Hart
A major accomplishment of this century is the doubling of the human life span (30). However, a downside of greater life expectancy is increased risk of cognitive impairment in late life (31,32). Motivated by evidence that significant potential exists to modify the structure and function of the aging human brain given intensive training (33–39), we are examining the cognitive gains and physical changes in the brain mediated by brain and physical training regimes. We incorporate a combination of cognitive testing, structural and functional brain imaging measures, and physical assessments as assessment tools.
In this synopsis, we briefly discuss previous pilot data as well as the preliminary findings from an ongoing randomized clinical trial of the differential and shared effects of short-term, intensive higher-order gist reasoning training or physical exercise in seniors as compared with a group of wait-list controls. The participants are between 60 and 75 years of age, are well screened to ensure normal cognitive function, and are established as non-exercisers prior to training.
Exercising the Mind
Gist reasoning represents the basis of the brain-training regimen and is a higher-order cognitive function that entails ability to bind details to form abstracted gist meanings (40,41). Individuals typically remember the gist of the information better than specific details (42,43). Gist processing promotes deeper understanding of commonly encountered information, and thus, is integral to everyday life skills. Three core cognitive capacities include strategic attention, integrated reasoning, and reasoning flexibility, each of which are essential components of the gist reasoning training program (44). Brain training participants are encouraged to incorporate the strategies as habitual practice whether listening to their financial advisor or physician, listening to news, reading a book, attending a lecture, to mention a few activities.
The brain exercise arm of the current randomized study is motivated by prior evidence from a feasibility study in 26 normally aging seniors that showed benefits of short-term intensive training (ie, 8 hours in 1 month) not only on trained gist-reasoning function but also generalized benefits to untrained measures of executive function (44). Specifically, the gist reasoning training was associated with improved performance on measures of concept abstraction, cognitive switching, and verbal fluency.
The brain-training group in the current randomized study is showing improvements similar to the feasibility study. The brain-training group (n = 7) is manifesting significant improvement in the trained areas of integrated reasoning with transfer effects on concept abstraction and verbal fluency as compared with both the control group (n = 13) and the physical training group (n = 13) (45). We are investigating individual differences by examining the relationship between the cognitive gains made and the brain changes observed. For example, we are asking whether high gist reasoners at baseline show reduced brain activation post training (perhaps implicating improved brain efficiency) or whether low gist reasoners show increased brain activation post training. We are also documenting the maintenance of the gains at 6 weeks post training.
Exercising the Body
For the physical training, participants are seen an equivalent number of sessions as the brain-training group (12 weeks). They work out with a trainer three times a week for 60 minutes at an intensity defined as 50%–70% of VO2 max for 12 weeks. Preliminary results from analyses of 13 participants as compared with the control group reveal three significant findings: increases in regional cerebral blood flow in the medial temporal lobe (a region linked to memory), concomitant cognitive increases in delayed memory function, and concept abstraction (46).
The current research elucidates promising ways to lengthen and strengthen cognitive functions with aging and suggests that brain training of gist reasoning and physical training may add differential benefits. Our goal is to identify ways to enhance cognitive functions in meaningful ways, not simply achieve higher performances on isolated tasks/skills within laboratory settings. Ideally, the training regimens should improve cognitive skills critical for everyday life functions such as reasoning, problem solving, goal management, and other executive functions. The economic, social, and emotional burden of cognitive impairment on society underscores the need to extend brain health expectancy, thereby bridging the gap between brain health expectancy and life expectancy.
CONCLUDING COMMENTS
The cutting edge studies of drug and behavioral interventions for cognitive aging presented in this final session of the Cognitive Aging Summit have implications for the development of strategies to improve the symptoms and course of Alzheimer's disease. The development of therapeutic interventions that address cognitive impairment associated with normal aging is also an active area of investigation. Indeed, an important clinical application of therapies for cognitive aging will likely prove to benefit Alzheimer's disease, either symptomatically or through delaying progression. Despite the current focus on “anti-amyloid” strategies as therapeutics for Alzheimer's disease, the causes and mechanisms of human cognitive aging have been too long neglected and underappreciated for their critical importance to quality of life in old age, and to our understanding and development of therapeutics for Alzheimer's disease.
References
- 1.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- 5.Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 7.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neurosci Biobehav Rev. 2002;26:733–741. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 9.Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten AF, Leslie FM. Behavioral and receptor binding analysis of the alpha 2-adrenergic agonist, 5-bromo-6 [2-imidazoline-2-yl amino] quinoxaline (UK-14304): evidence for cognitive enhancement at an alpha 2-adrenoceptor subtype. Neuropharmacology. 1991;30:1279–1289. doi: 10.1016/0028-3908(91)90024-6. [DOI] [PubMed] [Google Scholar]
- 11.Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav. 1996;55:415–422. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 12.Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- 13.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Ramos BP, Paspalas CD, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AF. Alpha2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Gamo NJ, Yang Y, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakala P, Riekkinen M, Sirvio J, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 19.Jakala P, Sirvio J, Riekkinen M, et al. Guanfacine and clonidine, alpha 2-agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacology. 1999;20:119–130. doi: 10.1016/S0893-133X(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 20.Muller U, Clark L, Lam ML, et al. Lack of effects of guanfacine on executive and memory functions in healthy male volunteers. Psychopharmacology (Berl) 2005;182:205–213. doi: 10.1007/s00213-005-0078-4. [DOI] [PubMed] [Google Scholar]
- 21.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 22.Scuteri A, Palmieri L, Lo Noce C, Giampaoli S. Age-related changes in cognitive domains. A population-based study. Aging Clin Exp Res. 2005;17:367–373. doi: 10.1007/BF03324624. [DOI] [PubMed] [Google Scholar]
- 23.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 24.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 26.Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15:685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- 27.Milgram NW, Head E, Zicker SC, et al. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Fahnestock M, Marchese M, Head E, et al. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol Aging. 2012;33:546–554. doi: 10.1016/j.neurobiolaging.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Head E, Nukala VN, Fenoglio KA, Muggenburg BA, Cotman CW, Sullivan PG. Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp Neurol. 2009;220:171–176. doi: 10.1016/j.expneurol.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United Nations. World Population Ageing 2007. New York, NY: United Nations; 2007. [Google Scholar]
- 31.Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 33.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 35.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: a review and future directions. Neuropsychol Rev. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebok GW, Carlson MC, Langbaum JB. Training and maintaining memory abilities in healthy older adults: traditional and novel approaches. J Gerontol B Psychol Sci Soc Sci. 2007;62(Spec No 1):53–61. doi: 10.1093/geronb/62.special_issue_1.53. [DOI] [PubMed] [Google Scholar]
- 38.Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: a meta-analytic study. Psychol Aging. 1992;7:242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- 39.Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 40. Chapman SB, Gamino JF, Mudar, RA. Higher-order strategic gist reasoning in adolescence. In: Reyna VF, Chapman SB, Confrey J, Dougherty M, eds. The Adolescent Brain: Learning, Reasoning, and Decision Making. New York, NY: American Psychological Association; 2011:123–151.
- 41.Reyna VF. Conceptions of memory development, with implication for reasoning and decision making. Ann Child Dev. 1996;12:87–118. [Google Scholar]
- 42.Gabrieli JD. Memory: Pandora's hippocampus? Cerebrum. 2004;6:39–48. [PubMed] [Google Scholar]
- 43.Lloyd FJ, Reyna VF. Clinical gist and medical education: connecting the dots. JAMA. 2009;302:1332–1333. doi: 10.1001/jama.2009.1383. [DOI] [PubMed] [Google Scholar]
- 44.Anand R, Chapman SB, Rackley A, Keebler M, Zientz J, Hart J., Jr Gist reasoning training in cognitively normal seniors. Int J Geriatr Psychiatry. 2011;26:961–968. doi: 10.1002/gps.2633. [DOI] [PubMed] [Google Scholar]
- 45. Keebler M, Bartz EK, Gardner C, et al. Cognitive changes after physical or mental exercise in healthy seniors. Program No.803.12. 2010 Neuroscience meeting planner. San Diego, CA; Society for Neuroscience, 2010 online.
- 46. Bartz EK, Keebler M, Gardner C, et al. Changes in the brain after physical or cognitive training in healthy seniors. Program No. 803.14. 2010 Neuroscience meeting planner. San Diego, CA; Society for Neuroscience, 2010 online.


