Abstract
Background.
Whey protein supplementation may augment resistance exercise-induced increases in muscle strength and mass. Further studies are required to determine whether this effect extends to mobility-limited older adults. The objectives of the study were to compare the effects of whey protein concentrate (WPC) supplementation to an isocaloric control on changes in whole-body lean mass, mid-thigh muscle cross-sectional area, muscle strength, and stair-climbing performance in older mobility-limited adults in response to 6 months of resistance training (RT).
Methods.
Eighty mobility-limited adults aged 70–85 years were randomized to receive WPC (40g/day) or an isocaloric control for 6 months. All participants also completed a progressive high-intensity RT intervention. Sample sizes were calculated based on the primary outcome of change in whole-body lean mass to give 80% power for a 0.05-level, two-sided test.
Results.
Lean mass increased 1.3% and 0.6% in the WPC and control groups, respectively. Muscle cross-sectional area was increased 4.6% and 2.9% in the WPC and control groups, respectively, and muscle strength increased 16%–50% in WPC and control groups. Stair-climbing performance also improved in both groups. However, there were no statistically significant differences in the change in any of these variables between groups.
Conclusions.
These data suggest that WPC supplementation at this dose does not offer additional benefit to the effects of RT in mobility-limited older adults.
Key Words: Nutrition, Physical function, Sarcopenia.
THE age-related loss of muscle mass, sarcopenia, is associated with declines in strength and physical functioning; a combination that is strongly predictive of future disability (1,2). Various interventions have been examined to delay progression of muscle loss and prolong independence among community-dwelling older adults. Studies examining changes in muscle CSA have observed that although older adults are responsive to resistance training (RT) stimuli (3,4), muscle hypertrophy is attenuated with aging (5–7).
Whey protein concentrate (WPC) contains a disproportionate amount of essential amino acids, is readily available commercially, and is palatable; thus, it may represent an ideal protein source to promote muscle anabolism in older individuals undergoing RT. Interestingly, in young healthy male adults, fat-free milk consumption has been shown to increase muscle size and muscle protein synthesis (MPS) in response to RT compared with isoenergetic soy protein (8) and casein, respectively (9). It is well recognized that leucine and the other branched chain amino acids (isoleucine and valine) are abundant in WPC and may be responsible for its enhanced ability to stimulate muscle protein anabolism, particularly in older adults (10,11).
To date, the effects of protein supplementation with RT on body composition, muscle hypertrophy, strength, and physical function in older adults have yielded mixed results (12–19). More importantly, most studies have focused on healthy older adults with very limited data from trials on mobility-limited older adults (15), and where nutritional and not specifically protein supplementation was a focus (19). Thus, the purpose of this study was to examine the chronic effects of WPC and RT in mobility-limited older adults on changes in whole-body lean mass, mid-thigh muscle CSA, muscle strength, and physical functioning. We hypothesized that compared with an isocaloric control, a daily 40g supplement of WPC would increase whole-body lean mass, mid-thigh muscle CSA, muscle strength, and physical functioning in older mobility-limited adults in response to 6 months of RT.
Methods
Participants
Eighty community-dwelling older adults participated in this randomized, double-blind, controlled study (Figure 1). Eligibility criteria are described (Supplementary Table 1). All participants were required to be sedentary (no structured exercise during the previous 6 months). Participants gave informed consent. Tufts University Health Sciences Campus Institutional Review Board approved this study. Allocation to the WPC (n = 42) or control (n = 38) group was conducted by a research assistant unaffiliated with this study who maintained the randomization schedule and communicated the randomization to the research dietitian. The randomization schedule was developed by the study statistician.
Figure 1.
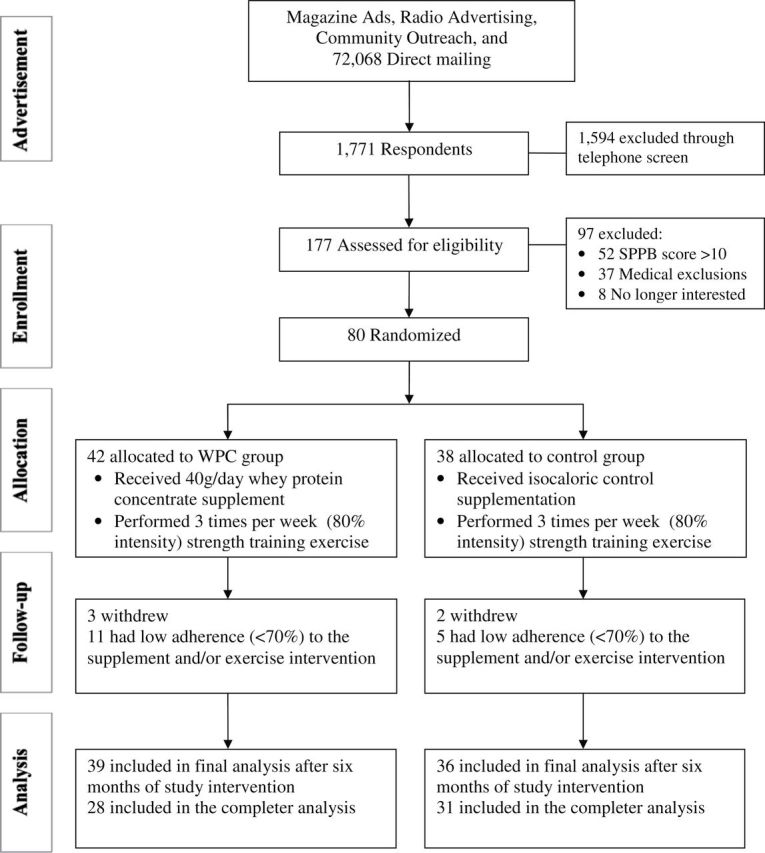
Consort diagram of study recruitment, enrollment, and randomization. SPPB = short physical performance battery.
Baseline Screening of Physical Functioning
All participants were required to have a short physical performance battery (SPPB) score ≤10. The SPPB consists of timed standing balance, gait speed, and timed chair-rise assessments (1). Performance for each of these tasks is scored between 0 and 4, with a summary score of 0–12.
RT Protocol
The RT protocol was a supervised progressive program, three times per week for 6 months, which entailed leg press, seated row, leg extension, chest press, and leg curl. Participants performed the training exercises progressing to 80% of their one repetition maximum (1-RM). The 1-RM was assessed at baseline and re-evaluated monthly. Participants initially performed 2 sets of 10 repetitions progressing to 3 sets of 12 repetitions throughout the 6-month intervention period. Each set was followed by a 1- to 2-min rest period. Resistance training was preceded by 5min of lower extremity “warm-up” (walking or stationary cycling). All training was performed on Cybex VR2 machines (Cybex International, Medway, MA). Adherence to RT was quantified by average attendance at scheduled sessions and the average exercise intensity (% 1-RM) for the knee extension and leg press exercises achieved over the length of the trial.
Whey Protein Supplementation
Innovative Food Processors Inc. (Faribault, MN) provided the WPC and control supplements. Participants were randomized to receive either 40g/day of WPC (one serving contains 20g protein, 25g maltodextrin, 1g fat; 189 kcal [791 kJ]) or an isocaloric control (45g maltodextrin, 1g fat; 189 kcal [791 kJ]). A research dietitian, blinded to the randomization schedule provided by the study statistician, distributed the supplements to participants weekly in exchange for returned packages to monitor adherence. Supplements were in powder form contained in numerically coded packages. Participants consumed two servings per day, one in the morning after breakfast and one in the evening after their evening meal. On the days that participants performed RT, one serving of the supplement was consumed immediately following RT. Participants were instructed to consume their regular food intake in addition to the supplements. To provide an additional measure of adherence, WPC and control also contained 200mg of para-aminobenzoic acid (PABA). A single random-spot urine sample was collected from each participant monthly to confirm adherence. Urinary PABA was measured by a modified colorimetric assay (20).
Determination of Dietary Intake
Participants recorded their food intake using a 3-day diet record at baseline and month 6. Dietary intake data were analyzed using Nutrition Data System for Research software version 2007 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). We reported dietary intake from food sources alone (excluding WPC/control supplement) and also reported dietary intake (including the WPC/control supplement as well as accounting for adherence).
Muscle Strength and Power
Leg press and knee extension strength and power were evaluated using pneumatic bilateral seated leg press and knee extension equipment (K400, Keiser Sports Health Equipment Inc., Fresno, CA). Strength defined as the 1-RM was measured for both the leg press and knee extensors, as reported previously (21).
Peak power was assessed for both knee extensors and leg press. Following a baseline 1-RM measurement, each participant performed five repetitions, as fast as possible, at 40% and 70% 1-RM. The highest power output achieved during each of these five repetitions by a participant was designated as their peak power. The test–retest reliability for this assessment in our laboratory is excellent (leg extensor: intraclass correlation [ICC] = 0.95) (22).
Body Composition
Dual energy x-ray absorptiometry.—
Whole body, regional fat, and lean mass were assessed using DXA (Hologic, Discovery A v12.3, Hologic Inc., Bedford, MA) following an 8-hour fast. The test–retest reliability of this method of estimating total lean mass is high (C.V. < 4%) (23).
Computed tomography.—
Regional changes in skeletal muscle CSA were measured by CT following an 8-hour fast. CT imaging was performed on the nondominant thigh, at the midpoint of the femur using a Siemens Somotom Scanner (Erlangen, Germany). Scans were analyzed for normal-density muscle (35–100 Houndsfield units [HU]), low-density muscle (0–34 HU), total muscle (normal-density muscle plus low-density muscle), subcutaneous, and intermuscular fat CSA by a technician in a blinded manner using SliceOmatic v4.2 software (Montreal, Canada), as described previously (24). Total muscle CSA was measured in the range of 0–100 HU and calculated as the sum of low-density muscle and normal-density muscle CSA.
Physical Function
Stair-climb and chair-rise performance.—
In addition to the SPPB, separate assessments of stair-climb and chair-rise times were performed. For the stair-climb assessment, participants ascended a 10-rise set of stairs as fast as possible. Participants were not permitted to hold on to the railing or use assistive devices. The average of two attempts was recorded. Repeated chair-rise time (10×) was determined on a standard chair with the participant holding their arms across the chest. The average of two attempts was recorded. The repeated chair-rise time has excellent reliability (ICC = .933 vs ICC = .124, respectively) (25).
Four-hundred-meter walk time.—
Participants were asked to walk 10 laps of a 20-m course at their usual pace. Rest intervals were permitted, while standing, for up to 60 seconds. Gait speed was calculated from the time and distance completed for each participant.
Statistics
The primary outcome for this study was the change in lean mass measured by DXA between WPC and control groups. Secondary outcomes included mid-thigh muscle CSA, muscle strength, stair-climb performance, and other measures of physical functioning. Sample sizes were calculated to give 80% power for a 0.05-level, two-sided test, using the above outcomes. All statistical analyses were performed at the two-tailed, .05 level of significance using version 11 of SYSTAT (Systat Software, Inc., San Jose, CA) and version 9.2 of SAS for Windows (SAS Institute, Cary, NC).
Continuous baseline characteristics (eg, age, BMI, and baseline strength) were compared across treatments using a two-sample t test. All outcomes were analyzed using analysis of covariance (ANCOVA) models in which the change from baseline was the response, use of WPC was the study factor, and the baseline value was the covariate. We also looked for sex differences and whether sex modified any effect of WPC by including a sex-by-whey interaction in the models. All data are reported as mean ± standard deviation. For all outcome variables, point estimates for the difference in mean change between WPC and control were calculated along with 95% confidence intervals.
The above-mentioned treatment group comparisons were carried out in the spirit of “Intent-to-Treat.” A secondary analysis was performed on those participants adherent with both the resistance exercise and nutritional intervention. Adherence was defined as participants that attended 70% of the scheduled resistance exercise sessions and did not have two consecutive negative PABA spot urine analyses during the trial. With the exception of normal-density muscle CSA and 400 m gait speed, the results did not differ for the subsequent “completers” analyses. Therefore, all data presented are based on the “Intent-to-Treat” analyses.
Results
Baseline Characteristics of Participants
Baseline characteristics of participants are displayed in Table 1. There were no differences between the groups for any of the baseline characteristics.
Table 1.
Baseline Participant Characteristics
| Characteristics | Whey (n = 42) | Control (n = 38) | p |
|---|---|---|---|
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
| Age | 78.0±4.0 | 77.3±3.9 | 0.43 |
| Height (cm) | 164.2±7.7 | 165.1±9.6 | 0.64 |
| Body mass (kg) | 73.0±10.8 | 73.8±11.2 | 0.75 |
| BMI (body mass/height2) | 27.0±3.2 | 26.9±3.1 | 0.93 |
| SPPB | 8.5±1.2 | 8.5±1.7 | 0.87 |
| Medical diagnoses* | 3 (0–6) | 3 (0–6) | 0.95 |
| Number of medications* | 4 (0–8) | 3 (0–10) | 0.46 |
| Gender, female | 25 (60%) | 22 (58%) | 1.00 |
| Ethnicity | 0.53 | ||
| Asian or Pacific Islander | 2 (5%) | 1(2%) | |
| Black, not of Hispanic origin | 6 (14%) | 7 (18%) | |
| White, not of Hispanic origin | 28 (67%) | 28 (74%) | |
| Other or unknown | 6 (14%) | 2 (5%) |
*Median (range).
Adverse Events and Intervention Adherence
The groups did not differ with respect to serious or nonserious adverse events (Supplementary Table 2). A total of five participants withdrew from the trial (three in WPC and two in control). The adherence to the supplement consumption based on the percentage of participants who did not have two consecutive negative PABA urine analyses was 67% and 84% in WPC and control, respectively. Adherence to the supplement consumption (measured by return of empty supplement packets) was similar between groups and averaged 72.1% ± 29.3% and 82.3% ± 21.9% in WPC and control, respectively. Adherence to the exercise sessions was 79.9% ± 20.8% and 80.2% ± 21.7% in WPC and control, respectively. The average exercise intensity achieved during training sessions for the knee extensors and leg press combined was 76% ± 17.4% and 80% ± 2.8% (% 1-RM) in WPC and control, respectively, and was not different between groups.
Dietary Intake
Three-day diet records (excluding supplement intake) revealed a significant decrease in total energy intake with no significant differences between groups (Table 2). The observed reductions in total energy intake appeared to be driven by small but significant decreases in total carbohydrate, protein, and total fat intake (Table 2). When dietary intake from food was combined with WPC/control supplement intake (adjusted for adherence), total energy intake was increased at 6 months in both groups (p < .001) and was not different between groups (p < .09). Total carbohydrate intake in control was 50g/day different than WPC at 6 months. Total protein was greater in WPC compared with control by 18g/day at 6 months (p < .001).
Table 2.
Three-Day Mean Dietary Intake* of Selected Nutrients in Total Sample
| Nutrient | Whey | Control | Point Estimates | P Value |
|---|---|---|---|---|
| (N = 30) | (N = 29) | (95% CI)† | Time | |
| Intake excluding whey/control | ||||
| Energy (total kcal/d) | .0159 | |||
| Baseline | 1639±376 | 1756±540 | −59 (−201,83) | |
| Six months | 1464±309 | 1580±488 | ||
| Carbohydrate (total g/d) | <.0001 | |||
| Baseline | 200±62 | 220±77 | −9 (−30,12) | |
| Six months | 176±45 | 193±63 | ||
| Protein (total g/d) | <.0001 | |||
| Baseline | 71±17 | 72±19 | −6 (−14,1) | |
| Six months | 64±15 | 68±18 | ||
| Fat (total g/d) | .0258 | |||
| Baseline | 64±27 | 64±27 | −3 (−12,5) | |
| Six months | 58±25 | 56±25 | ||
| Intake including whey/control | ||||
| Energy(total kcal/d) | <.0001 | |||
| Baseline | 1639±176 | 1756±540 | −128 (−276,20) | |
| Six months | 1702±324 | 1875±476 | ||
| Carbohydrate (total g/d) | <.0001 | |||
| Baseline | 200±62 | 220±77 | −50 (−73,−27) | |
| Six months | 208±47 | 262±63 | ||
| Protein (total g/d) | <.0001 | |||
| Baseline | 71±17 | 72±19 | 18 (8,29) | |
| Six months | 89±22 | 69±19 | ||
| Fat (total g/d) | .0153 | |||
| Baseline | 64±23 | 64±27 | −4 (−12,5) | |
| Six months | 58±22 | 60±25 | ||
*The average daily dietary intake was obtained from self-reported 3-d food logs.
†Mean change whey (6 month less baseline) minus change in control, and the corresponding 95% CI.
Body Composition (DXA and CT)
Total body lean mass increased 1.3% and 0.6% in the WPC and control groups, respectively, but was not significantly different between groups (p = .46). Total body mass (scale weight) increased in both groups over time with no significant difference between groups. Total body fat mass was also not affected over time in either group (Table 3).
Table 3.
Body Composition Analysis Using Dual X-Ray Absorptiometry and Mid-Thigh Muscle Cross-Sectional Area Using Computed Tomography
| Body Composition | Whey | Control | Point Estimates | P Value |
|---|---|---|---|---|
| (N = 42) | (N = 38) | (95% CI)* | Time | |
| Total body mass† (kg) | <.001 | |||
| Baseline | 73.5±10.8 | 73.7±11.4 | 0.05 (−1.24,1.33) | |
| Six months | 75.5±11.5 | 75.7±11.6 | ||
| Total lean mass (kg) | .01 | |||
| Baseline | 46.7±8.6 | 46.4±8.4 | 0.26 (−0.43,0.95) | |
| Six months | 47.3±8.6 | 46.7±8.4 | ||
| Total fat mass (kg) | .41 | |||
| Baseline | 25.9±6.9 | 25.6±7.2 | −0.12 (−0.87,0.64) | |
| Six months | 25.6±6.9 | 25.5±6.9 | ||
| Total mid-thigh CSA (cm2) | .138 | |||
| Baseline | 192.4±35.2 | 191.3±41.1 | 2 (−5,9) | |
| Six months | 196.4±39.4 | 192.0±36.3 | ||
| Total muscle CSA (cm2) | <.001 | |||
| Baseline | 96.3±21.8 | 97.3±25.0 | 1 (−1,4) | |
| Six months | 100.7±21.7 | 100.1±26.0 | ||
| Total normal-density muscle CSA (cm2) | .011 | |||
| Baseline | 70.3±19.7 | 72.0±26.8 | 3 (−2,8) | |
| Six months | 75.5±20.4 | 73.7±24.8 | ||
| Total low-density muscle CSA (cm2) | .75 | |||
| Baseline | 26.0±7.6 | 25.3±9.6 | −1 (−5,2) | |
| Six months | 25.2±8.6 | 26.4±9.7 | ||
| Total subcutaneous adipose tissue CSA (cm2) | .69 | |||
| Baseline | 83.6±41.9 | 82.8±42.6 | 3 (−2,7) | |
| Six months | 85.4±45.3 | 81.4±37.7 | ||
| Total intermuscular adipose tissue CSA (cm2) | .42 | |||
| Baseline | 4.8±2.8 | 4.7±2.5 | −0.2 (−0.7,0.2) | |
| Six months | 4.5±2.6 | 4.7±2.5 |
*Mean change whey (6 month less baseline) minus change in control, and the corresponding 95% CI.
†The total body mass was obtained from a portable digital scale.
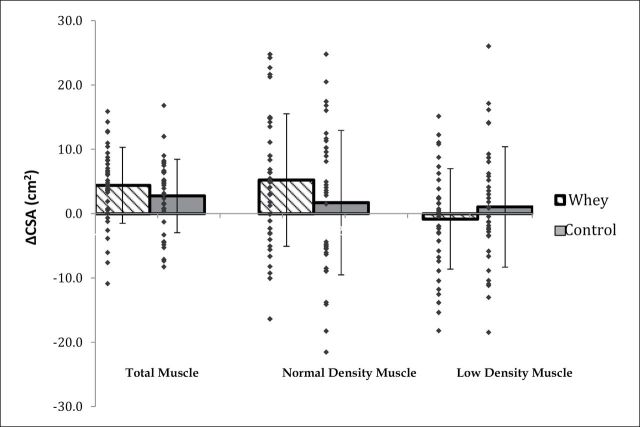
Analysis of the mid-thigh composition by CT scan revealed a statistically significant increase in total muscle CSA with no significant difference in change between groups (Table 3 and Figure 2). In both groups, normal- and low-density muscle CSA and intermuscular and subcutaneous fat were unchanged over time. Similar results were observed for the “completers” analysis with the exception of statistically significant increase over time in both groups for normal-density muscle CSA (8.0% and 4.3% in WPC and control, respectively; p = 0.005; data not shown).
Figure 2.
Plot of change in total, normal-density, and low-density muscle cross-sectional area (CSA, cm2) in whey and control.
Muscle Performance (1-RM Strength and Power)
Muscle strength as measured by the 1-RM increased significantly over time for all muscle groups (Table 4). However, there was no significant difference between the groups. Strength increases over time ranged from 16% to 50%.
Table 4.
Lower Extremity Strength (1-RM) and Power Measures for Total Sample
| Whey | Control | Point Estimates | P Value | |
|---|---|---|---|---|
| (N = 42) | (N = 38) | (95% CI)* | Time | |
| 1-RM (N) | ||||
| Double leg press | <.0001 | |||
| Baseline | 1223±380 | 1256±461 | 58 (−87,202) | |
| Six months | 1483±517 | 1465±528 | ||
| Unilateral right knee extension | <.0001 | |||
| Baseline | 286±115 | 297±145 | 10 (−31,52) | |
| Six months | 378±119 | 376±172 | ||
| Unilateral left knee extension | <.0001 | |||
| Baseline | 259±132 | 290±127 | 37 (−37,111) | |
| Six months | 386±212 | 373±158 | ||
| Peak power 40% (W) | ||||
| Double leg press | <.0001 | |||
| Baseline | 404±162 | 443±261 | 53 (−33,140) | |
| Six months | 569±256 | 549±266 | ||
| Unilateral right knee extension | <.0001 | |||
| Baseline | 108±47 | 113±61 | 15 (1,29) | |
| Six months | 140±53 | 132±64 | ||
| Unilateral left knee extension | <.0001 | |||
| Baseline | 94±47 | 109±48 | 15 (1,29) | |
| Six months | 131±53 | 131±61 | ||
| Peak power 70% (W) | ||||
| Double leg press | <.0001 | |||
| Baseline | 428±192 | 468±257 | 61 (−16,137) | |
| Six months | 592±235 | 567±293 | ||
| Unilateral right knee extension | <.0001 | |||
| Baseline | 126±58 | 135±73 | 27 (9,45) | |
| Six months | 161±68 | 146±68 | ||
| Unilateral left knee extension | <.0001 | |||
| Baseline | 111±56 | 128±53 | 30 (12,47) | |
| Six months | 151±66 | 139±67 | ||
Note: 1-RM = one repetition maximum.
*Mean change whey (6 month less baseline) minus change in control and the corresponding 95% CI.
Peak power measures increased over time for the double leg press at both 40% and 70% of the 1-RM with no difference in the change between groups (Table 4). The change in peak power at both 40% and 70% of 1-RM for the knee extensors increased significantly more in the WPC compared with control. The increases ranged from 28% to 38% in the WPC group and 8% to 21% in control.
Physical Performance Measures
With the exception of the 400-m walk, all measures of physical functioning improved significantly in both groups over time with no difference between groups (Table 5). Further examination of data from the “completers” analysis resulted in improvements in all measures of physical functioning in both groups over time but including 400-m walk time (p = .01; data not shown).
Table 5.
Physical Performance Measures for Total Sample
| Physical Function | Whey | Control | Point Estimates | P Value |
|---|---|---|---|---|
| (N = 42) | (N = 38) | (95% CI)* | Time | |
| Stair-climb time (s) | .03 | |||
| Baseline | 7.7 + 4.2 | 8.5 + 4.6 | 0.3 (−1.1,1.8) | |
| Six months | 7.1 + 3.9 | 7.0 + 3.2 | ||
| Chair-rise time (s; 10×) | .0001 | |||
| Baseline | 31.8 + 8.5 | 33.6 + 16.7 | −1.9 (−5.2,1.4) | |
| Six months | 24.9 + 6.9 | 26.8 + 5.9 | ||
| SPPB score | <.0001 | |||
| Baseline | 8.5 + 1.1 | 8.4 + 1.7 | 0.21 (−0.41,0.83) | |
| Six months | 10.3 + 1.5 | 10.0 + 1.8 | ||
| Four hundred-meter walk (gait speed; m/s) | .13 | |||
| Baseline | 1.1 + 0.2 | 1.0 + 0.2 | 0.08 (−0.02,0.19) | |
| Six months | 1.1 + 0.2 | 1.0 + 0.2 |
*Mean change whey (6 month less baseline) minus change in control, and the corresponding 95% CI.
Discussion
The main finding from this study was that total body lean mass increased in response to 6 months of progressive RT. Although the increase in lean mass in the WPC group was greater than control (1.3% vs 0.6%), these differences were not statistically significant. The analysis of changes in muscle strength, peak power, mid-thigh muscle CSA, stair-climb and chair-rise times, and SPPB score all revealed similar responses with WPC showing greater changes. However, with the exception of knee extensor power, there were no statistically significant differences in the change in measured variables between the groups. Additionally, with the exception of normal-density muscle CSA and 400 m gait speed, the results were similar when examining the “completers” analysis.
The increases in muscle strength and power, and physical functioning in response to RT were comparable to previous reports in this population (19,21,26). In addition, total muscle CSA and lean mass increased significantly in both groups. We also observed significant increases in normal-density muscle CSA over time in the “completers” analysis. Although there were no statistically significant differences between WPC and control, the reported changes all tended to favor WPC. The differences in change in muscle size could lead to important functional benefits over a longer time period.
Current data on the effects of protein supplementation with RT on body composition, muscle hypertrophy, strength, and physical function, from prior studies ranging in duration from 10 weeks to 18 months, have yielded mixed results (12–19). Furthermore, the protein dose delivered has varied between 7.4 and 15g per serving. These differences make comparisons between studies difficult. More importantly, most studies have examined healthy older adults with limited data on mobility-limited older adults (15), and where nutritional and not specifically protein supplementation was a focus (19).
Recent reports suggest that MPS is saturable in response to protein supplementation and overload individually. Cuthbertson et al. (27) observed a dose–response for amino acid supplementation with no added gains in MPS following supplementation exceeding 20 and 10g in young and old, respectively. In an investigation on younger adults, 20g of protein supplementation did not augment the effects of RT on MPS (28). The lack of MPS augmentation following 20g protein supplementation as reported in younger adults may be applicable to older adults also undergoing RT and protein supplementation simultaneously, regardless of physical functional status. Conversely, other observations indicate that at least 40g of protein per serving may be required to optimally augment MPS in older adults (29). We acknowledge that distributing the supplement into two 20g servings per day instead of a 40-g bolus may have blunted the optimal effects on skeletal muscle growth. In addition, the leucine content of WPC in this study may have been inadequate. Katsanos et al. (11) recently observed that a higher amount of leucine is needed for MPS in older relative to younger adults.
Although, a total of 40g per day of WPC was provided in this study, the average increase in total protein intake when supplement adherence and dietary intake of protein was accounted for was 18g per day in the WPC group. This was a result of the total dietary energy intake being reduced by approximately 180 kcal per day and adherence to the WPC supplement being approximately 72%. These data suggest that the supplemental WPC was used as a “partial” meal replacement and reduced voluntary food intake in this population. This is in contrast to the study of Fiatarone et al. (19) where a daily nutritional supplement had an isocaloric effect on voluntary food intake completely equivalent to the energy content of the supplement and which appeared to attenuate the influence of the supplement on exercise-induced changes in muscle mass and strength in institutionalized older adults. Despite previous studies examining the acute administration of dietary protein that have shown maximal stimulation of MPS with 40g of high-quality protein, the results of this study suggest that chronic administration of protein at these dose levels may be difficult as they may suppress energy and protein intake from other dietary sources. Although this lower-than-expected increase in the protein intake observed in the WPC may have attenuated the difference in response between WPC and control, this does not appear to be likely as there were no differences even in the “completer” analysis of participants with the highest adherence.
The dose of RT and session attendance was similar or better than previous exercise training studies in physical functionally impaired older adults (21,26,30). Adherence to the nutritional supplements was similar in both groups and virtually identical between PABA recovery and return of empty packets suggesting self-report of supplement consumption in this population is an acceptable means of tracking supplement adherence. Furthermore, clinically meaningful changes in physical function were reported in a number of the measures in this study suggesting the powerful impact of high-intensity RT in this population (31,32).
We acknowledge there are some limitations in the use of self-report of dietary intake to estimate total energy and protein intake in this study. Participants may have under-reported their dietary intake or even changed their intake as a result of participating in this trial. However, these effects are likely to have been balanced across both treatment arms of the study.
In summary, we observed significant increases in lean mass, muscle CSA, strength and power, and physical function following RT coupled with WPC or control in older mobility-limited adults. However, WPC did not significantly improve the response to 6 months of RT in this population. Future examinations on the efficacy of chronic higher doses of WPC, administered as a single-bolus serving versus multiple servings, or for longer periods of time on changes in lean mass and physical function in this population are warranted.
Funding
This work was supported by Dairy Research Institute (R.A.F.), Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679 to R.A.F.), the Boston Nutrition/Obesity Research Center (DK046200 to R.A.F.), a postdoctoral training grant (DK007651 to A.C.), and the U.S. Department of Agriculture under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
References
- 1. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995; 332: 556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill TM, Murphy TE, Barry LC, Allore HG. Risk factors for disability subtypes in older persons. J Am Geriatr Soc. 2009; 57: 1850–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melnyk JA, Rogers MA, Hurley BF. Effects of strength training and detraining on regional muscle in young and older men and women. Eur J Appl Physiol. 2009; 105: 929–938 [DOI] [PubMed] [Google Scholar]
- 4. Roth SM, Ivey FM, Martel GF, et al. Muscle size responses to strength training in young and older men and women. J Am Geriatr Soc. 2001; 49: 1428–1433 [DOI] [PubMed] [Google Scholar]
- 5. Chalé-Rush A, Morris EP, Kendall TL, Brooks NE, Fielding RA. Effects of chronic overload on muscle hypertrophy and mTOR signaling in young adult and aged rats. J Gerontol A Biol Sci Med Sci. 2009; 64: 1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol. 2005; 98: 557–564 [DOI] [PubMed] [Google Scholar]
- 7. Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci. 1996; 51: M270–M275 [DOI] [PubMed] [Google Scholar]
- 8. Hartman JW, Tang JE, Wilkinson SB, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007; 86: 373–381 [DOI] [PubMed] [Google Scholar]
- 9. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009; 107: 987–992 [DOI] [PubMed] [Google Scholar]
- 10. Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005; 135(suppl 6):1553S–1556S [DOI] [PubMed] [Google Scholar]
- 11. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006; 291: E381–E387 [DOI] [PubMed] [Google Scholar]
- 12. Kukuljan S, Nowson CA, Sanders K, Daly RM. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol. 2009; 107: 1864–1873 [DOI] [PubMed] [Google Scholar]
- 13. Holm L, Olesen JL, Matsumoto K, et al. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol. 2008; 105: 274–281 [DOI] [PubMed] [Google Scholar]
- 14. Iglay HB, Thyfault JP, Apolzan JW, Campbell WW. Resistance training and dietary protein: effects on glucose tolerance and contents of skeletal muscle insulin signaling proteins in older persons. Am J Clin Nutr. 2007; 85: 1005–1013 [DOI] [PubMed] [Google Scholar]
- 15. Rosendahl E, Lindelöf N, Littbrand H, et al. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Aust J Physiother. 2006; 52: 105–113 [DOI] [PubMed] [Google Scholar]
- 16. Bunout B, Barrera G, de la Maza P, et al. Effects of nutritional supplementation and resistance training on muscle strength in free living elders. Results of one year follow. J Nutr Health Aging. 2004; 8: 68–75 [PubMed] [Google Scholar]
- 17. Haub MD, Wells AM, Tarnopolsky MA, Campbell WW. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am J Clin Nutr. 2002; 76: 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol (Lond). 2001; 535(Pt 1):301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994; 330: 1769–1775 [DOI] [PubMed] [Google Scholar]
- 20. Bingham S, Cummings JH. The use of 4-aminobenzoic acid as a marker to validate the completeness of 24h urine collections in man. Clin Sci. 1983; 64: 629–635 [DOI] [PubMed] [Google Scholar]
- 21. Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002; 50: 655–662 [DOI] [PubMed] [Google Scholar]
- 22. Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007; 19: 194–199 [DOI] [PubMed] [Google Scholar]
- 23. Wang W, Wang Z, Faith MS, Kotler D, Shih R, Heymsfield SB. Regional skeletal muscle measurement: evaluation of new dual-energy X-ray absorptiometry model. J Appl Physiol. 1999; 87: 1163–1171 [DOI] [PubMed] [Google Scholar]
- 24. Frontera WR, Reid KF, Phillips EM, et al. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008; 105: 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001; 49: 1161–1167 [DOI] [PubMed] [Google Scholar]
- 26. Reid KF, Callahan DM, Carabello RJ, Phillips EM, Frontera WR, Fielding RA. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res. 2008; 20: 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005; 19: 422–424 [DOI] [PubMed] [Google Scholar]
- 28. Moore DR, Robinson MJ, Fry JL, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009; 89: 161–168 [DOI] [PubMed] [Google Scholar]
- 29. Yang Y, Breen L, Burd NA, et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012; 1–9 [DOI] [PubMed] [Google Scholar]
- 30. Fielding RA, Katula J, Miller ME, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007; 39: 1997–2004 [DOI] [PubMed] [Google Scholar]
- 31. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006; 54: 743–749 [DOI] [PubMed] [Google Scholar]
- 32. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011; 66: 1090–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.