Abstract
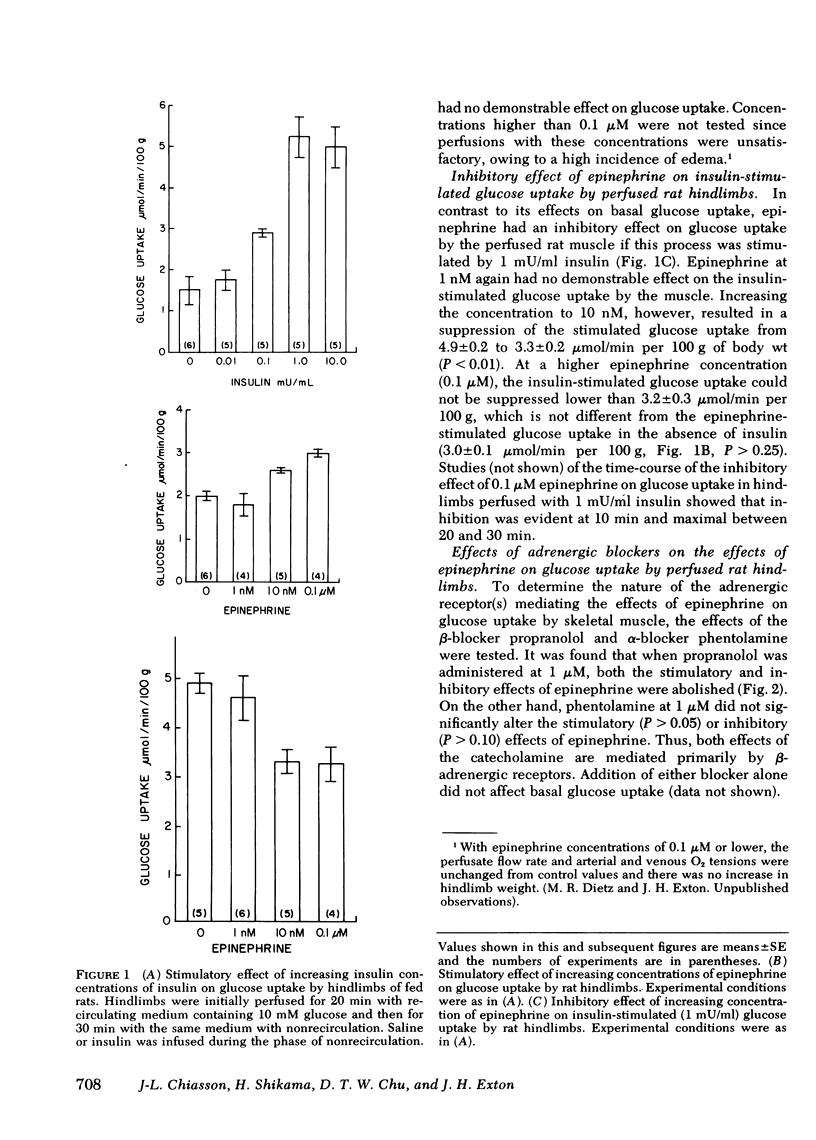
The effect of epinephrine on basal and insulin-stimulated glucose uptake in perfused hindlimbs of fed rats was studied. Insulin increased glucose uptake in a dose-dependent manner from a basal value of 1.5±0.3 up to a maximum value of 5.3±0.9 μmol/min per 100 g with 6 nM (1 m U/ml). Epinephrine at 10 nM and 0.1 μM also increased glucose uptake to 2.6±0.1 and 3.1±0.1 μmol/min per 100 g, respectively. These same concentrations of epinephrine, however, suppressed the insulin-stimulated glucose uptake to 3.2±0.3 μmol/min per 100 g. Both the stimulatory and inhibitory effects of epinephrine on glucose uptake were completely reversed by propranolol, but were not significantly altered by phentolamine.
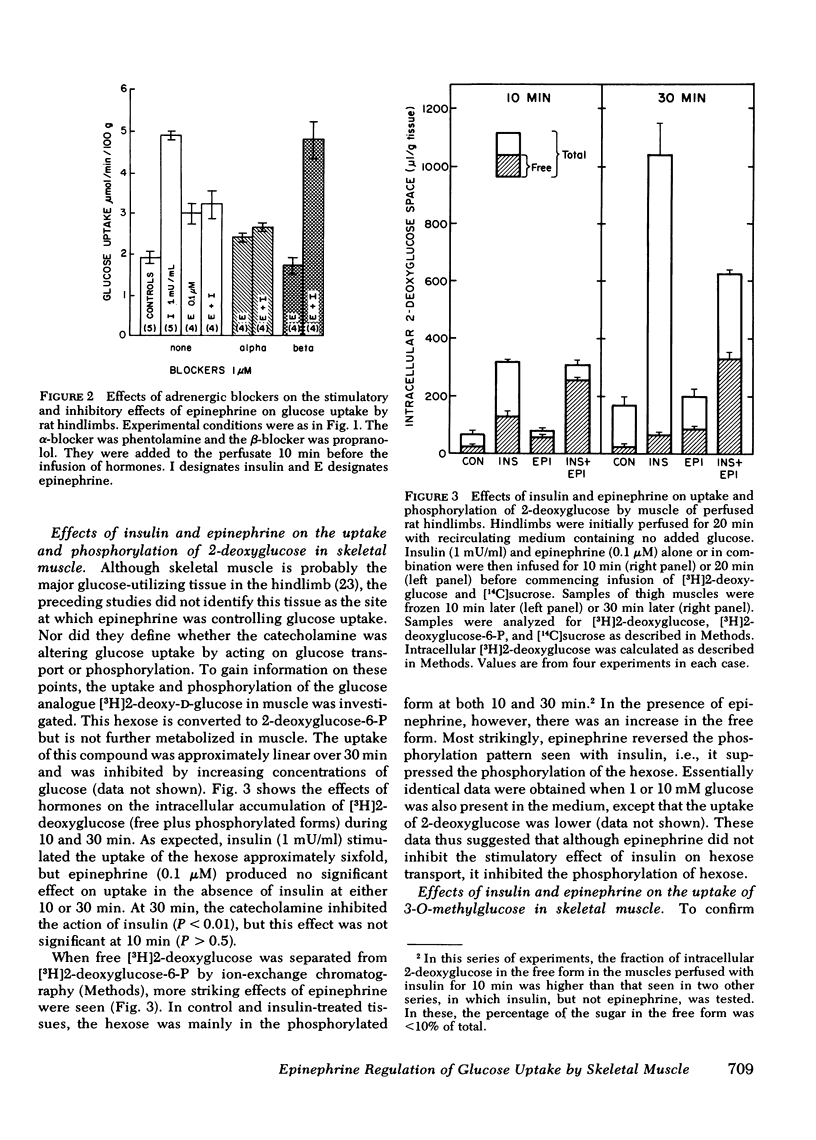
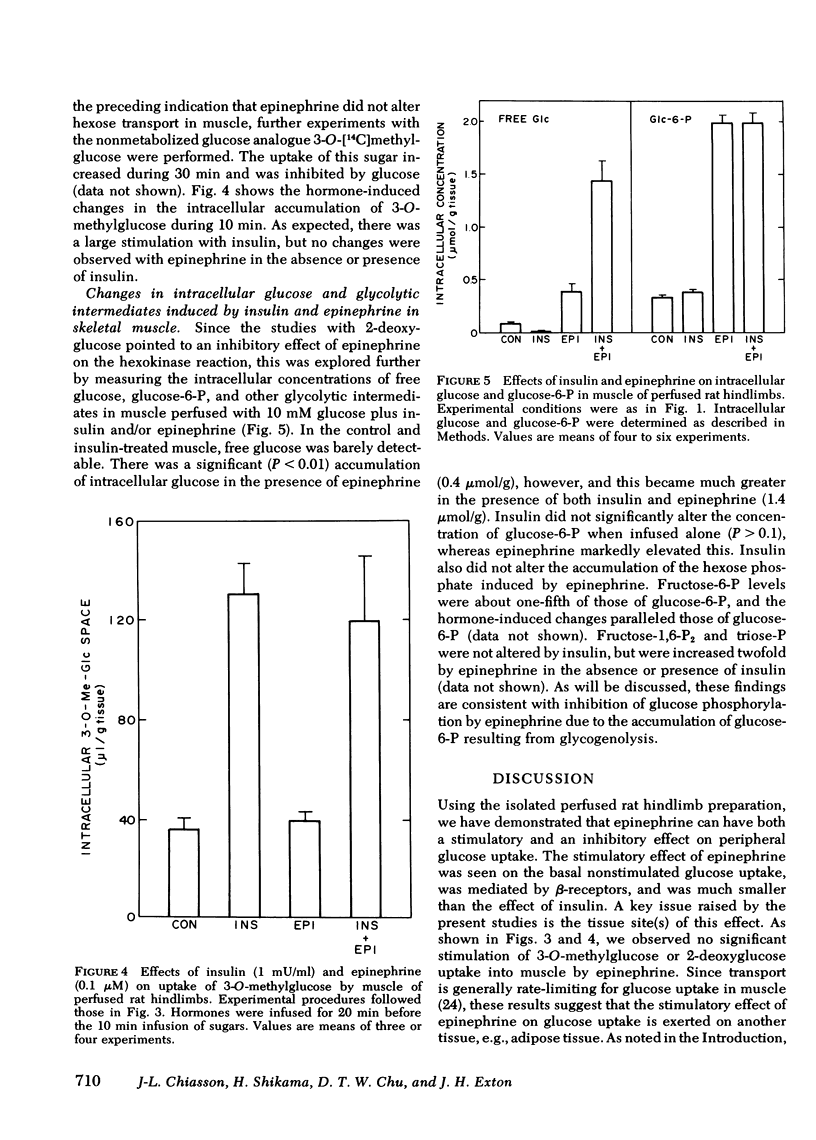
Uptake of 3-O-methylglucose and 2-deoxyglucose into thigh muscles of the perfused hindlimbs was stimulated fivefold by insulin, but was unaffected by epinephrine. Epinephrine also did not inhibit the stimulation of uptake by insulin. Epinephrine decreased the phosphorylation of 2-deoxyglucose, however, and caused the intracellular accumulation of free glucose. These last two effects were more prominent in the presence of insulin. Whereas epinephrine caused large rises in glucose-6-P and fructose-6-P, insulin did not alter the concentration of these metabolites either in the absence or presence of epinephrine.
These data indicate that: (a) epinephrine has a stimulatory effect on glucose uptake by perfused rat hindlimbs that does not appear to be exerted on skeletal muscle; (b) epinephrine does not affect hexose transport in skeletal muscle; (c) epinephrine inhibits insulin-stimulated glucose uptake in skeletal muscle by inhibiting glucose phosphorylation. It is hypothesized that the inhibition of glucose phosphorylation is due to the stimulation of glycogenolysis, which leads to the accumulation of hexose phosphates, which inhibit hexokinase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson E. A., Arky R. A. Role of beta-adrenergic receptors in counterregulation to insulin-induced hypoglycemia. Diabetes. 1968 Mar;17(3):141–146. doi: 10.2337/diab.17.3.141. [DOI] [PubMed] [Google Scholar]
- Bihler I., Sawh P. C., Sloan I. G. Dual effect of adrenalin on sugar transport in rat diaphragm muscle. Biochim Biophys Acta. 1978 Jul 4;510(2):349–360. doi: 10.1016/0005-2736(78)90035-4. [DOI] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, LEBOEUF B., FLINN R. B. Studies on rat adipose tissue in vitro. VI. Effect of epinephrine on glucose metabolism. J Biol Chem. 1960 May;235:1246–1250. [PubMed] [Google Scholar]
- CORI G. T., LARNER J. Action of amylo-1,6-glucosidase and phosphorylase on glycogen and amylopectin. J Biol Chem. 1951 Jan;188(1):17–29. [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Caldwell M. D., Lacy W. W., Exton J. H. Effects of adrenalectomy on the amino acid and glucose metabolism of perfused rat hindlimbs. J Biol Chem. 1978 Oct 10;253(19):6837–6844. [PubMed] [Google Scholar]
- Davidheiser S., Haugaard E. S., Haugaard N. Effects of epinephrine and the cyclic AMP phosphodiesterase inhibitor SQ 20009 on glucose and glycogen metabolism in skeletal muscle. Biochem Pharmacol. 1979 Mar 15;28(6):807–813. doi: 10.1016/0006-2952(79)90362-9. [DOI] [PubMed] [Google Scholar]
- Deibert D. C., DeFronzo R. A. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980 Mar;65(3):717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz M. R., Chiasson J. L., Soderling T. R., Exton J. H. Epinephrine regulation of skeletal muscle glycogen metabolism. Studies utilizing the perfused rat hindlimb preparation. J Biol Chem. 1980 Mar 25;255(6):2301–2307. [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- GROEN J., VAN DER GELD H., BOLINGER R. E., WILLEBRANDS A. F. The anti-insulin effect of epinephrine; its significance for the determination of serum insulin by the rat diaphragm method. Diabetes. 1958 Jul-Aug;7(4):272–277. doi: 10.2337/diab.7.4.272. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- HAGEN J. H., BALL E. G. Studies on the metabolism of adipose tissue. IV. The effect of insulin and adrenaline on glucose utilization, lactate production, and net gas exchange. J Biol Chem. 1960 Jun;235:1545–1549. [PubMed] [Google Scholar]
- HERMAN M. S., RAMEY E. R. Epinephrine action on glucose uptake by rat diaphragm; effect of ionic composition. Am J Physiol. 1960 Aug;199:226–228. doi: 10.1152/ajplegacy.1960.199.2.226. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- KIPNIS D. M., HELMREICH E., CORI C. F. Studies of tissue permeability. IV. The distribution of glucose between plasma and muscle. J Biol Chem. 1959 Jan;234(1):165–170. [PubMed] [Google Scholar]
- LYNN W. S., MACLEOD R. M., BROWN R. H. Effects of epinephrine, insulin, and corticotrophin on the metabolism of rat adipose tissue. J Biol Chem. 1960 Jul;235:1904–1911. [PubMed] [Google Scholar]
- Ludvigsen C., Jarett L., McDonald J. M. The characterization of catecholamine stimulation of glucose transport by rat adipocytes and isolated plasma membranes. Endocrinology. 1980 Mar;106(3):786–790. doi: 10.1210/endo-106-3-786. [DOI] [PubMed] [Google Scholar]
- Lueck J. D., Fromm H. J. Kinetics, mechanism, and regulation of rat skeletal muscle hexokinase. J Biol Chem. 1974 Mar 10;249(5):1341–1347. [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978 Apr 15;172(1):137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R., Haymond M., Cryer P., Gerich J. Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol. 1979 Oct;237(4):E356–E362. doi: 10.1152/ajpendo.1979.237.4.E356. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Felig P. Effect of sequential infusions of glucagon and epinephrine on glucose turnover in the dog. Am J Physiol. 1978 Sep;235(3):E287–E290. doi: 10.1152/ajpendo.1978.235.3.E287. [DOI] [PubMed] [Google Scholar]
- Saha J., Lopez-Mondragon R., Narahara H. T. Effect of epinephrine on permeability to sugar and on the production of free glucose in skeletal muscle. J Biol Chem. 1968 Feb 10;243(3):521–527. [PubMed] [Google Scholar]
- Saito Y., Itaya K., Ui M. Adrenergic alpha-receptor-mediated stimulation of the glucose utilization by isolated rat diaphragm. Biochim Biophys Acta. 1974 May 24;343(3):492–499. doi: 10.1016/0304-4165(74)90266-9. [DOI] [PubMed] [Google Scholar]
- Skikama H., Ui M. Metabolic background for glucose tolerance: mechanism for epinephrine-induced impairment. Am J Physiol. 1975 Oct;229(4):955–961. doi: 10.1152/ajplegacy.1975.229.4.955. [DOI] [PubMed] [Google Scholar]
- Sloan I. G., Sawh P. C., Bihler I. Influence of adrenalin on sugar transport in soleus, a red skeletal muscle. Mol Cell Endocrinol. 1978 Feb-Mar;10(1):3–12. doi: 10.1016/0303-7207(78)90054-0. [DOI] [PubMed] [Google Scholar]
- UI M. ACTION OF EPINEPHRINE ON MUSCLE GLUCOSE UPTAKE DEPENDING ON CA++ AND PHOSPHATE. Am J Physiol. 1965 Aug;209:359–364. doi: 10.1152/ajplegacy.1965.209.2.359. [DOI] [PubMed] [Google Scholar]
- WALAAS E. The effect of adrenaline on the uptake of glucose, mannose and fructose in the rat diaphragm. Acta Physiol Scand. 1955 Dec 31;35(2):109–125. doi: 10.1111/j.1748-1716.1955.tb01270.x. [DOI] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- Wohltmann H. J., Narahara H. T., Wesley M. E. Specificity of the effect of insulin on permeability of frog sartorius muscles to sugar. Diabetes. 1967 Jan;16(1):26–34. doi: 10.2337/diab.16.1.26. [DOI] [PubMed] [Google Scholar]


