Abstract
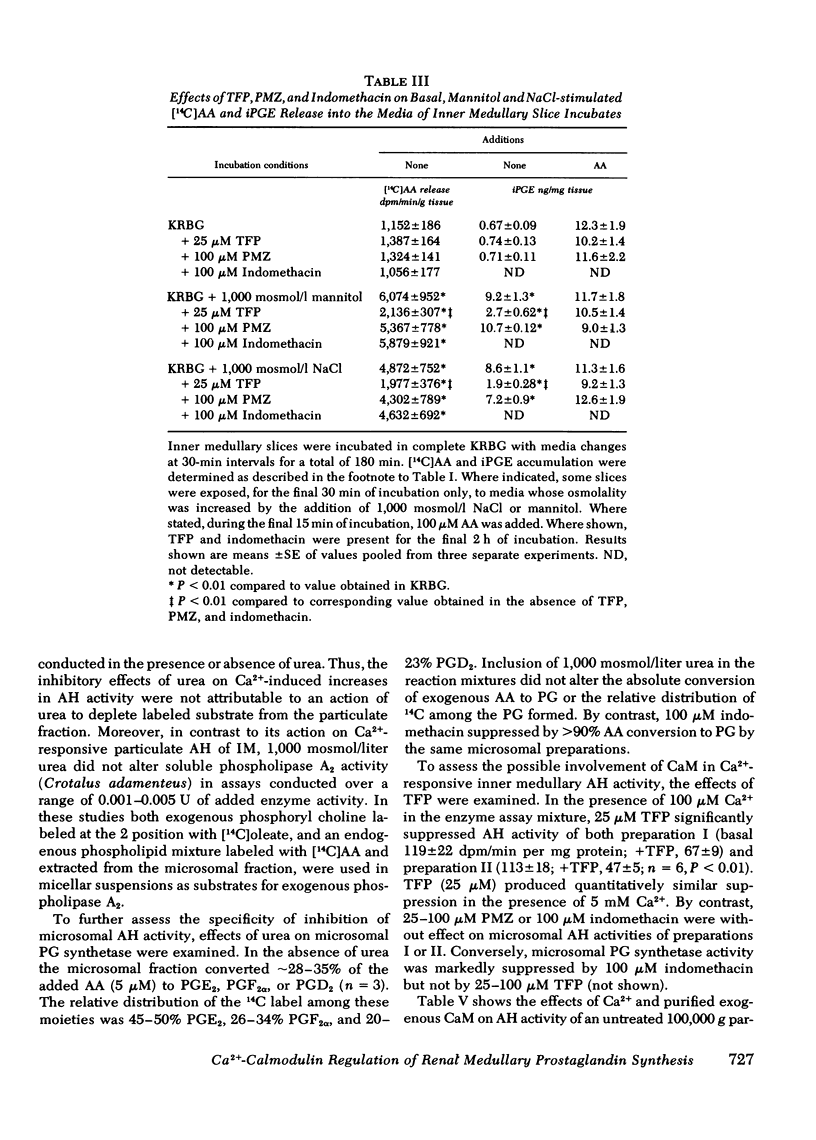
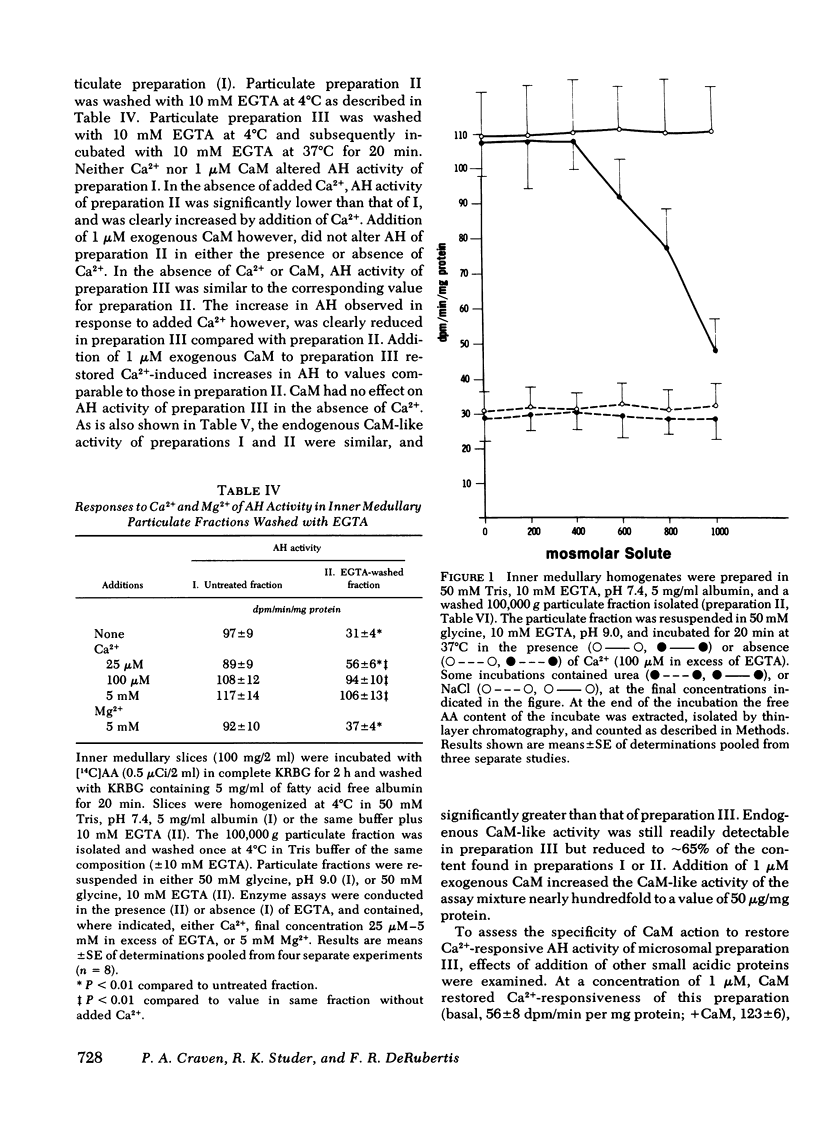
Previous studies have demonstrated that hyperosmolar NaCl and mannitol stimulate immunoreactive prostaglandin E (iPGE) production by slices of inner medulla (IM), whereas urea inhibits this process. In the present study, the roles of Ca2+ and calmodulin in the control of PGE synthesis in IM and the basis for the differential actions of solutes were examined. A23187 increased [14C]arachidonate (AA) release and iPGE accumulation in the presence but not in the absence of media Ca2+ whereas stimulation by hypertonic NaCl or mannitol was well expressed with Ca2+ or in Ca2+-free buffer containing 2 mM EGTA. Hypertonic urea and trifluoperazine (TFP), an inhibitor of actions of the Ca2+-CaM complex, suppressed increases in [14C]AA release and iPGE induced by A23187, NaCl, or mannitol. By contrast, increases in iPGE in response to exogenous AA were not altered by urea or TFP. Ca2+ (25-100 microM) increased acyl hydrolase (AH) activity in EGTA washed (4 degrees C) 100,000 g particulate fractions of IM threefold, thereby restoring AH activity to the higher basal values of particulate fractions not washed with EGTA. This action of Ca2+ was blocked by hypertonic urea of TFP, whereas AH activity was not influenced by NaCl or mannitol in the presence or absence of Ca2+. In contrast to their effects on AH activity, hypertonic urea and TFP did not alter conversion of AA to PGE2, PGF2 alpha, or PGD2 by IM microsomal fractions. Ca2+-induced increases in particulate AH were blunted after partial depletion of endogenous CaM-like activity. Ca2+ action was restored by addition of purified exogenous CaM, but not by addition of other small acidic proteins, including troponin C. The findings support a role for CaM in the regulation of PGE synthesis in the IM at the level of Ca2+-responsive AH activity. They further imply that urea suppresses PGE synthesis in IM through inhibition of AH and a reduction in the availability of endogenous AA for conversion to PGE.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELBOOM J. W., BRODSKY W. A., SCOTT W. N. EFFECT OF OSMOTIC DIURESIS ON INTRARENAL SOLUTES IN DIABETES INSIPIDUS AND HYDROPENIA. Am J Physiol. 1965 Jan;208:38–45. doi: 10.1152/ajplegacy.1965.208.1.38. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Berl T., McDonald K. D., Schrier R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J Clin Invest. 1975 Aug;56(2):420–426. doi: 10.1172/JCI108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl T., Raz A., Wald H., Horowitz J., Czaczkes W. Prostaglandin synthesis inhibition and the action of vasopressin: studies in man and rat. Am J Physiol. 1977 Jun;232(6):F529–F537. doi: 10.1152/ajprenal.1977.232.6.F529. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A., Wolff D. J. Calcium-dependent adenylate cyclase from rat cerebral cortex. Reversible activation by sodium fluoride. J Biol Chem. 1977 Aug 25;252(16):5677–5685. [PubMed] [Google Scholar]
- Craven P. A., Briggs R., DeRubertis F. R. Calcium-dependent action of osmolality on adenosine 3',5'-monophosphate accumulation in rat renal inner medulla: evidence for a relationship to calcium-responsive arachidonate release and prostaglandin synthesis. J Clin Invest. 1980 Feb;65(2):529–542. doi: 10.1172/JCI109697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Knapp H. R., Oelz O., Oates J. A. Stimulation of prostaglandin biosynthesis in the renal papilla by hypertonic mediums. Am J Physiol. 1978 Jan;234(1):F64–F67. doi: 10.1152/ajprenal.1978.234.1.F64. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Effects of osmolality and oxygen availability on soluble cyclic AMP-dependent protein kinase activity of rat renal inner medulla. J Clin Invest. 1978 Dec;62(6):1210–1221. doi: 10.1172/JCI109241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Sequential alterations in the hepatic content and metabolism of cyclic AMP and cyclic GMP induced by DL-ethionine: evidence for malignant transformation of liver with a sustained increase in cyclic AMP. Metabolism. 1976 Dec;25(12):1611–1625. doi: 10.1016/0026-0495(76)90114-1. [DOI] [PubMed] [Google Scholar]
- Derksen A., Cohen P. Patterns of fatty acid release from endogenous substrates by human platelet homogenates and membranes. J Biol Chem. 1975 Dec 25;250(24):9342–9347. [PubMed] [Google Scholar]
- EPSTEIN F. H., KLEEMAN C. R., PURSEL S., HENDRIKX A. The effect of feeding protein and urea on the renal concentrating process. J Clin Invest. 1957 May;36(5):635–641. doi: 10.1172/JCI103463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman M., Zia P., Zipser R. Contribution of urine volume to the elevated urinary prostaglandin E in Bartter's syndrome and central and nephrogenic diabetes insipidus. Adv Prostaglandin Thromboxane Res. 1980;7:1193–1197. [PubMed] [Google Scholar]
- Franson R., Weiss J., Martin L., Spitznagel J. K., Elsbach P. Phospholipase A activity associated with membranes of human polymorphonuclear leucocytes. Biochem J. 1977 Dec 1;167(3):839–841. doi: 10.1042/bj1670839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. A., Balsam P., Bourgoignie J. J. Reversible inhibition by lanthanum of the hydrosmotic response to serosal hypertonicity in toad urinary bladder. J Membr Biol. 1979 Jun 29;48(1):13–19. doi: 10.1007/BF01869254. [DOI] [PubMed] [Google Scholar]
- Kaye Z., Zipser R., Hahn J., Zia P., Horton R. Water diuresis is a major regulator of prostaglandin E excretion in man. Adv Prostaglandin Thromboxane Res. 1980;7:1017–1019. [PubMed] [Google Scholar]
- Kirschenbaum M. A., Serros E. R. Effects of alterations in urine flow rate on prostaglandin E excretion in conscious dogs. Am J Physiol. 1980 Feb;238(2):F107–F111. doi: 10.1152/ajprenal.1980.238.2.F107. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. The role of the renal concentrating mechanisms in the regulation of serum sodium concentration. Am J Med. 1977 Feb;62(2):165–169. doi: 10.1016/0002-9343(77)90312-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowe D. A., Richardson N. P., Taylor P., Donatsch P. Increasing intracellular sodium triggers calcium release from bound pools. Nature. 1976 Mar 25;260(5549):337–338. doi: 10.1038/260337a0. [DOI] [PubMed] [Google Scholar]
- Lum G. M., Aisenbrey G. A., Dunn M. J., Berl T., Schrier R. W., McDonald K. M. In vivo effect of indomethacin to potentiate the renal medullary cyclic AMP response to vasopressin. J Clin Invest. 1977 Jan;59(1):8–13. doi: 10.1172/JCI108624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk J. D., Waite M. Phospholipid hydrolysis by phospholipase A 1 and A 2 in plasma membranes and microsomes of rat liver. Biochim Biophys Acta. 1973 Mar 29;298(3):562–576. doi: 10.1016/0005-2736(73)90074-6. [DOI] [PubMed] [Google Scholar]
- Oelz O., Knapp H. R., Roberts L. J., Oelz R., Sweetman B. J., Oates J. A., Reed P. W. Calcium-dependent stimulation of thromboxane and prostaglandin biosynthesis by ionophores. Adv Prostaglandin Thromboxane Res. 1978;3:147–158. [PubMed] [Google Scholar]
- PRIVETT O. S., BLANK M. L., ROMANUS O. ISOLATION ANALYSIS OF TISSUE FATTY ACIDS BY ULTRAMICRO-OZONOLYSIS IN CONJUNCTION WITH THIN-LAYER CHROMATOGRAPHY AND GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1963 Jul;4:260–265. [PubMed] [Google Scholar]
- Patak R. V., Fadem S. Z., Rosenblatt S. G., Lifschitz M. D., Stein J. H. Diuretic-induced changes in renal blood flow and prostaglandin E excretion in the dog. Am J Physiol. 1979 May;236(5):F494–F500. doi: 10.1152/ajprenal.1979.236.5.F494. [DOI] [PubMed] [Google Scholar]
- Piascik M. T., Wisler P. L., Johnson C. L., Potter J. D. Ca2+-dependent regulation of guinea pig brain adenylate cyclase. J Biol Chem. 1980 May 10;255(9):4176–4181. [PubMed] [Google Scholar]
- Raz A., Erman A. Effects of divalent cations on prostaglandin biosynthesis and phospholipase A2 activation in rabbit kidney medulla slices. Adv Prostaglandin Thromboxane Res. 1980;6:231–234. [PubMed] [Google Scholar]
- Schwartzman M., Gafni Y., Raz A. Properties of prostaglandin synthetase of rabbit kidney medulla. Eur J Biochem. 1976 May 1;64(2):527–534. doi: 10.1111/j.1432-1033.1976.tb10332.x. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Activation of high levels of endogenous phospholipase A2 in cultured cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):195–199. doi: 10.1073/pnas.76.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylos W., Howard L., Ritzi E., Skarnes R. The preparation and characterization of prostaglandin E1 antiserum. Prostaglandins. 1974 Apr 10;6(1):1–13. doi: 10.1016/s0090-6980(74)80035-3. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Characterization of cyclic nucleotide phosphodiesterases of rat tissues. J Biol Chem. 1971 May 25;246(10):3145–3150. [PubMed] [Google Scholar]
- Vogt W. Role of phospholipase A2 in prostaglandin formation. Adv Prostaglandin Thromboxane Res. 1978;3:89–95. [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Wong P. Y., Cheung W. Y. Calmodulin stimulates human platelet phospholipase A2. Biochem Biophys Res Commun. 1979 Sep 27;90(2):473–480. doi: 10.1016/0006-291x(79)91259-2. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Davis B. B. Effects of calcium on prostaglandin E2 synthesis by rat inner medullary slices. Am J Physiol. 1978 Sep;235(3):F213–F218. doi: 10.1152/ajprenal.1978.235.3.F213. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Levitt M. J., Davis B. B. Effect of oxygen and solute on PGE and PGF production by rat kidney slices. Prostaglandins. 1977 Jan;13(1):143–151. doi: 10.1016/0090-6980(77)90051-x. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Steinhardt R. A., Winkler M. M. Intracellular calcium release and the mechanisms of parthenogenetic activation of the sea urchin egg. Dev Biol. 1978 Aug;65(2):285–295. doi: 10.1016/0012-1606(78)90028-3. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Brown C. A. Role of phospholipase in the regulation of prostaglandin E2 biosynthesis by rabbit renomedullary interstitial cells in tissue culture: effects of angiotensin II, potassium, hyperosmolality, dexamethasone, and protein synthesis inhibition. Adv Prostaglandin Thromboxane Res. 1980;6:243–248. [PubMed] [Google Scholar]


