Abstract
Impaired attentional set-shifting and inflexible decision-making are problems frequently observed during normal aging and in several psychiatric disorders. To understand the neuropathophysiology of underlying inflexible behavior, animal models of attentional set-shifting have been developed to mimic tasks such as the Wisconsin Card Sorting Task (WCST), which tap into a number of cognitive functions including stimulus-response encoding, working memory, attention, error detection, and conflict resolution. Here, we review many of these tasks in several different species and speculate on how prefrontal cortex and anterior cingulate cortex might contribute to normal performance during set-shifting.
Keywords: mPFC, prefrontal cortex, ACC, Anterior cingulate cortex, Attention, Set-shifting, Rule Learning, Behavioral Flexibility
INTRODUCTION
Cognitive rigidity is a hallmark of many human psychiatric disorders and is a frequent result of traumatic brain events[1-7]. Patients who suffer from deficits with behavioral flexibility are generally able to learn information and rules to guide behavior, but lack the ability to modify responding when the situation warrants such a change. One of the ways in which people assess deficits with behavioral flexibility is by studying selective attention. Attention is a cognitive process by which the brain dedicates sensory resources to particularly relevant stimuli, necessary to motivate behavior, and ignores other sensory input irrelevant to the current motivated goal [8-11]. Attention is context-dependent, and is an emergent property of semantic memory, working memory and reward related assessments of recent behaviors. Appropriate control and use of attention can lead to effective behavioral flexibility, enabling animals to successfully navigate in an ever changing world.
Rule learning and executive function in humans has been assessed successfully through several different behavioral methods, most notably the Wisconsin Card Sorting Task (WCST)[7, 12]. In the WCST, participants are presented with a series of cards with different shapes that vary in type, number, and color, and asked to sort them. Participants are not told how to sort the cards, but only to categorize them based on one of these dimensions in order to receive reward. Generally, people will quickly learn rules governing their card sorting and rapidly progress with effective sorting measures[11, 13]. Over time, the rule for sorting parameters is changed (unbeknownst to the participant) who then will need to determine the correct sorting parameters and shift their behavior from the previous rule to the current rule. The WCST test has proven to be an effective test of flexible learning and category learning in humans[14].
While the main strength of the WCST rests on its usability in assessing prefrontal damage in humans[7], both the WCST and other set-shifting paradigms are also highly effective at testing executive function in humans with psychiatric disorders[7, 15-17]. Impairments on the WCST in psychiatric patients and after brain damage have pointed to PFC as being critical for behavioral flexibility[16]. These deficits exist for disorders ranging from Alzheimer’s disease to schizophrenia, and even include individuals exposed to severe bouts of stress[2, 13, 18-28]. Although the proposed mechanisms of deficits in each of these disorders differ, deficits with behavioral flexibility regarding rule shifting likely originates, at least in part, from changes to the prefrontal cortex (PFC).
For example, it is thought that with patients who have schizophrenia, altered prefrontal gamma oscillations from deficits in parvalbumin expressing cortical interneurons may underlie the associated problems with behavioral flexibility[29, 30]. In people with a genetic risk factor for Alzheimer’s disease, the apolipoprotein E type 4 allele (ApoE4), there is a link between prefrontal cerebrovascular risk and degeneration due by blood pressure[31] which may lead to set-shifting deficits. Individuals with frontal lobe epilepsy also show deficits in set-shifting[3, 32-34]. Thus, it is of broad importance to try to understand the nature of the anatomy and circuitry behind set-shifting, and it is clear that we should be focusing on prefrontal cortex and its associated areas.
Animal variants are needed so that we can elucidate the neuroanatomical areas, connections and pharmacology that underlie particular cognitive functions and how these different neural substrates generate these cognitive functions[35]. One common behavioral method for assessing this is to use an attentional set-shifting paradigm from rodent to primate, which requires an initially learned rule to be shifted. Such tasks vary in their design, though they all maintain the same general principle requiring the learning of abstract rules to guide behavior, followed by a shift between available rules[11, 36, 37].
While the human WCST uses dimensions in which humans are well skilled to distinguish, animal variants use dimensions from modalities that are readily sensed and learned by the animal of interest. Primate tests generally take advantage of their advanced visual and spatial systems[38], and rodent tests use olfactory and texture cues or sets of simple visual cues that are easily dissociable and highly visible[36, 39, 40] .
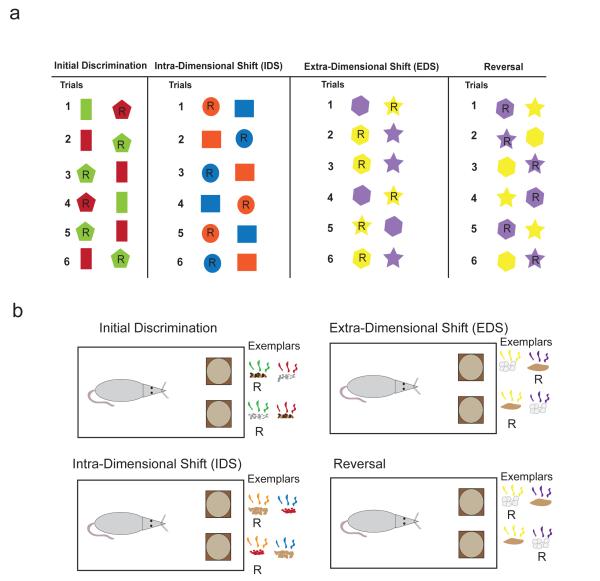
Key elements of set-shifting include intra-dimensional shifts (IDSs) and extra-dimensional shifts (EDSs). All set-shifting paradigms start with the formation of a rule (Fig. 1a; initial discrimination). For example, subjects may be presented with differently shaped stimuli of different colors and they must learn which object, when selected, produces a reward (e.g. money; food). In the example illustrated in Fig. 1a, during the initial discrimination, subjects learn that selection of the pentagon produces reward. This is true regardless of the color. Thus, subjects need to ignore the irrelevant dimension (color) and pay attention to the relevant dimension (shape), while following the rule of responding to the pentagon to obtain reward.
Figure 1.
Examples of set shifting tasks. a. Examples of different types of trials for a set shifting task between color and shape. Shapes with ‘R’ represent the ‘correct’ response, or a ‘reward’. Under the initial discrimination, the rewarded dimension is ‘shape’, not color, where pentagons are rewarded but never rectangles. During the IDS, the exemplar options are changed, but the rule remains the same, ‘shape’, where circles are rewarded but squares are not. The reversal trial reverses the previously correct stimuli, but keeping the rule the same ‘ie, follow shape’. For the EDS, the rewarded dimension shifts from the previous rule of ‘shape’ to the now correct ‘color’, rewarding yellow choices. b. A rodent version of this task, showing the same progression from a discrimination, through an IDS, reversal and an EDS. Exemplars are shown in the form of stylized digging media and odors, and correct pairings are shown with an ‘R’ under them. In this case, the rodent is trained to follow the rule ‘odor’, until the EDS, when the rodent has to shift from this rule to ‘media’.
For an IDS, the relevant dimension stays the same, but the exemplars change. In this case, participants must still focus on the same relevant dimension (shape) and ignore the irrelevant dimension (color). If participants are following the previously learned rule, performance on the IDS will include few errors. Thus, attention is still focused on the shape dimension while ignoring the irrelevant dimension (i.e. color). However, during an EDS, subjects must switch the dimension they are paying attention to because the relevant rule now resides in another dimension. In the example above, on EDS trials the shape of the object has no predictive power. Instead, one of the other exemplars from the other dimensions predicts reward, providing the new rule (Fig. 1a). Across tasks, dimensions vary (i.e. colors, shapes, space, textures, etc) depending on the subject being studied (i.e., rat, mouse, human, monkey, etc), but the general idea stays the same; rules are learned and reinforced within a dimension on IDS trials and rules are shifted across dimensions on EDS trials.
The IDS and EDS tasks differ from other classical reversal tasks, in that a reversal requires an animal to inhibit responding to a distinct stimulus which previously was instructive of a reward, and to drive responding towards a distinct, previously unrewarded stimulus (Figure 1). Reversals, therefore, test a more discrete form of cognition, whereas the IDS and especially the EDS test more abstract, rule-based learning. Animal models of set shifting are extremely powerful, as they combine elements of sensory perception, attention, and working memory to provide a more accurate representation of complex real life decisions. Set-shifting tasks have allowed animal researchers to test and tease apart prefrontal cognitive function, something that is difficult to do in humans with disease or non-localized brain damage. This is important because PFC consists of many brain regions, each with functionally different roles critical for accurate set-shifting performance.
In the rat, mPFC is broken into two different cytoarchitectonic regions: the prelimbic (plPFC) and infralimbic (ilPFC) regions. Both the plPFC and ilPFC are the recipients of direct projections from CA1 of the hippocampus[41, 42]. The ilPFC has efferent projections to plPFC, ACC, orbitofrontal cortex (OFC), dorsal and ventral striatum, central and basolateral amygdala, Medial Dorsal (MD) thalamus and ventral tegmental area/substantia nigra pars compacta (VTA/SNc), and shares many of the same projections as plPFC, with minor exceptions being projections to central, but not basolateral amygdala projections[43]. However, plPFC also is strongly innervated by CA1 projections and not CA2/CA3 or dentate gyrus[44], and plPFC cells can be excited by hippocampal stimulation at monosynaptic latencies[45]. Medial PFC (mPFC) as a whole is thus a strong recipient of CA1 projections, though interestingly mPFC does not reciprocally project directly to hippocampus[46, 47], but projects to hippocampus via parahippocamoal regions and the nucleus reunions [43, 48]. Rat mPFC is also strongly innervated by corticostriatal loops[49, 50] and receives direct dopaminergic projections from VTA[51, 52]. Indeed, dopamine receptor blockade in mPFC yields the same behavioral deficits as direct inactivation[53, 54]. Thus, rat mPFC is situated to effectively integrate context, current sensory stimuli, recent and past memories, value (both expected and unexpected) and recent motor plans.
In rodents and monkeys the ACC is often considered a sub section of the mPFC, along with the prelimbic and infralimbic cortices, however, these structures have been shown to serve different functions[55, 56], thus we will discuss them as different brain areas. In both rodents and monkeys, ACC is dorsal to plPFC (area 32 in monkeys), however, in many studies it is difficult to ascertain their dissociable roles because they are often lumped together and share many of the same connections[47, 57-60]. ACC receives projections from ilPFC and plPFC[61, 62] as well as motor and somatosensory cortices[63, 64]. Efferent projections from ACC are to sensorimotor and visual areas, while also projecting to caudal areas within the cingulate cortex[61] and to both plPFC and ilPFC[65]. ACC also projects to more basal and lateral areas of the amygdala, medial areas of the striatum, including both dorsal and ventral areas[66].
It is generally accepted that rodent mPFC and ACC are good anatomical homologues to the same regions found in primate PFC[65, 67-69]. Unlike rodent PFC, primate PFC is generally broken down also into a more dorsolateral region that is thought to be involved in more executive and cognitive-related functions as compared to more ventromedial portions (VMPFC) that are more related to emotional content and reward associations[58, 70, 71]. While there are no perfectly compatible dorsolateral neuroanatomical regions of the rodent PFC, a great body of work suggests that some of these functions might be under the control of rodent mPFC[43, 47, 63, 65, 68, 72, 73]. Certainly, the deficits observed after DLPFC lesions in monkeys and mPFC lesions/inactivation in rodents suggests they serve similar roles, at least during set-shifting as we will discuss below.
Set-shifting tasks identify a common property of cognition: that dynamic and complex processes underlie the cognitive function of set-shifting. When viewed broadly, understanding rule learning and shifting may appear simple, yet forming and shifting an established set requires the complex coordination of multiple underlying cognitive processes. These processes include competition between two behavioral responses (conflict), error detection, reward predictions and reinforcement history[74, 75]. These underlying cognitive processes all play major roles in the formation and shifting of an attentional set. Recent research has shown that many different regions in prefrontal cortex are critical for these functions to various degrees[74, 76-81]. Below we review several tasks that examine set-shifting in monkeys, rats, and mice, and how they have pointed to specific neuroanatomical regions being related to different aspects of set-shifting. Then, we explore what functions recently attributed to these areas might be critical for these aspects.
Monkey Visual Tasks
Primate research generally requires subjects to make visual saccades, or to manipulate objects to make selections. Primates possess great visual acuity, and attentional set-shifting tests for primates make use of this, generally presenting two complex and colorful visual cues, and requiring the monkey to make a response to one for a juice reward[38, 74, 82, 83]. The visual task is similar to the human set shifting tasks, in that it uses visual cues as the dimensions, including different colors, patterns and shapes which are frequently used as response options. Using a rule to guide behavior, the monkey must ignore the irrelevant stimulus dimension, but focus on the correct dimension.
Dias et al (1996) demonstrates in marmosets that lesions to OFC resulted in reversal deficits, while lesions to the lateral PFC lead to set-shifting deficits on EDS[38, 83] . These data utilized complex visual cues which shifted between using shape or lines to guide their behavior. Subsequent work demonstrated that inhibition of the serotonergic system in the cortex leads to reversal, but not attentional set-shifting deficits[38]. The learning of abstract categories relates to learning rules in that generalizable behavioral responses are created, based on rules that relate to future circumstances. Antzoulatos and Miller (2011) demonstrated that correlates of Stimulus-Response (S-R) learning occur earliest in the striatum of primates as they perform a categorization task, while the dorsolateral prefrontal cortex DLPFC more slowly encodes the developing associations[74]. Changes in activity in DLPFC predicted the shift in rules or responses better during category learning than S-R learning, which were better predicted by striatal activity. These data suggest that DLPFC is necessary for holding online a current and abstract rule to guide responding, while the striatum is more essential for behaving in a more stereotyped S-R manner, consistent with much previous literature[80, 82, 84, 85].
Unlike lateral PFC, lesions to ACC in monkeys do not yield large deficits on a set-shifting-like task per se, but rather a deficit related to error monitoring and sustaining attention to the task[86]. These data suggest that there are reliably dissociable functions of DLPFC and ACC in primates, whereby DLPFC has a more dedicated function towards forming attentional sets, and ACC is more directly activated under changing circumstances, such as altered response sets, changing rules or when there is conflict between responses. Notably, primate research on set-shifting has focused on dorsal and lateral parts of PFC, with few interference studies examining the role that mPFC (Brodmann areas 32 and 25) and ACC (Brodmann area 24) play in set-shifting [38, 56, 74, 85, 87] .
Digging Tasks
One method of testing rodents has been to use of a digging task as illustrated in Fig. 1b which combines attentional set-shifting with reversal learning [36]. Rodents are trained to dig in one of two bowls based on either an olfactory cue, or a media/texture cue, in cases where the two cues are presented together in random locations and combinations. Both rats and mice will readily dig for a food reward and quickly learn the associated rules, using either odors or digging media to guide decisions. In order to teach rodents a generalizable rule, different digging media and olfactory cues are used for multiple IDS tasks. Increased performance, measured as an enhanced learning rate, on new IDS tasks is generally taken as a measure of the rodent learning the ‘rule’ and applying this characterization rule to novel sets of exemplars in which the overarching ‘rule’ (ie, follow the odor, ignore the digging media) is followed. To avoid a potential confound with reversal learning on a known set of exemplars, new sets of exemplars are used for the EDS portion of the task, requiring animals to appropriately shift their response strategies on a novel set of discriminants, though the rule has shifted.
This is a robust task, allowing the testing of multiple rodent models for the purpose of examining multiple different topics. With the initial examination in rats, Birrell and Brown (2000) demonstrated that OFC and mPFC are necessary to effectively resolve reversal and set-shifting digging problems, respectively[36]. They demonstrated that rodent OFC and mPFC have independent and distinct roles during situations requiring flexible behavior, as described in primates.
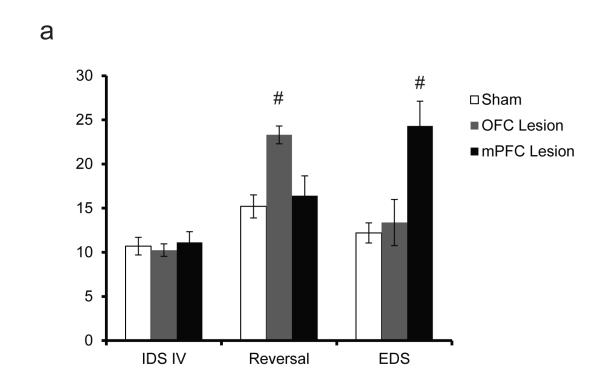
Subsequent work demonstrated that not only rats, but mice, had similar functional neuroconnectivity[39]. Bissonette et al demonstrated that excitotoxic lesions to OFC disrupted performance on a reversal digging task, while lesions to mPFC impaired performance on the EDS portion of the task (Fig. 2a). By demonstrating that mice had the capabilities to perform attentional set-shifting in a manner dissociable from reversal learning, Bissonette et al. opened the door for a myriad of transgenic testing[88-91]. Recently, they investigated the role of topiramate, an anti-seizure medication with mechanisms of action on GABAA receptors known to lead to impairments in humans[92] on set shifting in mice. Further research from their lab investigated the role of impaired cortico-striatal GABAergic inhibition on the digging task using a mutant of the autism associated gene, MET- and uncovered a reversal deficit similar to previous lesion work. Remarkably, they found that a loss of only 30% of the parvalbumin interneurons was sufficient to impair reversal learning[93]. The wealth of data originating in both the rat and mouse reports from this digging task should not be understated, as it remains a simple and relatively cost-effective task to perform, during which time many animals can be evaluated.
Figure 2.
Excitotoxic lesions to mouse OFC and mPFC yield different cognitive deficits. a, Lesions to mouse OFC drive reversal, but not set-shifting deficits, whereas mPFC lesions yield set-shifting, but not reversal deficits, when compared to sham animals (pound sign denotes p<0.05). N for Sham, OFC lesion and mPFC lesion were 10, 8 and 8, respectively. Modified from Bissonette et al, 2008[39]
Digging tasks have also been used to make dissociations between mPFC and ACC. Ng and colleagues demonstrated that ACC was critical when shifting attention between closely related meaning cues (ie, between two odors) within a perceptual dimension[37] on an IDS, whereas the mPFC was critical for shifting attention between disparate sets of meaningful cues (ie from odors to digging media) between perceptual dimensions on an EDS. This suggests that ACC is more critical for flexibility when cues are more closely related during IDS[37]. Although Ng et al. found that ACC was not critical for simple reversals, other groups have tested ACC inactivated rats on a 4-choice odor discrimination task with reversals and found impairments[94, 95]. Thus, unlike OFC, ACC is not critical for simple reversals but is necessary when reversals are more challenging, and unlike mPFC, ACC is necessary for discriminating between closely related cues, such as those found during an IDS, but not for more distinctly different cues, such as would be encountered on an EDS.
Maze Tasks
Strategy set-shifting is generally completed in an operant chamber or maze environment, typically a cross or Y maze[96, 97]. These tasks require animals to shift, on egocentric (left/right) or visual responses, though some tasks do use mazes contrasting visual brightness with tactile stimuli[98]. Operant tasks have the added advantage of using the same types of exemplars (egocentric direction and visual cues)[53, 96, 99, 100]. Errors in attentional set-shift paradigms are difficult to parse out, as they may be due to confounding sensory processing issues with novel stimuli. In a maze or operant task, since the stimuli are constant, the behavior tests conflict resolution more than investigative behaviors behind seeking a novel and successful behavioral strategy to solve a current problem.
Ghods-Sharifi et al, 2008, demonstrated the effectiveness of this type of task, by inactivating OFC and demonstrating impaired behavior on reversal trials, compared to strategy set-shifting trials for rats shifting between visual cued responses or egocentric responses[97]. These data continued to support dissociable roles for OFC and mPFC in mediating reversal or set-shifting type behaviors, respectively.
However, recent work has now suggested that OFC may play some role in mediating the formation of attentional sets on a rodent digging task. Worse performance on a repeated IDS task (using 4 ID tasks) correlated with severity of EDS deficit in an OFC lesioned animal[101]. This finding was replicated in a 7IDS task that included a reversal trial. OFC lesioned rats had a robust reversal deficit and reduced shift-costs, suggesting OFC plays an important role in clearly identifying the important relevant cues after a shift occurs, which generally leads to unexpected outcomes in performance during behavior. A deficit in identifying a relevant cue in an ambiguous situation may lead to an initial delay in determining what new rules may be important to guide behavior[101].
These maze tasks have allowed the testing of the role of many neuromodulators and have also revealed the functionality of different receptor subtypes. For example, Dalton et al (2011) demonstrated a set-shifting deficit stemming from the inactivation of the GluN2B subunit of the NMDA receptor in the PFC, while Floresco et al (2006) highlighted the fact that inhibiting multiple dopamine receptors (D1, D2 and D4) impaired performance in set-shifting tasks[53, 102]. Others have demonstrated the role of NMDA receptors in forming and assisting behavioral flexibility, where systemic injections MK801 disrupted both the formation and shifting of behavioral sets from egocentric to light rules[100] while local mPFC infusion MK801 just disrupted the set-shifting portion of the task.
Others have used similar tasks to examine specific subregions of mPFC. Oualian and Gisquet-Verrier (2010) found that lesions to either plPFC or ilPFC led to perseverative responding to previous rules during a necessary shift in strategy[103], reinforcing a role for ilPFC directly in mediating these shifts[96, 97]. Indeed, they also found that lesions encompassing both plPFC and ilPFC showed robust strategy set-shifting deficits, whereas lesions to plPFC or ilPFC yielded more moderate deficits. They go on to suggest that an intact ilPFC may assist animals in choosing a previous unrewarded strategy, whereas in an inactivation or lesion situation, a loss of the ilPFC region of the mPFC may inhibit their ability to directly choose a novel option[103].
One of the many additional advantages of these tasks is the ability to time-lock particular behaviors. With effective time-locking comes the ability to perform in vivo physiology with good temporal resolution to the actual behavioral occurrence. For example, Rich and Shapiro (2009) showed that plPFC and ilPFC may serve different functions related to initiating and establishing new strategies based on the dynamics of the neural response during learning[55]. Durstewitz et al (2010) investigated changes in neural ensemble firing patterns across shifts between visual and egocentric rules, and found distinct active states for neuronal ensembles during different rules[99]. Neuronal activity changed between states abruptly, suggesting that the ‘switch’ from one rule to the next may be more abrupt and more of an ‘ah ha!’ moment than a gradual process comparing previous behavioral failures and successes over time[99]. So far, such experiments have proven difficult to perform in mice. While mice readily perform digging tasks, these tasks are time consuming and require constant experimenter supervision making electrophysiological experiments more difficult (but possible) than more automated maze tasks. Implementation of mouse touchscreen tasks hold great potential to combine the power of precisely time locked behavior with specific genetic manipulations and single unit recordings[40, 104, 105].
Medial prefrontal cortex and functions related to set-shifting
From the studies described above, mPFC and ACC play different roles in set-shifting tasks than OFC, which mediates reversal learning and has been the topic of many reviews suggesting that reversal deficits are a result of lost reward expectancy signals[106-109]. Here, we focus our attention more to mPFC and ACC, and the functions that they might serve during set-shifting procedures. Set-shifting, as studied in all of these tasks, requires a number of fundamental functions to be performed accurately, all of which are thought to be under the control of mPFC and ACC to some degree. These include: 1) forming associations between stimuli, responses and outcomes, 2) detection of errors and conflict between rules, 3) tracking of reward history to determine which responses are no longer valid, and 4) enhanced attentional processes to resolve these issues when rules are violated. Unfortunately, very few studies have recorded from single neurons while animals perform set-shifting tasks, thus it is still unknown exactly how mPFC and ACC accomplish this task. Here, we speculate based on the current literature pertaining to neural correlates that likely underlie set-shifting behavior as examined in other tasks that tap into these functions.
Clearly, mPFC plays some role in forming associations between stimuli, responses and outcomes so that one can learn the contingencies necessary to perform the task initially[110, 111]. The plPFC in rats appears to be strongly associated with higher order cognition, requiring integration of past, current and future choices[43, 72, 112]. Rat plPFC mediates spatial working memory and visual object information, along with cross-modal switching involving spatial location, visual objects and spatial locations with motor responses[113-117].
Activity in mPFC has been shown to be linked to a variety of task correlates. Medial PFC appears to encode expected value, action selection, stimulus-response associations, and is spatially selective[81, 118-121]. Neural ensemble firing in mPFC reflects distinct active states during set-shifting, which is temporally related to behavioral performance[74, 79, 99]. Meanwhile, plPFC and ilPFC appear to subserve different functions in rule learning, with plPFC neurons encoding the initial learning of the rule, and ilPFC neurons lagging behind plPFC in encoding the relevant behavioral action[55]. Recordings in primate DLPFC have shown similar results, with multiple separate ensembles simultaneously representing categorization rules[79] and that DLPFC encodes, then represents abstract categories[74]. In addition to these correlates, mPFC may play an important role in monitoring performance via several functions including, maintaining information across delays, encoding correct and incorrect responses, and signaling reward-related feedback[118-120].
Lesions to mPFC do not prevent initial learning during set-shifting[36, 39, 83], suggesting that mPFC is not necessary for initial rule learning. Furthermore, the fact that performance in mPFC lesioned animals on EDS tasks is abnormal after shifts may be because mPFC cannot form new associations and that it is ‘stuck’ representing old contingencies. Recent research has demonstrated that mPFC is dependent on hippocampus for specific rule-related information. Navawongse and Eichenbaum (2013) show that inactivation of plPFC changes the location and reward based firing of hippocampal neurons, causing them to lose rule specificity[122]. Further evidence for this comes from the finding that rat mPFC contributes to maintenance of task switches, such that inactivation during a switched rule can be learned, but not recalled 24 hours later, an effect not observed when inactivating during reversals, task switching or switching between useful rules[123]. It has also been shown that primates, who have been previously trained on abstract rules, are quicker and better able to recall those familiar rules in the future[87].
The fact that rats with ACC lesions had no difficulty learning relevant cues or switching using different dimensions as relevant cues[37], but is critical for IDS[103], suggests that ACC might not be as critical for forming rules as does mPFC but may mediate other attentional mechanisms, especially when stimuli are perceptually similar. This behavioral deficit might reflect a deficit in a number of functions, including the inability to generalize within a dimension, increase learning sets[37], and reflect deficits in basic function such as resolving response conflict, detecting errors and attenuating attention away from the irrelevant dimension as we will discuss below.
In humans and primates, ACC is activated during scenarios of response conflict[75, 83, 86, 124-126] . Such competition requires increased attention so that engaged participants can override a stimulus-driven response in a situation where an alternative response is the more appropriate one. A classic example is the Stroop task, in which human participants are shown a word such as ‘red’ and asked to indicate the color of the font (e.g. ‘red’ written in green ink). The standard, stimulus-driven response is to read the word ‘red’, while the competing, task-imposed response is to name the color of the ink (i.e. green). On high conflict trials, participants are slower and less accurate, suggesting that it requires time and neural resources to resolve the conflict between the two competing responses. Neural activity in ACC has been shown to be positively correlated to the degree of conflict in the Stroop as well as a number of other tasks[127-138]. Thus, ACC’s role in mediating attention during set-shifting might be to signal when behaviors are in conflict with one another in terms of respective dimension, rather than identifying when a more global ‘rule’ is in conflict with current behavioral outcomes. Consistent with this idea, recent work suggests that ACC plays a role in conflict monitoring during automated retrieval of S-R maps, but not in forming or breaking S-R associations per se[139].
In addition to conflict monitoring function, ACC plays an important role in error detection. Originally this research focused on the role of ACC in detecting errors of commission[130, 140-142], but has also now been implicated in reporting errors in reward prediction. While the majority of this work has focused on the role of ACC in detecting errors, other studies suggest that ACC is important for signaling positive feedback[56, 111, 138].
New lines of research suggest that the role of ACC in signaling errors is related to providing an unsigned prediction error signal necessary for driving adjustments in behavior[143]. ACC neurons respond when outcomes are better and worse than expected. Hayden and colleagues demonstrated this in a task where rewards were delivered at predetermined probabilities[144]. Changes in ACC firing were observed regardless of the valence within single neurons, supporting the idea that primate ACC encodes unsigned reward prediction errors which would be important and useful for adjusting subsequent behavior[144].
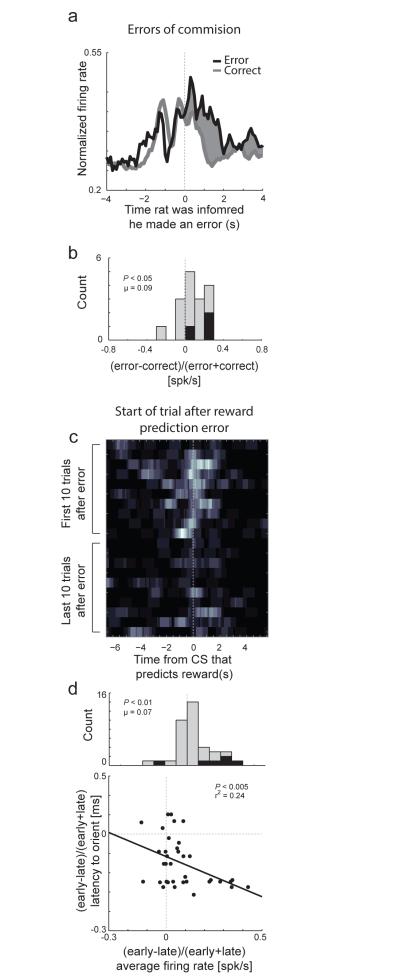
We have also reported similar results as illustrated in Figure 3. As has been shown numerous times in ACC[143], we found that many neurons in rat ACC fired when there were errors in reward prediction and errors of commission. More importantly, we demonstrated that activity was increased after reward prediction errors and that these activity changes were correlated to the amount of attention exhibited on subsequent trials during which rats correctly updated behavior[143]. These results suggest that activity in ACC does not simply signal reward prediction errors, but also signals the need for increased attention on subsequent trials so that learning can occur, reflecting past reward history. These data fit nicely with primate studies, where ACC appears to direct decisions with clear and obvious reinforcement histories, rather than more abstract rule scenarios[76].
Figure 3.
Activity in ACC was high after errors of commission, errors in reward prediction, and was high at the beginning of trials that followed errors. a, Average activity of error related (1s following well entry) neurons upon errors of commission. b, Distribution reflecting the difference in activity between forced-choice errors and forced-choice correct trials ((error −correct) /(error + correct)). Black bars represent the number of neurons that showed a significant difference between these responses (t-test; p < 0.05). c, Heat plot shows the average firing of a single ACC neuron when reward contingences unexpectedly change (reward prediction error). d. Activity of ACC neurons fire more strongly after reward prediction errors and was correlated with attention. Correlation between latency to initiate behavioral trial and firing rate (x-axis) either early or late during learning ((early-late)/(early+late)). N = 4 rats. Modified from Bryden et al, 2011[143]
Recent theoretical work has suggested that all of these functions in mPFC and ACC - error detection, reward prediction errors and conflict monitoring may reflect the same underlying process[145]. This work considers these signals to be part of a generalized surprise/attention system. In each of these cases, activity in mPFC and ACC might reflect unexpected non-occurrence of an expected outcome. The activity caused by unexpected outcomes could explain why several studies have suggested that mPFC and ACC activity is high when there are violations in expectations[130, 146-148].
Unfortunately, few studies have dissociated mPFC and ACC functions using the same behavioral tasks, making it difficult to pinpoint and tease apart their exact roles in set-shifting, as has been done for OFC and mPFC. Anatomical limitations exist as a natural barrier to this research, as most carefully run lesions studies of mPFC will undoubtedly impact the ACC, given their close anatomical proximity. However, genetic models may emerge in which the ACC and mPFC are differentially affected, perhaps an interneuron deficit in one region or targeted deletion of a neurotransmitter receptor. While the damage to ACC is minimal in recording studies, these provide a methodological route to separating the functions of ACC and mPFC in behaving animals. Both mPFC and ACC have been implicated in encoding associations between stimuli, responses and outcomes, and both have been discussed as being involved in reporting errors and signaling response conflict, albeit, to varying degrees[118, 119]. In fact, in many papers, mPFC and ACC are not distinguished when discussing function, which might not be so surprising considering they are so well interconnected and likely function as a unitary structure in many circumstances[149, 150].
With that said, the majority of the research into conflict monitoring and error detection processes have been directed towards ACC, rather than mPFC[75, 86, 140, 151] and some single unit studies suggested that ACC is more involved in detecting errors and is more strongly modulated during tasks switches relative to mPFC[147, 152]. Furthermore single unit studies in mPFC in rats and DLPFC in monkeys have shown clear correlations with rule-based decision-making and stimulus categorization [80-82, 87], and neural ensemble firing in mPFC is clearly related to shifts in behavioral performance during set-shifting tasks.
Together this suggests that mPFC may function solely to signal the rule and guide attention toward relevant dimensions as done in EDS, rather than representing error information, whereas ACC is most likely involved when a rule is violated and is critical for signaling the need for attentional shifts. In this model, mPFC functions as the region which sets the rule and ACC monitors the behavioral outcomes for favorable and unfavorable outcomes which have resulted from a behavioral action, guided by a particular rule. Although this model sits well with what happens after mPFC lesions, it does not fully explain why ACC is only critical for IDS and not EDS. Both would elicit errors and an induce response conflict. One possible remedy is that ACC is critical for reducing attention to irrelevant stimuli when contingencies change. When expected outcomes are violated, two processing are thought to occur; attention must be paid to the relevant dimension so that new association can be formed and attention to the irrelevant dimension is weakened. If attention to the irrelevant dimension is not weakened during the initial discrimination, then rats will still be equally attending to both dimensions, which might lead to impairment on IDS, but would not impact EDS[37]. Such a function fits with increased activity during conflict, on errors and on trials after errors. Typically, we think that this activity is critical for directing attention to stimuli so that new associations can be formed, but it might be equally necessary to weaken attention from irrelevant information.
Beyond this model and our knowledge obtained from single unit recordings, ACC and mPFC might encode different aspects of rules and sets. If ACC encodes the recent reward history, it could provide weighted information about which current rule possibilities will most likely yield a most desired outcome. In this regard, mPFC could store previously learned abstract rules and in the present, represent the current rule driving behavior. While mPFC represents the current rule, it would also be integrating the success of previous actions using reward history values from ACC. In this model, mPFC rule representations hold online the current rule that is the most effective, while receiving judgment advice from ACC regarding recent history and present behavioral choices. Familiar rules are stored in mPFC, making the recall of abstract rules easier for trained animals and leaving ACC to provide the information about which possible stored rule may provide the greatest maximal beneficial outcome[87]. Meanwhile, mPFC provides the rule instructions to hippocampus[122] to build the necessary spatial map required to engage the appropriate rule, as well as provides the correct rule to dorsal striatum, which enacts specific action policies when the animal encounters the necessary behavioral cues.
It is critical that we understand these functions in mPFC and ACC, and how they govern flexible and adaptive behavior because so many disorders impact this function though PFC/ACC pathophysiology. For example, in animal models of schizophrenia-like dysfunction, one mechanism by which disruption of set shifting may occur is through impairments of inhibitory neurons, in agreement with postmortem studies of patients with schizophrenia, which might lead to disorganized information processing in mPFC and ACC[21, 29, 30]. If inhibitory interneuron deficits in mPFC inappropriately tune the mPFC projection neurons that carry rule information to dorsal striatum, inflexibility may originate from a disorganized and weak rule signal emanating from mPFC. Conversely, if there is an enhancement of GABAergic inhibition tuning the local mPFC circuit, inflexible behavior may result from an overly strong and sustained rule signal. Similarly, if ACC suffers from weak tuning and organization from a deficit in cortical inhibition, weak or inaccurate information about recent reward history[153] would leave mPFC in a position to incorrectly continue to drive a rule that no longer corresponds to the desired outcome based on recent outcomes. All of these possibilities can lead to perseverative rule responding observed in set shifting deficits in animal models[154, 155], and would have strong implications for human disorders where reduced cortical synchrony is thought to underlie cognitive deficits, notable among them, schizophrenia[21, 29, 30, 156]. In the future, more precise testing and use of appropriate animal models will be necessary to help not only tease apart the roles of mPFC and ACC, but also the proposed underlying mechanisms behind cortical dysfunction in diseases which affect the human condition.
Conclusion
The study of attention and set-shifting is important on many fronts. Such elements of cognition are disturbed in both normal aging and in a myriad of disorders which affect both frontal and limbic structures, such as schizophrenia, bipolar disorder, affective and anxiety disorders [3, 29-34]. Although research has rapidly progressed on many fronts regarding the general circuitry involved in functions underlying learning and attention, there remains a good deal of unanswered questions. Multiple lines of evidence have robustly linked mPFC and ACC to different aspects of attention and set-shifting in several animal systems. The challenges, now, are determining exactly what is being represented by these critical regions during flexible decision-making and adaptive behavior. We must consider several functions, including error detection, conflict monitoring, stimulus-response learning and abstract rule representation. Furthermore, there has been surprising little work on understanding the role of prefrontal cortex modulation of downstream areas, such as striatum, during performance of these tasks. With such data in hand, we will gain a better understanding of the effects which occur in human disease states.
Highlights.
Thoroughly outline attentional set-shifting tasks across many animal models
Summarize research findings in set-shifting literature
Propose new directions and necessary experiments to fill in important gaps in literature
Figure 4.
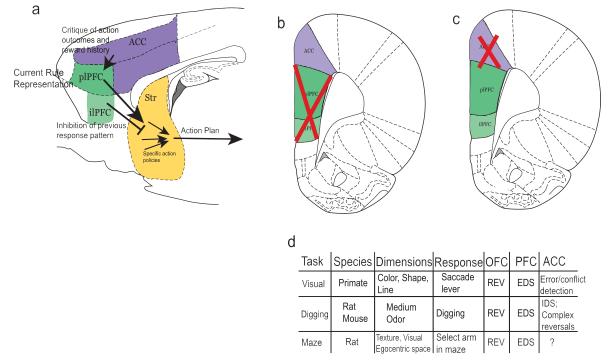
Circuit diagram highlighting main set-shifting pathways. a) Overall rule circuit, demonstrating running behavioral performance updates from ACC to mPFC, current best Rule strategy from dorsal mPFC (plPFC) to striatum, inhibitory input from ventral mPFC (ilPFC) to striatum and on to behavioral action. b) Atlas coronal slice of rat brain depicting representative areas destroyed in most mPFC lesion studies. c) Atlas section depicting representative region destroyed in ACC lesion studies. d) Summary table of behavioral designs across species and the general behavioral outcome of OFC, PFC or ACC lesions.
ACKNOWLEDGEMENTS
This work was supported by a grant from the NIDA (R01DA031695, MR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Archives of general psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- [2].Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain : a journal of neurology. 2001;124:1492–508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- [3].McDonald CR, Delis DC, Norman MA, Wetter SR, Tecoma ES, Iragui VJ. Response inhibition and set shifting in patients with frontal lobe epilepsy or temporal lobe epilepsy. Epilepsy & behavior : E&B. 2005;7:438–46. doi: 10.1016/j.yebeh.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [4].Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:1036–47. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Halleland HB, Haavik J, Lundervold AJ. Set-shifting in adults with ADHD. Journal of the International Neuropsychological Society : JINS. 2012;18:728–37. doi: 10.1017/S1355617712000355. [DOI] [PubMed] [Google Scholar]
- [6].Podell JE, Sambataro F, Murty VP, Emery MR, Tong Y, Das S, et al. Neurophysiological correlates of age-related changes in working memory updating. NeuroImage. 2012;62:2151–60. doi: 10.1016/j.neuroimage.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berg EA. A simple objective technique for measuring flexibility in thinking. The Journal of general psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- [8].James W. The principles of psychology. H. Holt and company; New York: 1890. [Google Scholar]
- [9].Buccafusco JJ. Methods of behavior analysis in neuroscience. 2nd ed. CRC Press; Boca Raton: 2009. [PubMed] [Google Scholar]
- [10].Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and biobehavioral reviews. 2002;26:631–64. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- [11].Reverberi C, Gorgen K, Haynes JD. Compositionality of rule representations in human prefrontal cortex. Cereb Cortex. 2012;22:1237–46. doi: 10.1093/cercor/bhr200. [DOI] [PubMed] [Google Scholar]
- [12].Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prentice KJ, Gold JM, Buchanan RW. The Wisconsin Card Sorting impairment in schizophrenia is evident in the first four trials. Schizophrenia research. 2008;106:81–7. doi: 10.1016/j.schres.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain and cognition. 2009;71:437–51. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- [15].Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex; a journal devoted to the study of the nervous system and behavior. 1976;12:313–24. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- [16].Shamay-Tsoory SG, Tomer R, Goldsher D, Berger BD, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. Journal of clinical and experimental neuropsychology. 2004;26:1113–27. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- [17].Jacobs R, Anderson V. Planning and problem solving skills following focal frontal brain lesions in childhood: analysis using the Tower of London. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2002;8:93–106. doi: 10.1076/chin.8.2.93.8726. [DOI] [PubMed] [Google Scholar]
- [18].Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophrenia research. 1999;37:251–70. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- [19].Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biological psychiatry. 2009;66:586–93. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cools R, Brouwer WH, de Jong R, Slooff C. Flexibility, inhibition, and planning: frontal dysfunctioning in schizophrenia. Brain and cognition. 2000;43:108–12. [PubMed] [Google Scholar]
- [21].Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews Neuroscience. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- [22].Elliott R, McKenna PJ, Robbins TW, Sahakian BJ. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychological medicine. 1995;25:619–30. doi: 10.1017/s0033291700033523. [DOI] [PubMed] [Google Scholar]
- [23].Elliott R, Sahakian BJ. The neuropsychology of schizophrenia: relations with clinical and neurobiological dimensions. Psychological medicine. 1995;25:581–94. doi: 10.1017/s0033291700033493. [DOI] [PubMed] [Google Scholar]
- [24].Gauntlett-Gilbert J, Roberts RC, Brown VJ. Mechanisms underlying attentional set-shifting in Parkinson’s disease. Neuropsychologia. 1999;37:605–16. doi: 10.1016/s0028-3932(98)00049-9. [DOI] [PubMed] [Google Scholar]
- [25].Josiassen RC, Curry LM, Mancall EL. Development of neuropsychological deficits in Huntington’s disease. Archives of neurology. 1983;40:791–6. doi: 10.1001/archneur.1983.04050120041005. [DOI] [PubMed] [Google Scholar]
- [26].Traykov L, Raoux N, Latour F, Gallo L, Hanon O, Baudic S, et al. Executive functions deficit in mild cognitive impairment. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2007;20:219–24. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- [27].Traykov L, Rigaud AS, Cesaro P, Boller F. [Neuropsychological impairment in the early Alzheimer’s disease] L’Encephale. 2007;33:310–6. doi: 10.1016/s0013-7006(07)92044-8. [DOI] [PubMed] [Google Scholar]
- [28].Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural plasticity. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, et al. Interactive effects of apolipoprotein E type 4 genotype and cerebrovascular risk on neuropsychological performance and structural brain changes. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2010;19:261–8. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy research. 2012;98:1–13. doi: 10.1016/j.eplepsyres.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hudson JM, Flowers KA, Walster KL. Attentional control in patients with temporal lobe epilepsy. Journal of neuropsychology. 2013 doi: 10.1111/jnp.12008. [DOI] [PubMed] [Google Scholar]
- [34].Longo CA, Kerr EN, Smith ML. Executive functioning in children with intractable frontal lobe or temporal lobe epilepsy. Epilepsy & behavior : E&B. 2013;26:102–8. doi: 10.1016/j.yebeh.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [35].Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biological psychiatry. 2006;59:1198–207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12123–31. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral neuroscience. 1996;110:872–86. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- [39].Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, et al. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behavioural brain research. 2006;171:181–8. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- [41].van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. The Journal of comparative neurology. 1990;302:515–28. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- [42].Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. The Journal of comparative neurology. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [43].Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- [44].Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. The Journal of comparative neurology. 1991;313:574–86. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- [45].Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neuroscience letters. 1990;114:184–90. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- [46].Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain research. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- [47].Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. The Journal of comparative neurology. 2002;442:163–87. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- [48].Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. The Journal of comparative neurology. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [49].Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. The Journal of comparative neurology. 1990;294:607–22. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- [50].Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Progress in brain research. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion −8. [DOI] [PubMed] [Google Scholar]
- [51].Berger B, Thierry AM, Tassin JP, Moyne MA. Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain research. 1976;106:133–45. doi: 10.1016/0006-8993(76)90078-0. [DOI] [PubMed] [Google Scholar]
- [52].Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–24. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- [53].Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- [54].Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7208–19. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nature neuroscience. 2006;9:940–7. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- [57].Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behavioural brain research. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- [58].Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- [59].Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Progress in brain research. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- [61].Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of comparative neurology. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- [62].Room P, Russchen FT, Groenewegen HJ, Lohman AH. Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. The Journal of comparative neurology. 1985;242:40–55. doi: 10.1002/cne.902420104. [DOI] [PubMed] [Google Scholar]
- [63].Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. The Journal of comparative neurology. 1995;352:567–93. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- [64].Van Eden CG, Lamme VA, Uylings HB. Heterotopic Cortical Afferents to the Medial Prefrontal Cortex in the Rat. A Combined Retrograde and Anterograde Tracer Study. The European journal of neuroscience. 1992;4:77–97. doi: 10.1111/j.1460-9568.1992.tb00111.x. [DOI] [PubMed] [Google Scholar]
- [65].Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and biobehavioral reviews. 2003;27:555–79. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [66].Ding DC, Gabbott PL, Totterdell S. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain research. 2001;917:81–9. doi: 10.1016/s0006-8993(01)02912-2. [DOI] [PubMed] [Google Scholar]
- [67].Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- [68].Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural brain research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- [69].van Eden CG, Kros JM, Uylings HB. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Progress in brain research. 1990;85:169–83. doi: 10.1016/s0079-6123(08)62680-1. [DOI] [PubMed] [Google Scholar]
- [70].Fuster JM. Memory networks in the prefrontal cortex. Progress in brain research. 2000;122:309–16. doi: 10.1016/s0079-6123(08)62147-0. [DOI] [PubMed] [Google Scholar]
- [71].Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Current opinion in neurology. 2005;18:734–9. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- [72].Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- [73].Preuss TM. Do Rats Have Prefrontal Cortex? The Rose-Woolsey-Akert Program Reconsidered. Journal of cognitive neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- [74].Antzoulatos EG, Miller EK. Differences between neural activity in prefrontal cortex and striatum during learning of novel abstract categories. Neuron. 2011;71:243–9. doi: 10.1016/j.neuron.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in cognitive sciences. 2004;8:410–7. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [76].Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Current opinion in neurobiology. 2007;17:220–7. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [77].Miller EK. The prefrontal cortex and cognitive control. Nature reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- [78].Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- [79].Roy JE, Riesenhuber M, Poggio T, Miller EK. Prefrontal cortex activity during flexible categorization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8519–28. doi: 10.1523/JNEUROSCI.4837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. Journal of neurophysiology. 2003;90:1790–806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- [81].Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–11. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- [82].Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of neurophysiology. 2003;90:3419–28. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- [83].Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- [84].Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63:244–53. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cromer JA, Roy JE, Miller EK. Representation of multiple, independent categories in the primate prefrontal cortex. Neuron. 2010;66:796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rushworth MF, Hadland KA, Gaffan D, Passingham RE. The effect of cingulate cortex lesions on task switching and working memory. Journal of cognitive neuroscience. 2003;15:338–53. doi: 10.1162/089892903321593072. [DOI] [PubMed] [Google Scholar]
- [87].Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. Journal of cognitive neuroscience. 2006;18:974–89. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- [88].Montgomery KS, Simmons RK, Edwards G, 3rd, Nicolle MM, Gluck MA, Myers CE, et al. Novel age-dependent learning deficits in a mouse model of Alzheimer’s disease: implications for translational research. Neurobiology of aging. 2011;32:1273–85. doi: 10.1016/j.neurobiolaging.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends in neurosciences. 2009;32:347–58. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual review of neuroscience. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–20. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- [92].Bissonette GB, Lande MD, Martins GJ, Powell EM. Versatility of the mouse reversal/set-shifting test: Effects of topiramate and sex. Physiology & behavior. 2012;107:781–6. doi: 10.1016/j.physbeh.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Martins GJ, Shahrokh M, Powell EM. Genetic disruption of Met signaling impairs GABAergic striatal development and cognition. Neuroscience. 2011;176:199–209. doi: 10.1016/j.neuroscience.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ragozzino ME, Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behavioral neuroscience. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- [95].Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- [96].Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural brain research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- [97].Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiology of learning and memory. 2008;89:567–73. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [98].Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behavioral neuroscience. 2003;117:728–37. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- [99].Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–48. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- [100].Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behavioural pharmacology. 2008;19:225–34. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chase EA, Tait DS, Brown VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. The European journal of neuroscience. 2012;36:2368–75. doi: 10.1111/j.1460-9568.2012.08141.x. [DOI] [PubMed] [Google Scholar]
- [102].Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology. 2011;216:525–35. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- [103].Oualian C, Gisquet-Verrier P. The differential involvement of the prelimbic and infralimbic cortices in response conflict affects behavioral flexibility in rats trained in a new automated strategy-switching task. Learn Mem. 2010;17:654–68. doi: 10.1101/lm.1858010. [DOI] [PubMed] [Google Scholar]
- [104].Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, et al. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–63. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bussey TJ, Saksida LM, Rothblat LA. Discrimination of computer-graphic stimuli by mice: a method for the behavioral characterization of transgenic and gene-knockout models. Behavioral neuroscience. 2001;115:957–60. doi: 10.1037//0735-7044.115.4.957. [DOI] [PubMed] [Google Scholar]
- [106].Roesch MR, Calu DJ, Esber GR, Schoenbaum G. All that glitters … dissociating attention and outcome expectancy from prediction errors signals. Journal of neurophysiology. 2010;104:587–95. doi: 10.1152/jn.00173.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Takahashi YK, Roesch MR, Wilson RC, Toreson K, O’Donnell P, Niv Y, et al. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nature neuroscience. 2011;14:1590–7. doi: 10.1038/nn.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–6. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews Neuroscience. 2009;10:885–92. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Boettiger CA, D’Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2723–32. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. Journal of cognitive neuroscience. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- [112].Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. The Journal of comparative neurology. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- [113].Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behavioral neuroscience. 1995;109:1063–73. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- [114].Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–8. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- [115].Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behavioral neuroscience. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- [116].Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4585–94. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behavioral neuroscience. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- [118].Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 2009;164:444–56. doi: 10.1016/j.neuroscience.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Horst NK, Laubach M. Working with memory: Evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. Journal of neurophysiology. 2012 doi: 10.1152/jn.01192.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Narayanan NS, Laubach M. Methods for studying functional interactions among neuronal populations. Methods Mol Biol. 2009;489:135–65. doi: 10.1007/978-1-59745-543-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Navawongse R, Eichenbaum H. Distinct pathways for rule-based retrieval and spatial mapping of memory representations in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1002–13. doi: 10.1523/JNEUROSCI.3891-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4747–55. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. Journal of cognitive neuroscience. 2002;14:1139–50. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- [125].Rushworth MF, Kennerley SW, Walton ME. Cognitive neuroscience: resolving conflict in and over the medial frontal cortex. Current biology : CB. 2005;15:R54–6. doi: 10.1016/j.cub.2004.12.054. [DOI] [PubMed] [Google Scholar]
- [126].Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain research. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- [127].De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–7. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain research. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- [130].Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6418–26. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–8. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- [132].Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- [133].Badgaiyan RD, Posner MI. Mapping the cingulate cortex in response selection and monitoring. NeuroImage. 1998;7:255–60. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- [134].Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:256–9. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. NeuroImage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- [136].Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who’s in control? Nature neuroscience. 2000;3:421–3. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- [137].Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rothe M, Quilodran R, Sallet J, Procyk E. Coordination of high gamma activity in anterior cingulate and lateral prefrontal cortical areas during adaptation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11110–7. doi: 10.1523/JNEUROSCI.1016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Brass M, Wenke D, Spengler S, Waszak F. Neural correlates of overcoming interference from instructed and implemented stimulus-response associations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1766–72. doi: 10.1523/JNEUROSCI.5259-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–55. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–2. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- [142].Quilodran R, Rothe M, Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57:314–25. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- [143].Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR. Attention for learning signals in anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18266–74. doi: 10.1523/JNEUROSCI.4715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4178–87. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature neuroscience. 2011;14:1338–44. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- [147].Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–21. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 148].Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature reviews Neuroscience. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- [149].Totah NK, Jackson ME, Moghaddam B. Preparatory Attention Relies on Dynamic Interactions between Prelimbic Cortex and Anterior Cingulate Cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wilson CR, Gaffan D, Browning PG, Baxter MG. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends in neurosciences. 2010;33:533–40. doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [152].Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–62. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- [153].Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia bulletin. 2008;34:835–47. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Astrocyte-mediated hepatocyte growth factor/scatter factor supplementation restores GABAergic interneurons and corrects reversal learning deficits in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2918–23. doi: 10.1523/JNEUROSCI.5268-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:17102–10. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3022–31. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]