Abstract
The survival factor Bcl-2 is a cyclic AMP response element-binding protein (CREB) gene product implicated in mediating some of estrogen’s effects on neuroprotection. Previously, we showed an effect of estradiol benzoate (E) on numbers of neuron-specific protein (NeuN)- and phosphorylated CREB (pCREB)-positive cells in medial (MeA), but not central (CeA), amygdala of ovariectomized rats. To determine whether these effects are accompanied by an E-induced increase in Bcl-2, we examined the effects of E on levels of Bcl-2 protein and mRNA in MeA and CeA of ovariectomized rats treated with E regimens resulting in moderate (2.5μg E for 4 or 14 days) or high (10μg E for 14 days) plasma estradiol levels. As a physiological control, we showed that all E treatments increased uterine wet weight relative to vehicle; 10μg E for 14 days also increased uterine weight compared to that seen with lower E levels. Western blot analysis revealed that all E groups displayed an increase in uterine Bcl-2 protein levels compared to vehicle. We found that 2.5μg and 10μg E for 14 days increased levels of Bcl-2 gold immunolabeling compared to vehicle and 2.5μg E for 4 days in MeA, but not CeA. We measured Bcl-2 mRNA levels in vehicle and 2.5μg E for 14 days groups. There was a significant increase in Bcl-2 mRNA levels in MeA, but not CeA, of E-treated ovariectomized rats compared with vehicle controls. The E-induced increase in protein and mRNA levels of Bcl-2 in MeA may be important for neuroprotection in this region.
Keywords: Estrogen, Bcl-2, Amygdala, Uterus, Immunocytochemical studies, in situ RT-PCR
INTRODUCTION
The effects of estrogens on neuroprotection may involve cyclic AMP response element-binding protein (CREB)-related genes, such as that for the anti-apoptotic protein Bcl-2. Estrogen effects on Bcl-2 expression has been documented in intact and ovariectomized animals treated with 17β-estradiol- or estradiol benzoate (E) (Garcia-Segura et al., 1998; Tsukahara et al., 2008), as well as those subjected to ischemia (Dubal et al., 1999; Jover-Mengual et al., 2007), and in hippocampal neurons exposed to glutamate- (Nilsen and Brinton, 2003; Zhao et al., 2004) and β-amyloid-induced (Nilsen et al., 2006) toxicity. In addition, the estrogen receptor (ER)α agonist propylpyrazole triol (PPT) prevented a decrease in the ratio of Bcl-2 to the pro-apoptotic protein BAD in mice treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (D’Astous et al., 2006; cf. Morissette et al., 2008). Garcia-Segura et al. (1998) demonstrated an increase in Bcl-2 immunolabeling in the arcuate nucleus during estrus, and a decrease in this protein in other stages of the estrus cycle and as a result of ovariectomy. A single dose of 17β-estradiol (10μg or 100μg) increased Bcl-2 immunolabeled cells compared to vehicle or 1μg 17β-estradiol; progesterone injected with estradiol had a negative effect on the numbers of Bcl-2 immunolabeled cells.
We showed in a series of studies that E (10 μg E for 14 days) increased the function of the CREB signaling pathway in the medial amygdala (MeA). The MeA is a brain area involved in reproduction (Swanson and Petrovich, 1998), and has abundant ERs (Shughrue et al., 1997, 1998; Österlund et al., 1998). It has been implicated with pCREB in endocrine responses to emotional stress (Kuipers et al., 2006) and is a target of antidepressant action (Kosten et al., 2008). E effects include those on the upstream regulator of CREB, Ca2+/calmodulin-dependent protein kinase IV (CaMK IV) (Zhou et al., 2001), CREB, phosphorylated CREB (pCREB), brain-derived neurotrophic factor (BDNF) (Carlstrom et al., 2001; Zhou et al., 2005) and the phosphatase calcineurin (Zhou et al., 2004). These effects were specific to the medial and basomedial, but not central (CeA) or basolateral, amygdala. Moreover, we recently showed an E-induced increase in the mean number of neuron-specific protein (NeuN)- and pCREB-positive cell numbers and increase in brain region volume in the MeA, but not CeA (Fan et al., 2008). Although, there are no reports of estrogen effects on Bcl-2 in the amygdala, Bcl-2 is expressed in the MeA of monkeys (Fudge, 2004) and rats (Kosten et al., 2008). The increased neuronal number may therefore be due to an effect of E on cell survival, possibly mediated by its effect on Bcl-2. We therefore examined the effects of E treatment on Bcl-2 protein and mRNA levels in the MeA and CeA of ovariectomized rats. As a physiological control, we also determined levels of Bcl-2 protein in the uterus of ovariectomized rats treated with estrogen.
MATERIALS AND METHODS
Animals
Ovariectomized Sprague Dawley rats (200–250 g) were obtained from Harlan (Madison, WI). Following adaptation to a reverse 12/12-hr light/dark cycle (lights on at 22.0 hours) for 19 days, females were tested for completeness of ovariectomy (during rats’ dark period, at approximately 11:00AM) using the lordosis test (Kow and Pfaff, 1975) for six days. Rat chow and water were available ad libitum. Immediately following the adaptation period, the animals were randomly divided into four groups and, approximately forty-eight hours after lordosis testing (and approximately 28 days following ovariectomy), injected subcutaneously as follows: the vehicle sesame oil (SO) for 10 days followed by 2.5 μg estradiol benzoate (E) for 4 days (2.5μg/4d); 2.5 μg E 14 days (2.5μg/14d), or 10 μg E for 14 days (10μg/14d); SO was the control. The regimen of 2.5 μg E for 4 days was selected based the observation that disparate responses to E treatment are obtained with either high doses or when the hormone treatment is extended for more than 4 days compared to doses which are in the physiological range (cf. Becker, 1999). The 10 μg E for 14 days protocol was based on data from our laboratory (Carlstrom et al., 2001; Zhou et al., 2001, 2004, 2005), as well as those of others (Gu et al., 1996), to optimize the detection of hormonal action on cellular responses and relate our finding to previously published data on estrogen effects on the CREB signaling cascade (Gu et al., 1996; Zhou et al., 1996; Ábrahám et al., 2003; Szegö et al., 2006; cf. also review in Rønnekleiv et al., 2007). Because the rodent estrus cycle is characterized by increases in plasma estradiol levels for less than 24 hours, the timing with which these injections were given did not replicate the physiological condition. Because of the rapid clearance of estradiol and since plasma estradiol levels were measured a day after the last injection, the observed levels provide a relative, but not precise, indication of circulating estradiol levels during the experiment.
On the next day after the last injection, one group of animals was decapitated, uteri were immediately dissected out at 4°C, free of fat and connective tissues, and measurements of uterine wet weight were taken. The uteri from another group of rats were taken and placed in storage at −80°C and used for Western blot analysis. Another group of animals was perfused for immunocytochemistry or in situ RT-PCR (see below). Guidelines for the humane and proper treatment of animals were followed. All possible steps were taken to avoid discomfort by the animals.
Plasma Estradiol Measurements
Plasma was obtained in the early afternoon of the next day after the last E injection. Blood was collected in BD Vacutainer 10 ml sterile plastic vials with sodium heparin during perfusion by cutting the left ventricle and was allowed to sit at room temperature (RT) between 30–60 min, then spun down for 30 min at 2,000 rpm in the cold. Plasma was siphoned off the top, placed into three Eppendorf tubes, and frozen at −80°C. Radioimmunoassays (RIAs) for plasma estradiol levels were performed at the Endocrinology Laboratory of the Cornell New York State Animal Health Diagnostic Laboratory, College of Veterinary Medicine (Ithaca, NY) using a Coat-A-Count Kit (Diagnostic Products Corp. [DPC], Los Angeles, CA) with modifications (Stephen V. Lamb, Endocrinology Laboratory of the Cornell New York State Animal Health Diagnostic Laboratory, College of Veterinary Medicine, Ithaca, NY, personal communication; cf. also Reimers et al., 1991). Their procedure results in a minimum detectable dose of 7.1 pg/ml estradiol, an intra-assay coefficient of variance of less than 15%, and an interassay coefficient of variance of 10% or lower (Stephen V. Lamb, Endocrinology Laboratory of the Cornell New York State Animal Health Diagnostic Laboratory, unpublished observations) (cf. also Fan et al., 2008).
Western Blot Analysis
Uterine tissue was prepared as follows and used for Western blot analysis according to standard procedures with antibodies to Bcl-2 or β-actin (Pandey et al., 2001). Uterine tissues were homogenized in lysis buffer (40 mM Tris-HCl; 4 mM EGTA; 9 mM EDTA; 500 mM sucrose; pH 7.5), with dithiothreitol (DTT) (2 mM), aprotinin (10 μg/ml), pepstatin A (1 μg/ml), leupeptin (10 μg/ml) and phenylmethylsulfunylfluoride (PMSF) (0.1 mg/ml) (Sigma, Saint Louis, MO) added just before use. Homogenates were incubated for 10 min on ice and then centrifuged at 10,000 rpm for 15 min at 4°C. Supernatants were collected and stored at −80°C. Protein concentrations were determined by the Lowry method (Lowry et al., 1951). A volume containing 20 μg protein was diluted 1:1 with 2X Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8; 25% glycerol; 2% sodium dodecyl sulfate; 0.01% Bromophenol Blue; 5% β-mercaptoethanol). After being denatured in a water bath for 5 min at 100°C, samples were loaded on 12% sodium dodecyl sulfate-polyacrylamide gels followed by electrophoresis. Gels were electroblotted to Immobilon-P polyvinylidene fluoride (PVDF) transfer membranes (Millipore) for 2 hr at 4°C. Blots were blocked in 5% nonfat dry milk/0.1% Tween@20 PBS (PBST) overnight at 4°C. After washing, blots were incubated with rabbit polyclonal anti-Bcl-2 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) (1:500 in PBST) or mouse anti-β-actin antibody (Sigma, Saint Louis, MO) (1:10,000 in PBST) for 1 hr at RT. Blots were washed and incubated in goat anti-rabbit IgG-horse radish peroxidase (HRP) (Santa Cruz Biotechnology Inc.) (1:5,000 in PBST) or goat anti-mouse IgG-HRP (Santa Cruz Biotechnology Inc.) (1:10,000 in PBST) for 1 hr at RT. After washes, blots were incubated in Amersham ECL Plus™ chemiluminescent detection substrates (Amersham Biosciences, Pittsburgh, PA) for 2 min. Membranes were exposed to X-OMAT AR film (Kodak Molecular Imaging, New Haven, CT) for 1 sec to 5 min. Bands on the autoradiograms were quantified by using the ImageJ image analysis software (Abramoff et al., 2004), and values were normalized to β-actin immunoreactivity in each sample and expressed as percentage of control.
Gold immunolabeling of Bcl-2 in rat amygdala
Gold immunolabeling was performed according to procedures as described by us previously (Zhou et al., 2001, 2004, 2005). Briefly, rats were anesthetized and perfused intracardially with normal saline, followed by ice cold paraformaldehyde in 0.1M phosphate buffer. For the animals perfused for in situ RT-PCR, the perfusion solutions were made from diethylpyrocarbonate (DEPC)-treated water. Brains were removed, processed and cut into 20μm coronal sections at identical bregma levels (Paxinos and Watson, 1998). Sections were immunolabeled with antibodies to Bcl-2 (1:1,000) in 1% BSA prepared as described or, as a negative control, with 1% BSA in phosphate buffered saline (PBS) containing 0.25% Triton X-100, processed for immunogold labeling and silver enhancement and viewed with a light microscope. Quantification of gold immunolabeled particles was performed with the Loats Image Analysis System (Westminster, MD) connected to a light microscope that calculated the number of immunogold particles/100μm2 area of defined amygdaloid structures at high magnification (×100). The threshold of each image was set up in two ways: one within the section itself; areas without labeling gave zero counts and were then crossed check with negative sections. Three matching sections from each hemisphere for each rat were used and three fields from each section (for each area) were calculated. Thus, from one rat, data were pooled from 18 fields (from both hemispheres) of five to six sections, the number of which depended upon bregma levels. The values are presented as number of immunogold particles/100 μm2 area.
Characterization of the antibodies
Rabbit polyclonal Bcl-2 antibody (Santa Cruz Biotechnology Inc. [N-19]) was raised against a peptide mapping at the N-terminus of human Bcl-2. Western blots of U-937, HL-60 cells and Jurkat whole cell lysates show a major band at 26 kDa. These antibodies were used to demonstrate the 17β-estradiol regulation of Bcl-2 in adult brain neurons (Garcia-Segura et al., 1998). We determined the specificity of the antibody by preabsorption with Bcl-2 blocking peptide (Santa Cruz Biotechnology Inc., Bcl-2P) on Western blots and tissue sections as described below. Mouse anti-β-actin antibody (Sigma) was raised from the AC-15 hybridoma produced by the fusion of mouse myeloma cells and splenocytes from an immunized mouse. It recognizes an epitope located at the N-terminus of the β-isoform of actin. We used this antibody successfully for Western blotting (Zhou et al., 2001).
In situ RT-PCR for Bcl-2 mRNA levels
Two of the four aforementioned treatment groups were used in this experiment: the vehicle SO and 2.5μg/14d groups. Rat brain sections were used to determine mRNA levels of Bcl-2 using in situ RT-PCR as described by Pandey et al. (2003, 2004) and Zhang and Pandey (2003). We used the non-radioactive in situ method because of its higher sensitivity compared to conventional in situ methods, with the potential of being eight times more sensitive than conventional methods (Bates et al., 1997). We have successfully used this method for quantification of BDNF mRNA (Pandey et al., 2004; Zhou et al., 2005) and neuropeptide Y (NPY) mRNA (Pandey et al., 2003, 2004; Zhang and Pandey, 2003). Others have used in situ RT-PCR for the quatification of levels of Bcl-2 mRNA and Bax mRNA (Yoshimura et al., 2007).
Forty micrometer sections were cut on a cryostat and sections of interest, as described above, were processed for in situ RT-PCR as described below. Sections were washed with PBS, incubated in proteinase K (1 μg/ml in 1×PBS, 0.05% Triton X-100) for 15 min at 37°C, and the reaction was stopped by 0.1 M glycine on ice (20 min). Sections were then washed with 1XPBS and incubated with DNase digestion solution (2 μl RNase inhibitor, 8 μl water, 80 μl RQ1RNase free DNase and 10 μl DNase 10×buffer l) overnight (12–16 hr) at RT. Sections were then washed four times with 1XPBS, transferred to 100 μl reverse transcriptase reaction mixture (20 μl 25 mM MgCl2 solution, 10 μl 10 X PCR buffer, 15 μl DEPC-treated water, 10μl dCTP, 10μl dATP, 10 μl dTTP, 10 μl dGTP, 5 μl RNase inhibitor, 5 μl MuLV reverse transcriptase and 5 μl Oligo d(T)16 (Applied Biosystems, Foster City, CA) and reverse transcribed for 60 min at 42°C (Applied Biosystems, Foster City, Calif., USA). For negative control sections, DEPC-treated water replaced reverse transcriptase enzyme. Sections were transferred to PCR reaction mixtures containing DIG-11-dUTP instead of dUTP. PCR was performed with 0.5 μl Taq DNA polymerase enzyme and 1μl (36–37 pmol) each of Bcl-2 primer. Primers were as follows: forward primer: 5′-CCGGGAGAGAACAGGGTATGAT-3′; backward primer: 5′-CAGGTATGCACCCAGAGTGA-3′ (Yao et al., 2007). PCR conditions are: 95°C for 3 min; 95°C for 45 sec, 55°C for 45 sec, 72°C for 45 sec for a total of 30 cycles, and then 72°C for 7 min.
After PCR, sections were mounted on slides and Bcl-2-positive cell bodies were detected by using alkaline phosphate-conjugated anti-digoxigenin antibody followed by staining of the complex with specific substrate nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) (Roche Molecular Biochemical, Mannheim, Germany). The optical density (OD) of positive areas containing cell bodies was calculated with an image analyzer (Loats Associates). The OD of negative sections was subtracted from the OD of the target area containing positive cell nuclei. For MeA and CeA, three randomly selected areas were analyzed. The OD of positive cell bodies was calculated by the image analysis system, and values were averaged for each rat. The mRNA results were given as mean OD/100 pixel area.
Statistical analysis
For uterine weight, plasma estradiol levels and immunogold labeling data, values are the mean ± standard error of mean (S.E.M). For these data, differences between vehicle and E-treated rats were evaluated by one-way analysis of variance with Fisher’s least significant difference post hoc tests to determine which means differed from one another. For Western blot analyses, values are the mean ± S.E.M. and are expressed as a percentage of the control. The non-parametric Kruskal-Wallis test was first used to determine the significance among groups. Mann-Whitney U-test was then used to determine the significance between any two groups. For in situ RT-PCR data, mRNA results were represented as mean OD/100 pixel area. The differences between vehicle and E-treated rats were determined using Student’s test. A P-value less than 0.05 was considered statistically significant. The number of animals in each hormone treatment group is represent by “n.” All analyses were performed with SPSS 12.0 for Windows (SPSS, Chicago, IL) or GraphPad Prism®4 (GraphPad Software, San Diego, CA).
RESULTS
Effects of various regimens of E on plasma estradiol levels and uterine weight
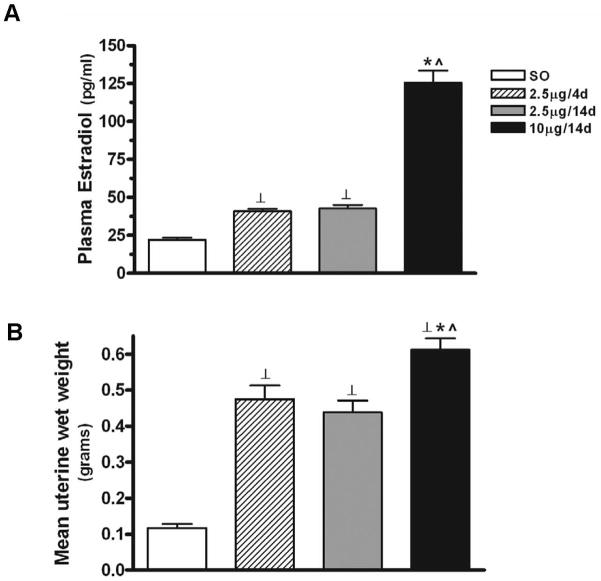
All E treatments had a significant effect on plasma estradiol levels (F3,72=126.84, P<0.0001) (Fig. 1A). Both 2.5μg for 4 (n=15) (40.84±1.65) and 14 (n=23) (42.6±2.31) days resulted in similar levels of plasma estradiol (pg/ml), and are consistent with levels obtained during proestrus (Goodman, 1978); both regimens resulted in estradiol levels that were significantly higher than that seen with SO (n=21) (22.12±1.33) (P<0.01 in both cases). Ten μg E for 14 days (n=17) resulted in plasma estradiol levels (125.5±8.03) that were about five times higher than that seen with SO (P<0.0001) and 2.5 times higher than those obtained with 2.5μg/4d (P<0.0001) or 2.5μg/14d (P<0.0001).
Figure 1.
Comparison of mean plasma estradiol levels and uterine wet weights following different E regimens. (A) All E treatments had a significant effect on plasma estradiol levels (F3,72=126.84, P<0.0001). The regimens of 2.5μg/4d and 2.5μg/14d showed no differences in estradiol levels, but both regimens resulted in levels that were significantly higher than that seen with SO (P<0.01). Ten μg E for 14 days resulted in estradiol levels that were higher than that seen with SO (P<0.0001) and 2.5μg/4d or 2.5μg/14d (P<0.0001). Results are expressed as mean±S.E.M. ⊥: P<0.01; *: P<0.0001, compared with SO; ^: P<0.0001, compared with 2.5μg/4d and 2.5μg/14d. (B) Quantitative analysis revealed that all of the E regimens showed a greater mean uterine wet weight than sesame oil alone (F3, 14=44.05, P<0.0001). Both 2.5μg/4d and 2.5μg/14d regimens resulted in greater uterine wet weight than SO (P<0.001 in both cases). The regimen of 10μg/14d resulted in greater uterine weight than either the regimes of SO (P<0.001), 2.5μg/4d (P<0.05) and 2.5μg/14d (P<0.01). Results are expressed as mean±S.E.M. ⊥: P<0.001, compared with SO; *: P<0.05, compared with 2.5μg/4d; ^: P<0.01 compared with 2.5μg/14d.
All E treatments increased uterine wet weight (grams) relative to vehicle (F3, 14=44.05, P<0.0001) (Fig. 1B). No differences in uterine weight were seen between 2.5μg/4d (n=4) (0.48±0.04) and 2.5μg/14d (n=5) (0.44±0.03). However, significant increases in uterine weight were noted between these two regimens compared to SO (n=4) (0.12±0.01) (P<0.001 in both cases). The E regimen of 10μg/14d (n=5) (0.61±0.03) increased uterine weight compared to that seen with SO (P<0.001) and 2.5μg/4d (P<0.05) and 2.5μg/14d (P<0.01).
Effects of E on protein levels of Bcl-2 in whole uterine extracts
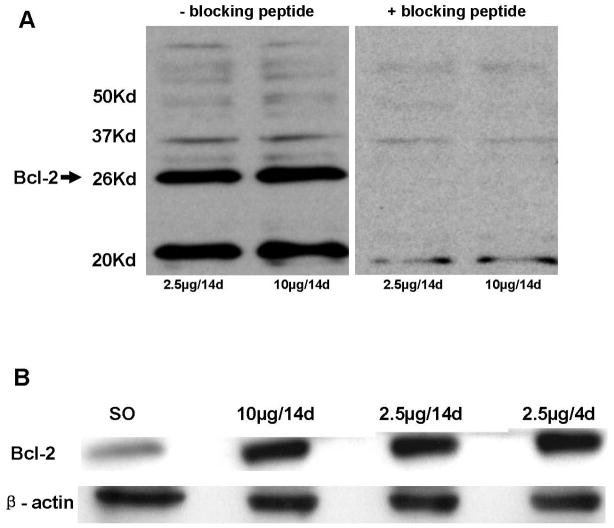
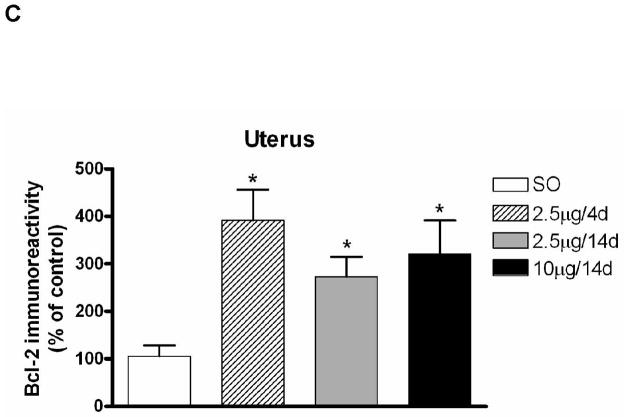
We examined the effect of various E regimens on Bcl-2 immunolabeling in whole uterine extracts. First, we characterized the antibody to Bcl-2 using Western blot, by preincubating the Bcl-2 antibody with blocking peptide Bcl-2P 1:1 (v:v) in PBST (1:500) for 2 hr at RT. We then incubated the blot of uterine extracts from animals treated with the regimens of 2.5 μg and 10 μg E for 14 days with this solution as described above. Specificity of the antibody for the 26 kDa band was evidenced by disappearance of this band upon incubation of the antibody (blocked with blocking peptide) with the membrane (Fig. 2A). Confirmation of the specificity of the Bcl-2 antibody on tissue sections is given below. Figure 2B shows a representative Western blot of Bcl-2 demonstrating an increase in Bcl-2 immunolabeling in whole uterine extracts of animals treated with the E regimens or vehicle. Significant differences were seen in Bcl-2 immunolabeling in SO (n=5) compared to the E groups (n=8/group) (Kruskal-Wallis, P<0.01, H=12.27, d.f.=3; Mann-Whitney U-test, P<0.01 in all cases; U values=2, 2, 0 respectively); no differences were seen among E groups (Fig. 2C).
Figure 2.
Comparison of levels of Bcl-2 protein in uterus following various E regimens on Western blots. (A) Western blots of uterine extracts demonstrating the specificity of the antibody to Bcl-2. Bcl-2 antibody was preincubated with the blocking peptide Bcl-2P as described in Materials and Methods. Blots of uterine extracts from animals treated with 2.5 μg and 10 μg E for 14 days were treated with the antibody-antigen solution displayed no immunolabeling for the Bcl-2 band at 26 kDa. (B) Representative Western blot of Bcl-2 and β-actin in whole uterine extracts from animals treated with the various E regimens. Proteins (20μg) were resolved on SDS-PAGE (12.5%) and were then transferred to polyvinylidene fluoride transfer membranes using electrophoresis. Detection and quantification of proteins were performed as described in Materials and Methods. All E treatments resulted in an increase in Bcl-2 immunolabeling relative to SO. (C) Effects of various E regimens on Bcl-2 protein levels in whole uterine extracts. E treatment groups are significantly different from the control group SO (U values=2, 2, 0 respectively; P<0.01). Values are mean ± S.E.M, and are represented as the percentage of control. *: P<0.01, compared with SO.
Effects of E on Bcl-2 immunolabeling and levels of Bcl-2 mRNA signal in the MeA and CeA
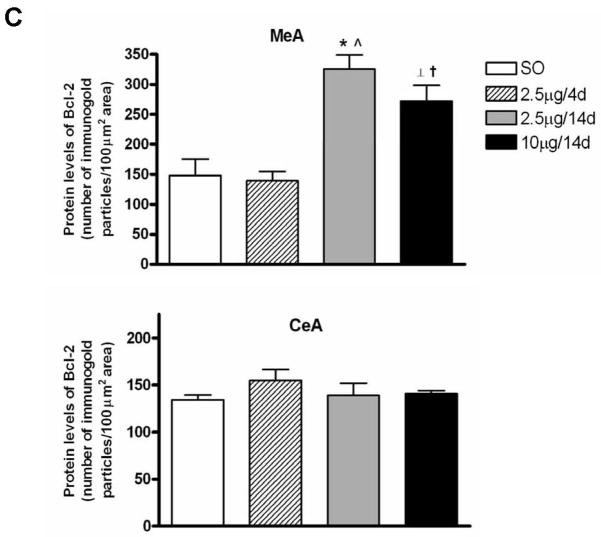
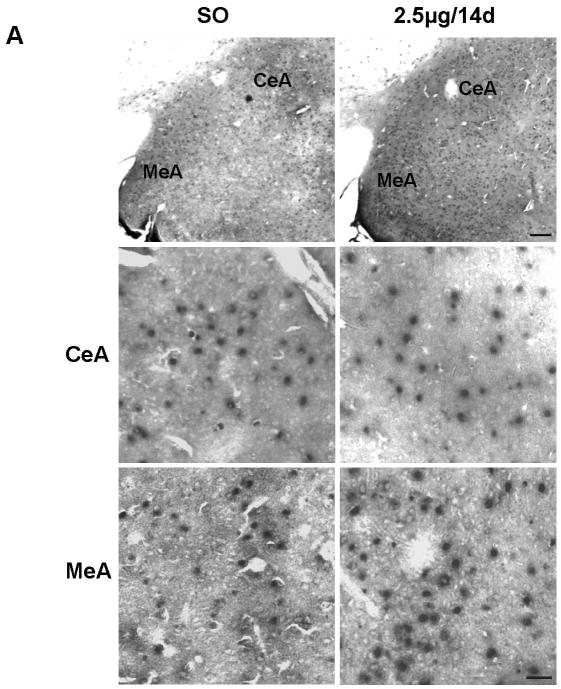
Gold immunolabeling histochemical procedure was used to detect and quantify Bcl-2 protein levels within the amygdala. Immunolabeling was detected in cytoplasm, as previously reported (Garcia-Segura et al., 1998). First, we confirmed the specificity of the antibody by preincubating Bcl-2 antibody with the blocking peptide Bcl-2P 1:1 (v:v) in 1% BSA-PBS (1:1,000) for 2 hr at RT; incubation of sections with this solution abolished gold immunolabling (Fig. 3A). In MeA, the E regimens of 2.5μg/14d (n=6) and 10μg/14d (n=6) increased Bcl-2 immunolabeling compared to SO (n=7) and 2.5μg/4d (n= 7), with no differences in CeA (Fig. 3B). Overall, there were significant differences among groups in MeA (F3, 22=15.4, P<0.001) (Fig. 3C). Moreover, Bcl-2 protein levels were similar between SO and 2.5μg/4d groups. Significant increases in Bcl-2 immunolabeling were seen in 2.5μg/14d (325.1±24) versus SO (148.3±27.01) and 2.5μg/4d (139.4±15.36) (P<0.0001, in both cases). The 10μg/14d (272.4±25.87) regimen also increased Bcl-2 immunolabeling compared with SO (148.3±27.01) and 2.5μg/4d (139.4±15.36) (P<0.01, in both cases). No differences in Bcl-2 immunolabeling were seen among the groups in CeA (Fig. 3C).
Figure 3.
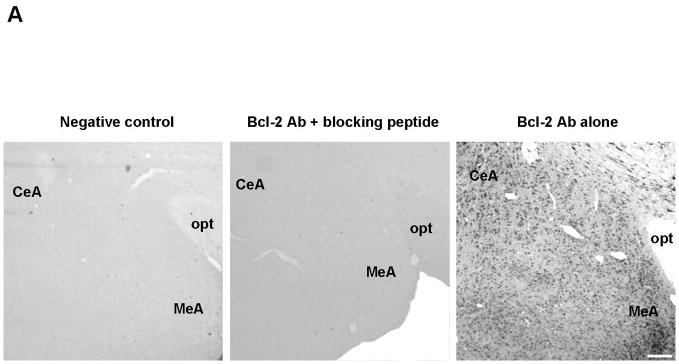
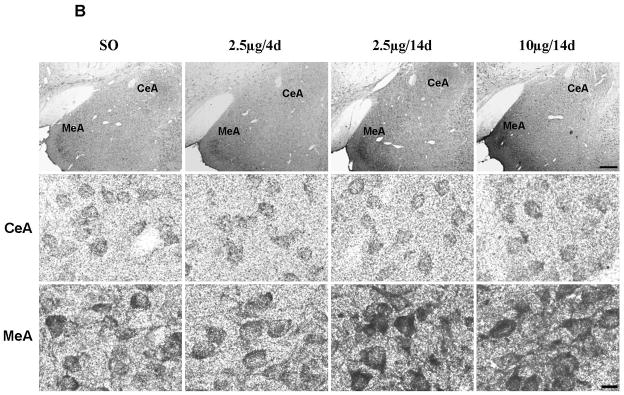
Comparison of levels of Bcl-2 protein following various E regimens by gold immunolabeling in the medial (MeA) and central (CeA) amygdala. (A) Low magnification light micrographs of tissue sections from MeA and CeA of rats treated with 2.5μg/14d demonstrating the specificity of the antibody (Ab) to Bcl-2. In the negative control on the left, the primary antibody was omitted and replaced by 1% BSA in PBST containing 0.25% Triton X-100. No labeling is visible. In the preabsoption control in the middle, the antibody was preabsorbed with Bcl-2 blocking peptide and this solution was incubated with the tissue section, as described in the Results; no labeling is visible on the section. Incubation of the section with the antibody to Bcl-2 resulted in labeling of cell bodies throughout the section on the right. opt, Optic nerve. Scale bar = 150 μm. (B) Low and high magnification light micrographs of Bcl-2 immunolabeling in the CeA and MeA of ovariectomized E- and vehicle-treated rats. Top: Low magnification light micrographs of Bcl-2 immunoreactivity in the CeA and MeA of rats treated with the indicated regimens. Scale bar = 250μm. Bottom: Bcl-2 immunopositive cell bodies in the CeA and MeA at high magnification Scale bar = 10μm. (C) Quantitation of Bcl-2 immunogold labeling (number of immunogold particles per 100μm2 area) in MeA (top) and CeA (bottom). There was a significant difference among treatment groups in MeA (F3, 22=15.4, P<0.001). Significant increases in immunolabeling were seen in the 2.5μg/14d versus SO (P<0.0001) and 2.5μg/4d (P<0.0001) groups and 10μg/14d versus SO (P<0.01) and 2.5μg/4d (P<0.01) groups. None of the groups displayed differences in gold immunolabeling in the CeA. Values are the mean ± S.E.M. *: P<0.0001; ⊥: P<0.01, compared with SO; ^: P<0.0001; †: P<0.01, compared with 2.5μg/4d.
To determine the effects of E on Bcl-2 mRNA levels in the MeA and CeA, we compared the regimen of 2.5μg E for 14 days (n=7) to vehicle (n=6) using in situ RT-PCR. Positive cells bodies in the MeA and CeA showing the signal for Bcl-2 mRNA are shown in Figure 4A. Examples of high magnification light micrographs of the MeA and CeA after E or vehicle treatment are provided in Figure 4A. Quantitative analysis of Bcl-2 mRNA levels in MeA and CeA reveals a significant increase in mRNA levels of Bcl-2 in the E- compared to the vehicle- treated group (12.76±0.60 versus 8.58±0.87) (P<0.01); no differences were seen in CeA between E- and vehicle-treated groups (Fig. 4B).
Figure 4.
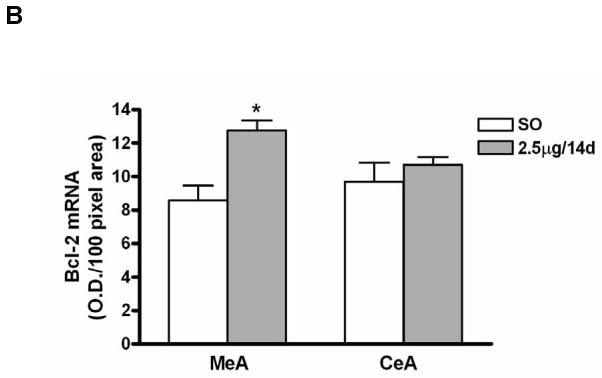
(A) Photomicrographs of Bcl-2 mRNA signal (in situ RT-PCR) in the medial (MeA) and central (CeA) amygdala from ovariectomized E- and vehicle-treated rats. Top: Light micrographs at low magnification of Bcl-2 mRNA positive cells in the CeA and MeA from E- and vehicle (sesame oil [SO])-treated rats. Scale bar = 150μm. Photomicrographs from the CeA (middle) and MeA (bottom) from SO- and E-treated rats shows the Bcl-2 mRNA positive cells at higher magnification. Scale bar = 20μm. (B) Quantification of Bcl-2 mRNA signal in the MeA and CeA amygdala of vehicle (sesame oil [SO])- and E- (2.5μg for 14 days [2.5μg/14d]) treated ovariectomized rats. There was a significant increase in levels of Bcl-2 mRNA in the E-treated group compared to the vehicle control in MeA (P<0.01), but not CeA. Values are the mean ± S.E.M. *: P<0.01.
DISCUSSION
We demonstrate time-dependent, E-induced changes in levels of Bcl-2 immunolabeling and mRNA in the MeA, but not CeA, of ovariectomized rats. These effects correspond to those seen in our studies, where components of the CREB signaling cascade, including BDNF, and/or NeuN-positive cell numbers are increased over controls with E regimens of 10μg for 14 days (Carlstrom et al., 2001; Zhou et al., 2001, 2004, 2005) or 2.5μg for 14, but not 4, days (Fan et al., 2008). We also report an E-induced increase in Bcl-2 in uterus of ovariectomized rats.
Cyclic changes in human endometrial growth and regression may be controlled by alterations in the ratio of Bcl-2 and related proteins (Tao et al., 1997; cf. also Otsuki et al., 1994). We have shown an increase in Bcl-2 immunolabeling with all of the aforementioned E regimens compared to vehicle. These results are consistent with others showing a decrease in Bcl-2 in the uterus during the transition from proestrus to estrus, and a corresponding increase after its nadir (Mendoza-Rodriquez et al., 2003). Bcl-2 is a CREB-related gene product, but it was also shown that the bcl-2 gene exhibits an estrogen responsive element (ERE) in its coding region (Perillo et al., 2000). Nevertheless, at least in breast cancer cells, 17β-estradiol-induced transactivation of Bcl-2 depends upon a CREB-response element (CRE) motif in the bcl-2 gene promoter (Dong et al., 1999).
The time related increase in Bcl-2 immunolabeling may be due to an increase in the number of cells expressing Bcl-2 or an upregulation of Bcl-2 in cells already expressing this protein. We presented evidence for E action on the CREB signaling pathway that may lead to the persistence of pCREB in the MeA, but not CeA. The regimen of 10μg E for 14 days resulted in increased levels of the upstream regulator of CREB, CaMK IV (Zhou et al., 2001), and a decrease in levels of calcineurin (Zhou et al., 2004) in the MeA, but not CeA, of ovariectomized rats. Calcineurin can dephosphorylate pCREB, and others observed a negative effect of proestrus or E on calcineurin mRNA levels (Funabashi et al., 1995) and/or protein levels and activity (Sharrow et al., 2002) in the ventromedial hypothalamus and hippocampus, respectively. Because of the length of time of the 2.5μg or 10μg 14 day E treatment, the increase in Bcl-2-immunolabeled cells may be mediated via a nuclear ER and gene transcription. As mentioned above, the bcl-2 gene has both an ERE and CRE motif (Dong et al., 1999), so that E may upregulate Bcl-2 directly or indirectly via an effect on CREB or other transcription factors, such as STAT-3 (Dziennis et al., 2007). 17β-estradiol may also activate the phosphatidyl inositol-3 kinase (PI3K)/Akt pathway, thereby, inhibiting the proapoptoic protein glycogen synthase kinase 3 (GSK3) (D’Astous et al., 2006). The extra time of E treatment may result in indirect E actions on second messenger systems via other receptors, resulting in upregulation of CREB signaling and transcription of the bcl-2 gene. 17β-estradiol-induced Ca2+ influx via the L-type Ca2+ channel is necessary for 17β-estradiol activation of the Src/ERK/CREB/Bcl-2 signaling cascade in hippocampal neurons; CREB siRNA reduced Bcl-2 protein levels in hormone-treated and untreated groups (Wu et al., 2005).
We noted an effect of E regimens of 2.5μg/14d and 10μg/14d on Bcl-2 immunolabeling and/or mRNA in the MeA, but not CeA, of ovariectomized rats. Whether the effect is dependent on ERα or ERβ or another ER subtype is a subject for future investigation. Nevertheless, it is notable that physiological effects of E or 17β-estradiol have been reported in the CeA (Jasnow et al., 2006; Foradori et al., 2007) and both ERα mRNA and ERβ mRNA have been reported in MeA and CeA (Simerly et al., 1990; Li et al., 1997; Shughrue et al., 1997; Österlund et al., 1998), suggesting that these two subtypes may be differentially modulated (Dubal et al., 1999) in these two regions. Hormonal states of the animals studied and the heterogeneity of the MeA, itself, may also confound a precise comparison of ER mRNA and/or receptor distribution in the MeA and CeA (Simerly et al., 1990; Li et al., 1997; Shughrue et al., 1997, 1998; Laflamme et al., 1998; Österlund et al., 1998; Greco et al., 2001; Isgor et al., 2002). In regard to CeA, Österlund et al. (1998) and Simerly et al. (1990) reported ERα mRNA expression in the CeA. Shughrue et al. (1998) showed no ERβ mRNA in the CeA, but Österlund et al. (1998) note its presence in that region. Other factors that may affect E’s efficacy in the MeA versus CeA include: relative levels of ERα or ERβ and their ability to homo- or heterodimerize within a cell; receptor variants; levels of coregulators and signal transduction cascades; the conformation of the ER-ligand complex; and/or ERβ modulation of ERα activity (Nomura et al., 2003) (cf. reviews in Weihua et al., 2003; Turgeon et al., 2004; Bjornstrom and Sjoberg, 2005).
ERs have been shown to mediate estrogenic effects on Bcl-2 in the brain. Low, physiological levels of 17β-estradiol regulate the expression of Bcl-2 in the cerebral cortex following cerebral ischemia, an effect that may be mediated via ERβ (Dubal et al., 1999). The injury-induced downregulation of ERβ in the ipsilateral part of the brain was averted by 17β-estradiol in a manner similar to the regulation of Bcl-2. 17β-estradiol increased the ratio of ERβ/ERα in this region, underscoring the importance of the relative levels of ER subtypes in estrogen action. By using selective ERα or ERβ agonists, Zhao et al. (2004) showed that activation of either subtype enhanced Bcl-2 expression in hippocampal neurons in a manner comparable to the neuroprotective ability of the ER subtypes against glutamate excitotoxicity. The two subtypes were effective in attenuating an excitotoxic glutamate-induced intracellular Ca2+ rise, with the ERβ-selective agonist displaying greater efficacy than the ERα agonist in potentiating a physiological concentration of glutamate-induced intracellular Ca2+ rise in hippocampal neurons (Zhao and Brinton, 2007). The nuclear ER antagonist, ICI 182,780, failed to block neuroprotection mediated by these agonists, suggesting involvement of a non-nuclear ER-mediated rapid signaling cascade. In striatum, ERα mediated the increased ratio of Bcl-2/BAD in mice treated with MPTP (D’Astous et al., 2006).
Wise and colleagues (Dubal et al., 2001 and Wise et al., 2001) noted that physiological levels of 17β-estradiol usually require receptor-mediated genomic or non-genomic action for mediation of 17β-estradiol neuroprotection, but that pharmacological levels of 17β-estradiol protect by non-ER-mediated antioxidant and/or membrane channel effects. We show that regimens resulting in both proestrus and supraphysiological E levels increased Bcl-2 immunolabeling and/or mRNA in the MeA. Both regimens increased NeuN-positive cell numbers in this region (Fan et al., 2008).
The effect of E on Bcl-2 in the MeA may signify neuroprotection as it relates to stress, depression and anxiety behavior. Kosten et al. (2007) have shown upregulation of Bcl-2 mRNA with various antidepressant drug treatments in the rat MeA and CeA following repeated unpredictable stress. An increase in Bcl-2 mRNA was also seen following chronic electroconvulsive therapy in the CeA, but not MeA. Mice displaying a targeted mutation of the Bcl-2 gene exhibit anxiety-like (fear) behavior (Einat et al., 2005). On the other hand, a reduction in anxiety-like behavior was seen in mice with overexpression of Bcl-2 (Rondi-Reig et al., 1997). 17β-estradiol has been implicated in anxiolysis via ERβ (Walf and Frye, 2007) and, as noted above, in the regulation of Bcl-2. In future studies we will determine if an E-induced increase in Bcl-2 protein and mRNA expression in the MeA is associated with anxiolytic properties of estrogen in ovariectomized rats. In summary, data collected here suggest that an increase in Bcl-2 mRNA and protein levels in the MeA during E exposure may be responsible for increases in NeuN-positive neurons in the MeA (Fan et al., 2008).
Acknowledgments
Grant information: This work was supported by National Institute of Mental Health grant MH065990 and funds from the University of Illinois Earl M. Bane Charitable Trust (to R. S. C.), and the National Institute on Alcohol Abuse and Alcoholism grant AA-010005; AA-013341 and Veterans Affairs Merit grant and VA Career Scientist Award (to S. C. P.).
We thank Dr. Huaibo Zhang and Dr. Handojo Kusumo for their helpful assistance with in situ RT-PCR protocol. Submitted in partial fulfillment for the degree of Doctor of Philosophy to L. Fan at the University of Illinois at Chicago.
References
- Ábrahám IM, Han S, Todman MG, Korach KS, Herbison AE. Estrogen receptor {beta} mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Bates PJ, Sanderson G, Holgate ST, Johnston SL. A comparison of RT-PCR, in-situ hybridization and in-situ RT-PCR for the detection of rhinovirus infection in paraffin sections. J Virol Methods. 1997;67:153–160. doi: 10.1016/s0166-0934(97)00095-5. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Carlstrom L, Ke ZJ, Unnerstall JR, Cohen RS, Pandey SC. Estrogen modulation of the cyclic AMP response element-binding protein pathway. Effects of long-term and acute treatments. Neuroendocrinology. 2001;74:227–243. doi: 10.1159/000054690. [DOI] [PubMed] [Google Scholar]
- D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta -estradiol in breast cancer cells. J Biol Chem. 1999;274:32099–32107. doi: 10.1074/jbc.274.45.32099. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziennis S, Jia T, Rønnekleiv OK, Hurn PD, Alkayed NJ. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci. 2007;27:7268–7274. doi: 10.1523/JNEUROSCI.1558-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: Further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res. 2005;165:172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Fan L, Hanbury R, Pandey SC, Cohen RS. Dose and time effects of estrogen on expression of Neuron-specific Protein and cyclic AMP response element-binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology. 2008;88(2):111–126. doi: 10.1159/000129498. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Lund TD, Nagahara AH, Koenig JI, Handa RJ. Corticotropin-releasing hormone heterogeneous nuclear RNA (hnRNA) and immunoreactivity are induced in extrahypothalamic brain sites by kainic-acid-induced seizures and are modulated by estrogen. Brain Res. 2007;1164:44–54. doi: 10.1016/j.brainres.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Fudge JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Brooks PJ, Kleopoulos SP, Grandison L, Mobbs CV, Pfaff DW. Changes in preproenkephalin messenger RNA level in the rat ventromedial hypothalamus during the estrous cycle. Brain Res Mol Brain Res. 1995;28:129–134. doi: 10.1016/0169-328x(94)00191-g. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen J. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroendocrinology. 1998;9(4):593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology. 1978;102(1):42–150. doi: 10.1210/endo-102-1-142. [DOI] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Huang GC, Akil H, Watson SJ. Correlation of estrogen β-receptor messenger RNA with endogenous levels of plasma estradiol and progesterone in the female rat hypothalamus, the bed nucleus of stria terminalis and the medial amygdala. Molecul Brain Res. 2002;106:30–41. doi: 10.1016/s0169-328x(02)00407-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148(3):1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Galloway MP, Duman RS, D’Sa C. Repeated unpredictable stress and antidepressants differentially regulate expression of the BCl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology online. 2008;33(7):1545–1558. doi: 10.1038/sj.npp.1301527. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Induction of lordosis in female rats: two models of estrogen action and the effect of adrenalectomy. Horm Behav. 1975;6:259–276. doi: 10.1016/0018-506x(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Kuipers SD, Trentani A, Westenbroek C, Bramham CR, Korf J, Kema IP, Ter Horst GJ, Den Boer JA. Unique patterns of FOS, phospho-CREB and BrdU immunoreactivity in the female rat brain following chronic stress and citalopram treatment. Neuropharmacology. 2006;50:428–440. doi: 10.1016/j.neuropharm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36(3):357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor-beta-like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66(2):63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mendoza-Rodriguez CA, Monroy-Mendoza MG, Morimoto S, Cerbon MA. Pro-apoptotic signals of the bcl-2 gene family in the rat uterus occurs in the night before the day of estrus and precedes ovulation. Molecul Cellul Endocrinol. 2003;208:31–39. doi: 10.1016/s0303-7207(03)00258-2. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Molecul Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neuroscience. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor β (ERβ) protein levels in neurons depend on estrogen receptor α (ERα) gene expression and on its ligand in a brain region-specific manner. Molec Brain Res. 2003;110:7–14. doi: 10.1016/s0169-328x(02)00544-2. [DOI] [PubMed] [Google Scholar]
- Österlund M, Kuiper GGJM, Gustafsson J, Hurd YL. Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Molecul Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Otsuki Y, Misaki O, Sugimoto O, Ito Y, Tsujimoto Y, Akao Y. Cyclic bcl-2 gene expression in human uterine endometrium during menstrual cycle. Lancet. 1994;344(8914):28–29. doi: 10.1016/s0140-6736(94)91051-0. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat cortex. J Pharmacol Exp Ther. 2001;296(3):857–868. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate response element-binding protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta -Estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20:2890–2901. doi: 10.1128/mcb.20.8.2890-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers TJ, Lamb SV, Bartlett SA, Matamoros RA, Cowan RG, Engle JS. Effects of hemolysis and storage on quantification of hormones in blood samples from dogs, cattle, and horses. Am J Vet Res. 1991;7:1075–1080. [PubMed] [Google Scholar]
- Rondi-Reig L, Dubreuil YL, Martinou J-C, Delhaye-Bouchaud N, Caston J, Mariani J. Fear decrease in transgenic mice over-expressing bcl-2 in neurons. Neuroreport. 1997;8(11):2429–2432. doi: 10.1097/00001756-199707280-00004. [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25(3):165–177. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- Sharrow KM, Kumar A, Foster TC. Calcineurin as a potential contributor in estradiol regulation of hippocampal synaptic function. Neuroscience. 2002;113:89–97. doi: 10.1016/s0306-4522(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence of the colocalization of estrogen receptor-{beta} mRNA and estrogen receptor-{alpha} immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Szegö EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Ábrahám IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Tilly KI, Maravei DV, Shifren JL, Krajewski S, Reed JC, Tilly JL, Isaacson KB. Differential expression of members of the bcl-2 gene family in proliferative and secretory human endometrium: glandular epithelial cell apoptosis is associated with increased expression of bax. J Clin Endocrinol Metab. 1997;82:2738–2746. doi: 10.1210/jcem.82.8.4146. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Hojo R, Kuroda Y, Fujimaki H. Estrogen modulates Bcl-2 family protein expression in the sexually dimorphic nucleus of the preoptic area of postnatal rats. Neurosci Lett. 2008;432(1):58–63. doi: 10.1016/j.neulet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Andersson S, Cheng G, Simpson ER, Warner M, Gustafsson J. Update on estrogen signaling. FEBS Letters. 2003;546:17–24. doi: 10.1016/s0014-5793(03)00436-8. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17[beta]-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and Bcl-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J Neurosci. 2007;27(6):1422–33. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Masui A, Jinde S, Kanai H, Kato N, Okawa M. Influence of age or circadian time on Bcl-2 and Bax mRNA expression in the rat hippocampus after cortisone exposure. Brain Res Bull. 2007;73(4-6):254–258. doi: 10.1016/j.brainresbull.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides. 2003;24:1397–1402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu T, Brinton R. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cohen RS, Pandey SC. Estrogen affects the expression of Ca2+/calmodulin-dependent protein kinase IV in amygdala. Neuroreport. 2001;12:2987–2990. doi: 10.1097/00001756-200109170-00046. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pandey SC, Cohen RS. Estrogen decreases levels of calcineurin in rat amygdala and hippocampus. Neuroreport. 2004;15:2437–2440. doi: 10.1097/00001756-200410250-00027. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Watters J, Dorsa D. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]