Abstract
Tamoxifen, a selective estrogen receptor modulator, is widely used in research and clinically in patients. We find that treatment of normal mice with a single ≥ 3mg/20g body weight dose of tamoxifen leads to apoptosis of > 90% of all gastric parietal cells and metaplasia of zymogenic chief cells within 3 days. Remarkably, gastric histology returns to nearly normal by 3 weeks. Tamoxifen toxicity occurs by oral and intraperitoneal administration, in both sexes, in multiple strains, and does not depend on estrogen, though acid secretion inhibition is partially protective. Thus, substantial gastric toxicity is a heretofore unappreciated tamoxifen side effect.
Tamoxifen is widely used to spatio-temporally delete mouse genes using the CreERT/loxP system1. Tamoxifen is also used clinically as a selective estrogen receptor (ER) modulator, in chemotherapeutic, anti-osteoporotic, and several other therapeutic regimens2,3. Some reports suggest tamoxifen also increases risk for subsequent gastric cancer4,5. Most gastric cancers occur in stomachs colonized by Helicobacter pylori6. Precancerous effects of bacterial colonization include: death (atrophy) of acid-secreting gastric parietal cells (PCs), differentiation changes (metaplasia) in the digestive-enzyme secreting zymogenic (chief) cell lineage (ZC) and increased stem cell proliferation7,8.
In control experiments for tamoxifen induction of Cre-recombinase activity9, we noticed that tamoxifen injection (3 consecutive days, intraperitoneal, 5mg/20g mouse body weight) caused dramatic rearrangement of the gastric mucosa with loss of > 90% of PCs, a 6-fold increase in proliferation in stem/progenitor cells, and morphological changes in the ZCs in the bases of gastric-units (Fig. 1A-D). By 14–21 days, the epithelium recovered (Fig. 1A). No other organs had marked phenotypes at this dose or time-schedule (Supp. Fig. 1). Even a single dose of tamoxifen, by intraperitoneal injection or oral gavage, from two different commercial suppliers in three different strains of mice caused similar effects in n>63 mice (Fig. 1E, Supp. Fig. 2A-F). By qRT-PCR, PC-specific transcripts (Atp4a) and markers of ZC maturation (Mist1 aka Bhlha15, Pgc, GIF)10 were significantly reduced by d3, whereas the surface/foveolar lineage marker (Tff1) and transcripts for gastrin were unchanged (Fig. 1F, see Supp. Fig. 2G for western blots of GIF and ATP4A).
Fig. 1.
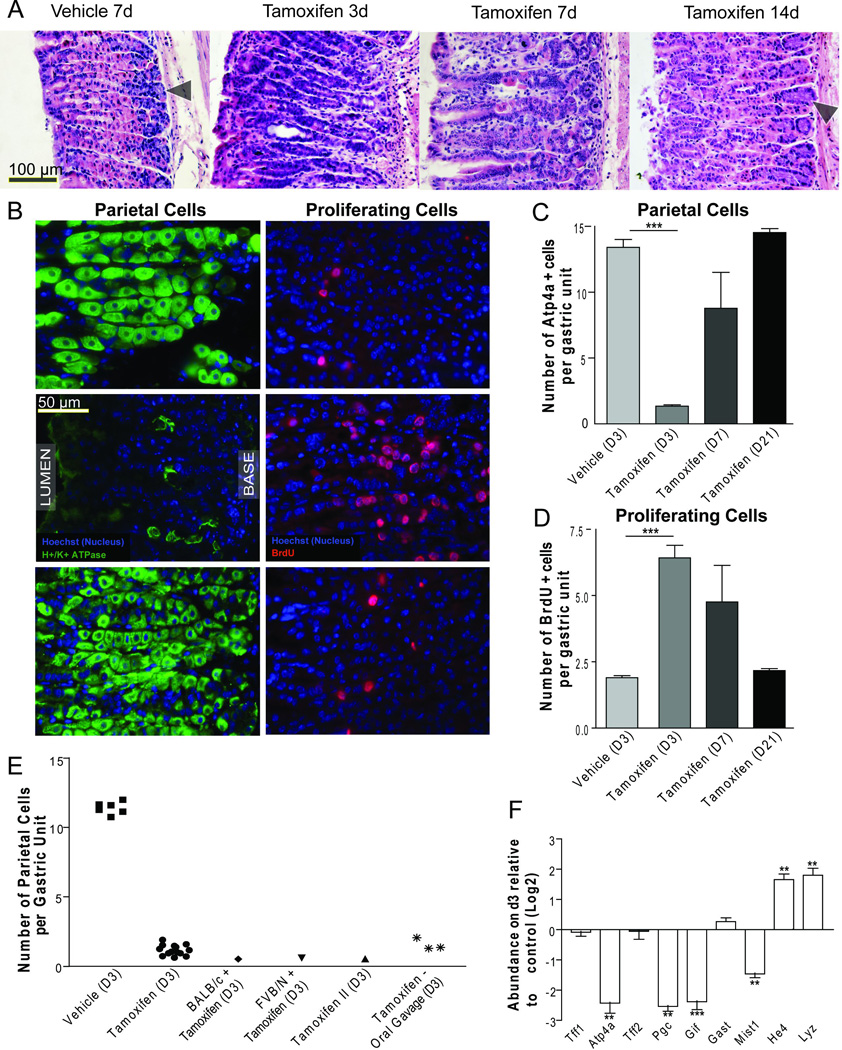
A) H&E of wild-type mice following intraperitoneal (i.p.) injection of vehicle at day 7 or 5mg/20g body weight tamoxifen at 3, 7, and 14 days following injection. B) Immunofluorescence (green: anti-ATP4A; red:anti-BrdU) at d3, quantified in C,D. E) Quantification of mean PCs/unit/individual mouse by H&E; unless otherwise indicated, mice were C57/B6 strain; “Tamoxifen II”: tamoxifen from another supplier. F) Whole stomach qRT-PCR (expressed as Log2 scale. *P<0.05;**P<0.01;***P<0.001)
Increased progenitor cell proliferation and changes in ZC differentiation are characteristic of spasmolytic polypeptide expressing metaplasia (SPEM)8,11. In SPEM, expression of mucous neck cell markers (like spasmolytic polypeptide, aka TFF2) occurs in the base of glands, where ZCs normally reside10,11. Consistent with the SPEM phenotype, tamoxifen caused a marked increase in basal neck cell marker expression, (Supp. Fig. 3). Additionally, tamoxifen increased two SPEM-specific transcripts, He4 and Lyz8; however, transcript levels for spasmolytic polypeptide(Tff2) itself were unchanged (Fig. 1F). SPEM is usually diagnosed by histopathological criteria and not transcriptionally7,8,10–12, but we cannot rule out the possibility that the lack of increased Tff2 indicates that tamoxifen-induced metaplasia is a SPEM variant.
In humans and mice, metaplasia always occurs in the setting of PC atrophy8,10. To assess PC death, we crossed Atp4b-Cre mice, whose PCs constitutively express Cre13, to nuclear lacZ-R26R mice. In these mice, all mature PCs had, as expected, nuclear lacZ expression (Fig. 2A). Tamoxifen caused near complete loss of lacZ, indicating that PCs died and did not give rise to other cells with different morphological or molecular characteristics. TUNEL-positive PCs were not observed in the vehicle treated controls, whereas they were common within 12 hours after a single injection of 5mg/20g tamoxifen (Fig. 2B). By 12h, cytochrome C staining could now be found leaked into the cytoplasm of the majority of PCs, consistent with early aptoptosis; in controls, distribution was still punctate, consistent with retention in the mitochondria (Fig. 2B). By transmission electron microscopy, PCs showed neither vacuolization nor organellar swelling, characteristics of necrotic death, but had apoptotic features like electron-dense inclusions in mitochondria and peripheral chromatin condensation (Fig. 2D, E). Finally, only tamoxifen-treated stomachs showed Caspase 3 cleavage (Fig. 2C).
Fig. 2.

A) Nuclear LacZ labeled PCs following tamoxifen treatment. B) Top: TdT-mediated dUTP nick-end labeling shows dying PCs (arrowheads). Below: Cytochrome C staining is punctate, consistent with mitochondrial localization in vehicle-treated (below left) and dispersed throughout the cytoplasm tamoxifen-treated PCs (below right) C) Cleaved caspase 3 western blot with tubulin loading control. D) At 2.5d following tamoxifen, PCs show chromatin condensation (arrows with black outline), consistent with early apoptosis. E) Another degenerating PC exhibits mitochondria ranging in morphology from normal (dashed arrow) to electron-dense-inclusion-containing (white solid arrow) to electron-dense and degenerating (arrowheads).
Tamoxifen can function as both an estrogen receptor (ER) agonist and antagonist depending on tissue type; however, neither treatment with the pure ER agonist 17-β-estradiol nor the specific antagonist fulvestrant induced atrophy/metaplasia. And neither blocked tamoxifen effects (Supp. Fig. 4A, B). The sex of the mice also did not affect tamoxifen effects (Supp. Fig. 4C), nor did ovarectomy of females to block endogenous estrogen production (Supp. Fig. 4C; data not shown). However, tamoxifen effects could largely be reversed by blocking the PC proton pump with omeprazole (Supp. Fig. 5), suggesting a role for active acid secretion in tamoxifen toxicity to PCs. Finally, another SERM family member, raloxifene, which also has both pro and anti-estrogenic effects, did not cause atrophy up to a dose of 5mg/20g (Supp. Fig. 4D), indicating toxicity is not a general feature of SERMs. On the other hand, intraperitoneal injection of raloxifene induced Cre recombinase-mediated lacZ activation in mice expressing Rosa26-Cre fused with a modified ER (Supp. Fig. 4E), indicating that raloxifene can be used to induce Cre recombinase activity to obviate the off-target toxicity of tamoxifen in Cre-loxP inducible systems.
Tamoxifen is a chemotherapeutic drug that has toxic effects on cancer cells from diverse tissues. In osteoclasts (which, like PCs, are large, mitochondria- and proton pump-rich cells), toxicity is caused by disrupting proton gradients and, thereby intracellular pH14. The drug DMP-777 causes PC death the same way12. Omeprazole is partially protective against both DMP-777 and tamoxifen toxicity, suggesting a similar mode of action12. The minimal dosing that causes metaplasia in the current study is an order of magnitude more than the equivalent (40 mg/day) used therapeutically in humans15. Acute loss of PCs in patients taking tamoxifen might be beneficial for acid-reflux associated illness but could also predispose, long-term to gastric cancer. Further experiments are clearly needed to address effects of tamoxifen on the human stomach.
Supplementary Material
Acknowledgments
Grant support: JCM (ACS DDC-115769, NIH DK079798-1,2; Funderburg Research Scholar Award; Digestive Diseases Research Core Center: NIH 2P30 DK052574-12), WJH, SK and JHG were supported in part by the Cancer Biology Pathway Program, Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes Jewish Hospital.
Abbreviations
- SPEM
spasmolytic polypeptide expressing metaplasia
- ZC
zymogenic (chief) cell
- tEM
transmission electron microscopy
- BrdU
Bromodeoxyuridine
- ER
estrogen receptor
- SERM
selective estrogen receptor modulator
Footnotes
The authors disclose no conflicts of interest.
Contributions: WJH and SSK (study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis), JHG (analysis and interpretation of data; statistical analysis); KK (acquisition of data); RAW (acquisition of data); JCM (study concept and design; analysis and interpretation of data; statistical analysis; obtained funding; study supervision)
References
- 1.Feil R, et al. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan VC. Cancer. 1992;70:977–982. [PubMed] [Google Scholar]
- 3.Love RR, et al. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 4.Chandanos E, et al. Br J Cancer. 2006;95:118–122. doi: 10.1038/sj.bjc.6603214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuyama Y, et al. Ann Oncol. 2000;11:1537–1543. doi: 10.1093/oxfordjournals.annonc.a010406. [DOI] [PubMed] [Google Scholar]
- 6.Group HaCC. Gut. 2001;49:347–353. [Google Scholar]
- 7.Lennerz JK, et al. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, et al. Gastroenterology. 2010;139:213 e3–225 e3. [Google Scholar]
- 9.Huh WJ, et al. Am J Physiol Gastrointest Liver Physiol. 2010;299:G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredemeyer AJ, et al. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quante M, et al. Gastroenterology. 2010;139:2018 e2–2027 e2. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa M, et al. Dig Dis Sci. 2006;51:431–439. doi: 10.1007/s10620-006-3151-x. [DOI] [PubMed] [Google Scholar]
- 13.Syder AJ, et al. Proc Natl Acad Sci U S A. 2004;101:4471–4476. doi: 10.1073/pnas.0307983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehenkari P, et al. J Bone Miner Res. 2003;18:473–481. doi: 10.1359/jbmr.2003.18.3.473. [DOI] [PubMed] [Google Scholar]
- 15.Reagan-Shaw S, et al. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


