Abstract
Interoception is theorized to be an important process mediating substance use disorders, and the insular cortex is recognized as a core neural region supporting interoception. The purpose of this study was to compare the integration of the insular cortex into prefrontal-related resting-state networks between individuals with cocaine dependence and healthy controls. 41 participants with cocaine dependence and 19 control participants underwent a resting-state 3T fMRI scan. Individuals with cocaine dependence demonstrated altered functional connectivity of the insular cortex, predominantly the right insular cortex, with all eight prefrontal-related resting-state networks identified through Independent Component Analysis (ICA). A conjunction analysis demonstrated that the right insular cortex was the neural region with the highest number of common group differences across the networks. There was no evidence that insular cortex connectivity commonly differed between groups for non-prefrontal-related networks. Further, seed-based functional connectivity analyses extended the network analyses and indicated that cocaine dependence was associated with greater connectivity of the right insula with the dorsomedial PFC, inferior frontal gyrus, and bilateral dlPFC. These data support the hypothesis that cocaine dependence is related to altered functional interactions of the insular cortex with prefrontal networks. The results suggest possible neural mechanisms by which the insular cortex and interoceptive information influence cognitive control and decision-making processes presumably mediated by prefrontal networks in the cocaine dependence process.
Keywords: cocaine, addiction, insular cortex, cognitive control, neuroimaging, independent component analysis
1. Introduction
Cocaine use is associated with high rates of drug dependence, making cocaine dependence among the more prevalent substance use disorders (SAMHSA, 2010; Wagner and Anthony, 2002). Drug dependence is associated with conditioned incentive salience of drug-related cues, a process believed to be mediated by drug-induced sensitization of mesocorticolimbic systems (Robinson and Berridge, 2000, 2003, 2008). While incentive sensitization helps explain many aspects of drug dependence, additional core processes including cognitive control, response inhibition, and decision making are altered in drug dependence and theorized to increase the impact of incentive salience on decision making around drug use (Goldstein and Volkow, 2002; Robinson and Berridge, 2003). The combined views of incentive sensitization and cognitive control deficits posit that compulsive drug use results from increased response tendencies elicited by drug cues in tandem with decreased cognitive control ability to modulate these response tendencies. Here, we purposefully use the broad term ‘cognitive control’ to refer to the overarching key functional role of the prefrontal cortex: to control the coordination and biasing of information processing across distributed brain regions based on current context and goals (Miller and Cohen, 2001).
It has recently been proposed that interoception may play a key function in the drug use disorder process that integrates incentive salience with cognitive control and decision making processes (Naqvi and Bechara, 2009, 2010; Naqvi et al., 2007). Interoception refers to the neural representation of hedonic bodily states (Craig, 2002), such as internal state changes related to pain, temperature, pleasure, craving, or withdrawal. According to one theory, interoception involves two major processes: neural representation of hedonic bodily states, and conscious/subjective awareness of these bodily states (Craig, 2002, 2009). These processes can be dissociated at the neural processing level, with the dorsal posterior insular cortex hypothesized to represent the actual changes in bodily states and the anterior insular cortex hypothesized to mediate subjective awareness of the bodily states (Craig, 2002, 2009; Craig et al., 2000). This hypothesized differential role of the posterior and anterior insular cortex is consistent with anatomical studies showing heterogeneous structural connectivity across the insular cortex (Augustine, 1996) and human neuroimaging studies showing heterogeneous activation and connectivity across the insular cortex (Craig, 2002, 2009; Deen et al., 2011; Nanetti et al., 2009). These differing patterns of functional and structural connectivity support differential integration of the anterior and posterior insula into large scale neural networks. The neural substrates mediating interoception can be differentiated from neural substrates mediating the commonly theorized processes involved in drug use disorders: reward/valuation and cognitive control. Reward valuation involves a network of regions including the orbitofrontal cortex (OFC), basolateral amygdala, and ventral striatum (Goldstein and Volkow, 2002; Robinson and Berridge, 2001). Cognitive control is a more heterogeneous construct that involves several spatially and functionally distinct networks including the dorsolateral PFC, ventral lateral PFC, anterior cingulate, and parietal cortex. For example, a network involving the dorsolateral PFC and anterior cingulate is commonly implicated in conflict monitoring and attentional control (Botvinick et al., 2001; Kerns et al., 2004), the inferior frontal gyrus (IFG), dorsal medial PFC and subthalamic nucleus are commonly implicated in response inhibition (Aron et al., 2003; Aron et al., 2004; Hampshire et al., 2010; Sharp et al., 2010), while regions within medial and ventral medial PFC (rostral and subgenual cingulate, medial PFC) are implicated in emotional control, salience attribution, and self-referential processing (Benoit et al., 2010; Etkin et al., 2006; Johnstone et al., 2007; Sajonz et al., 2010).
According to one recent model (Naqvi and Bechara, 2009), interoceptive processes mediate the relationship between incentive/motivational-related bodily changes associated with detecting drug cues and the disruption of on-going behavior in order to engage in drug use. Neurobiologically, the OFC and amygdala appear essential for detecting and processing the motivational significance of drug cues, the posterior insular cortex codes bodily changes associated with changes in motivational states, and the anterior insular cortex mediates subjective awareness of the craving state. This information is transmitted to the anterior cingulate cortex, dorsal lateral PFC, and likely also the IFG, that gate whether to maintain on-going behavior or disrupt it and engage in drug use (Naqvi and Bechara, 2009). From this model, it can be seen how interoceptive processes, either the degree of the representation of changes in craving states or the degree of subjective awareness of the craving state, can influence the subsequent control of behavior. Thus, the model predicts that drug dependence should be associated with altered functional connectivity of the insular cortex within cognitive control processes, and therefore, with prefrontal networks. Indeed, a substantive body of research links the insular cortex with cognitive control processes (Medford and Critchley, 2010; Menon and Uddin, 2010). For example, a prior study suggested that interactions between the right insular cortex and anterior cingulate cortex were critical for switching between default mode and cognitive control networks (Sridharan et al., 2008). Other regions implicate the anterior insula as critical in mediating uncertainty processing and tracking variance in risk (Behrens et al., 2007; Bossaerts, 2010; Preuschoff et al., 2008; Rushworth and Behrens, 2008). Findings such as these are consistent with one hypothesized role of the anterior insula in mediating awareness and salience detection (Craig, 2009) and highlights the integration of the insular cortex into cognitive control processes.
Given the predominant role of the prefrontal cortex in mediating cognitive control processes (Miller and Cohen, 2001), we focus analyses here on resting-state networks with peak loadings in the prefrontal cortex derived from independent component analysis. Though a growing body of research is examining functional connectivity in drug use disorders (Ma et al., 2011), and cocaine dependence in particular (Camchong et al., 2011; Gu et al., 2010; Kelly et al., 2011; Tomasi et al., 2010; Wilcox et al., 2011), there have been no prior studies explicitly testing insular cortex connectivity.
2. Methods
2.1 Participants
Participants included 41 current cocaine dependent individuals (9 female) and 19 control participants (11 female) with no history of drug use disorders. Demographic variables are provided in Table 1. Clinical interviews were conducted by a trained masters-level clinical research coordinator using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID;(First, 2002)) to establish current diagnoses of cocaine dependence. Inclusion criteria were: age between 18 and 65, no MRI contraindications or pregnancy, ability to read and write in English, and current cocaine dependence. Exclusion criteria were: serious medical conditions, current mood or anxiety disorder, current or past psychotic symptoms (hallucinations or delusions), loss of consciousness greater than 10 minutes, traumatic brain injury, comorbid drug use disorders except alcohol, nicotine, or marijuana.
Table 1.
Demographic and clinical characteristics of the sample.
| Measure | Cocaine Dependent Participants (N=41) |
Control Participants (N=19) |
P value of group differences |
|---|---|---|---|
| Age | 42.71 (7.71) | 31.68 (9.06) | 0.005* |
| Race | 17% | 58% | 0.001* |
| Caucasian | Caucasian | ||
| 81% Black | 37% Black | ||
| 2% other | 5% other | ||
| Sex | 22% female | 58% female | 0.001* |
| Education | 12.33 (1.38) | 14.74 (2.28) | <0.001 |
| Current Psychotropic Drug Use | 0 | 0 | n/a |
| Positive cocaine urinanalysis | 78% | 0 | <0.001 |
| Positive marijuana urinanalysis | 10% | 0 | 0.16 |
| Days Since Last Use | 8.34 (27.84) | 0 | n/a |
| Days Used in Last 30 days | 13.95 (9.77) | 0 | n/a |
| Days Used in Last 7 days | 2.85 (2.42) | 0 | n/a |
| Years of Cocaine Use | 16.02 (8.02) | 0 | n/a |
| Cigarette Use | 85.4% | 5.26% | <0.001 |
| Cigarettes per day | 10.33 (6.51) | 0.79 (3.44) | <0.001 |
| Years of Cigarette Use | 23.94 (8.97) | 1.05 (4.59) | <0.001 |
Note. Due to group differences in demographic variables of age, sex, race, and education, these variables were used as covariates in all between-group comparisons.
2.2. fMRI acquisition
A Philips 3T Achieva X-series MRI system using an 8-channel head coil (Philips Healthcare, USA) was used to acquire imaging data. Anatomic images were acquired with a MPRAGE sequence (matrix=192×192, 160 sagittal slices, TR/TE/FA=min/min/90°, final resolution=1×1×1mm3 resolution). As participants were recruited as part of two separate studies, the echo planar imaging sequences used to collect the functional images differed slightly between participants. One study (N = 23) used the following sequence parameters: TR/TE/FA=2000ms/30ms/90°, FOV(field-of-view)=240×240mm, matrix=80×80, 37 oblique slices (parallel to AC-PC plane to minimize OFC sinal artifact), slice thickness=3 mm, final resolution 3×3×3 mm3. The other study used the following sequence parameters: TR/TE/FA=2000ms/30ms/90°, FOV=192×192mm, matrix=64×64, 34 oblique slices (parallel to AC-PC plane to minimize OFC sinal artifact), slice thickness=3 mm, final resolution 3×3×3 mm3. As can be seen, the only difference across the two sequence parameters was the number of slices collected, which only affected coverage of the ventral cerebellum and ventral brain stem that represent brain areas that were not of interest to the present study. The resting-state scans lasted 7.2 minutes, during which participants were instructed to lie passively in the scanner and to refrain from thinking about anything specific.
2.3. Image preprocessing
Image preprocessing followed standard steps and was completed using AFNI (Cox, 1996) software. In order, images underwent despiking, slice timing correction, deobliquing, motion correction using rigid body alignment, alignment to participant’s normalized anatomical images, spatial smoothing using a 5 mm FWHM Gaussian filter, were passed through a low-frequency bandpass filter (0.01–0.1 Hz), and rescaled into percent signal change. Images were normalized using the MNI 452 template brain. Additionally, to correct for respiratory and cardiovascular artifacts, fluctuations in white matter voxels and CSF were regressed out of time courses from grey matter voxels following segmentation using FSL(Smith et al., 2004) and using restricted maximum likelihood to account for autocorrelation. This step was implemented directly after motion correction and normalization of the EPI images in the preprocessing stream. Resulting images for each participant were inspected for artifacts and accuracy of normalization. Eleven additional subjects were scanned but excluded from analyses (and demographics not reported) due to excessive head motion or uncorrectable scanner artifacts.
2.4. Independent Component Analysis
Independent Component Analysis (ICA) is a blind source separation technique first applied to neuroimaging by McKeown and colleagues(Calhoun et al., 2009; McKeown et al., 1998). ICA seeks to identify and isolate the independent signals from a recording of a mixture of signals (e.g., individual speaking voices recorded during a cocktail party) given little or no information about the signal(s). In the context of fMRI analyses, spatial ICA seeks to identify the higher-order latent spatial patterns (components) that explain temporal fluctuations in the voxels’ timecourses and, by providing voxel-wise coefficient loadings indicating the degree to which the component explains temporal fluctuations in each voxel, can identify spatially distributed and independent networks of temporal co-activation. We implemented the infomax algorithm for ICA within the widely used Group Independent Component Analyses for fMRI Toolbox (GIFT(Calhoun et al., 2001)). We implemented GIFT with the following additional parameters: group ICA (GICA) using the ICASSO method to assess component estimation reliability with 20 iterations, removing the mean per voxel as an initial preprocessing step, and dimension reduction using singular value decomposition with three reduction steps. One free parameter that must be selected when conducting ICA is the number of components for which to solve (Calhoun et al., 2009). We initially solved for 75 components (i.e., model order of 75) and then, based on the interpretability of identified networks (e.g., canonical components, such as default mode network and motor network, splitting into separate networks) and component separation indicated in the similarity matrices produced through ICASSO iterations, iteratively reduced the model order until a balance was reached between maximizing both interpretability and the number of components. The final ICA was conducted with a model order of 35. Individual spatial component maps for each participant were additionally computed in GIFT using the GICA back-reconstruction approach (Calhoun et al., 2001).
We chose a priori to focus on networks with peak component loadings in the prefrontal cortex in order to test hypotheses about the insular cortices functional integration into prefrontal networks.
2.5. Between-Group Comparisons of ICA Components
The back-reconstruction GICA approach provides a three-dimensional component map for each individual for each network. This component map indicates each voxel’s coefficient loading onto the component; that is, it indicates the degree to which each voxel is integrated into the network for that individual. The coefficient loading is indicated by a z-score, with greater z-scores indicating greater integration into the network. In order to test the hypothesis that cocaine dependence is associated with altered integration of the insular cortex into prefrontal networks, we conducted a series of two-sample t-tests in which each voxel within a component map is compared between cocaine dependent individuals and controls, with voxels compared separately for each component. This analysis provides a three-dimensional statistical map of t-scores indicating the degree to which the groups differed on each voxel, which indicates the degree to which the groups differ in the functional integration of that voxel into the network of interest. To control for multiple comparisons, we set a corrected alpha level of P < 0.05 by creating a cluster-size threshold of 17 contiguous voxels surviving an uncorrected voxel-level threshold of p < 0.005 (based on Monte Carlo simulations). All t-tests also included age, race, education, and biological sex as covariates.
We tested for areas of common group differences across the prefrontal networks with a conjunction analysis. In this analysis, we identify in how many different networks each voxel survives our statistical threshold for group differences. This analysis provides a three-dimensional statistical map indicating the percentage of all the networks tested in which the group’s differed for each voxel. While the focus of this study was on prefrontal networks, in order to test whether functional co-activation for the insular cortex was specifically altered in prefrontal networks versus generally altered across all networks identified by the ICA, we additionally compared the groups on all 15 of the non-prefrontal networks identified via ICA. For space reasons we do not report the results of group differences on every network and instead focus on just the prefrontal networks.
Finally, we supplemented the network-level analyses provided by the ICA with more focused seed map analyses of the effect of cocaine dependence on insula functional connectivity. We identified two regions of the right anterior/mid insula that differed between groups across the prefrontal networks (see below). Seed maps were created for each individual by extracting timecourses from each voxel with 4 mm spherical ROIs centered on these two insular cortex areas to generate seed regions and assessed voxel-wise those regions that exhibited correlated activity. Singular value decomposition was used to extract the timecourse component explaining the greatest proportion of variance in the voxels’ fluctuations. These two timecourses were simultaneously regressed onto the timecourses of all other voxels, providing partial regression coefficients of the relationship between the seed regions’ timecourses and each other voxel’s timecourse, and accounting for serial correlation using restricted maximum likelihood. The resulting seed maps for each individual were z transformed to improve normality. Univariate t-tests were used to compare connectivity with each voxel between groups with a corrected alpha level of P < 0.05 defined by a cluster-size threshold of 17 contiguous voxels surviving an uncorrected voxel-level threshold of p < 0.005 (based on Monte Carlo simulations). All t-tests also included age, race, education, and sex as covariates. These analyses identify specific functional connectivity between the seed regions and each voxel in the brain that might differ between groups. Whereas group differences in the ICA-derived components indicate altered connectivity of the insular cortex with functional networks, these seed map analyses identify altered connectivity of the seed regions with specific nodes. Thus, the seed map analyses supplement the ICA-based analyses by providing inferences regarding specific alterations in interregional functional connectivity.
3. Results
3.1. Prefrontal Networks Identified with ICA
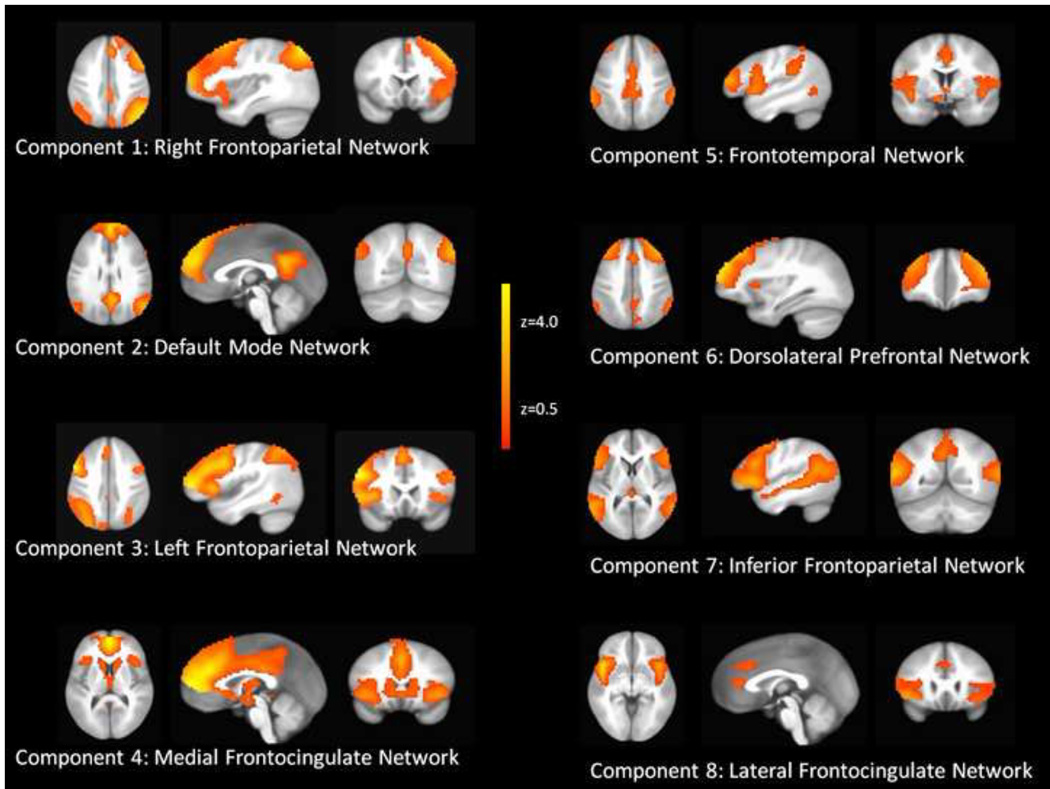
Of the 35 components obtained by the ICA, 12 were interpreted as artifacts (e.g., networks primarily in ventricles and/or white matter, motion artifacts, etc) and 23 were interpreted as meaningful (i.e., anatomically and functionally attributable) neural network components. Of these 23 networks, eight had peak voxel coefficient loadings in the PFC and were labeled as putative prefrontal networks. Figure 1 illustrates each of the eight prefrontal networks identified with ICA. Component 1 was consistent with a right frontoparietal network. Component 2 was consistent with the default mode network (Raichle et al., 2001). Component 3 was consistent with a left frontoparietal network. Component 4 consisted of medial PFC and bilateral anterior insular cortex (medial frontocingulate network). Component 5 consisted of cingulate, lateral PFC, parietal and temporal cortex (frontotemporal network). Component 6 consisted of mostly anterior lateral PFC and dorsal cingulate/dorsal medial PFC (dorsolateral PFC network). Component 7 consisted of bilateral IFG, dorsal lateral PFC, and parietal cortex (inferior frontoparietal network). Component 8 consisted of rostral and dorsal cingulate and bilateral insular cortex (lateral frontocingulate network). The remaining 15 networks were labeled as non-prefrontal-related networks and consisted of visual (dorsal and ventral networks), somatosensory, auditory and language, motor (primary motor and premotor cortices), limbic, basal ganglia, and brain stem networks.
Figure 1.
ICA component maps for the eight prefrontal-related independent components. The maps indicate the degree to which each voxel contributes to the prefrontal network of activation (colors represent z-scores), thresholded at z = 0.5.
3.2. Group Differences in Prefrontal Network Organization
Compared to healthy comparison subjects, cocaine addicts demonstrated altered integration of the insular cortex, and predominantly the right insular cortex, into each of the prefrontal networks. Table 2 provides coordinates of each area of the insular cortex exhibiting significant (P < 0.05, corrected) altered functional connectivity with the PFC networks from the voxel-wise comparison of group differences.
Table 2.
Anatomical coordinates of areas within the insular cortex showing group differences in functional connectivity with the PFC networks
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Component | Region | X | Y | Z | Peak voxel t value |
Size of cluster (number of voxels) |
| 1 ‘Right Frontoparietal Network’ | Left posterior insula | −52.5 | −13.5 | 17.5 | −4.32 | 44 |
| Right posterior insula | 34.5 | −19.5 | 23.5 | −4.44 | 29 | |
| 2 ‘Default Mode Network’ | Right mid-insula | 43.5 | −4.5 | −0.5 | −4.35 | 30 |
| Right mid-insula | 40.5 | 4.5 | 14.5 | −5.33 | 25 | |
| 3 ‘Left Frontoparietal Network’ | Right mid-insula | 42 | −3 | 0 | 4.93 | 80 |
| Left posterior insula | −31.5 | −31.5 | 20.5 | −4.75 | 25 | |
| 4 ‘Medial Frontocingulate Network’ | Right anterior insula | 40.5 | 4.5 | −9.5 | 4.63 | 45 |
| 5 ‘Frontotemporal Network’ | Right anterior insula | 37.5 | 16.5 | −6.5 | 4.12 | 39 |
| Right posterior insula | 43.5 | −7.5 | 8.5 | −5.18 | 20 | |
| Right mid-insula | 37.5 | 1.5 | 14.5 | −4.93 | 24 | |
| 6 ‘Dorsolateral Prefrontal Network’ | Left anterior insula | −43.5 | 22.5 | 5.5 | 4.66 | 64 |
| 7 ‘Inferior Frontoparietal Network’ | Left mid-insula | −34.5 | −4.5 | −3.5 | −5.03 | 83 |
| Left posterior insula | −49.5 | −25.5 | 11.5 | 5.71 | 64 | |
| Right posterior insula | 37.5 | −19.5 | 11.5 | 4.32 | 41 | |
| 8 ‘Lateral Frontocingulate Network’ | Right mid-insula | 43.5 | −4.5 | 2.5 | −6.57 | 106 |
| Left Anterior insula | −40.5 | 10.5 | −9.5 | −5.86 | 103 | |
Note. Coordinates provided in MNI space. Positive t values indicate greater integration into the network among cocaine addicts; negative t values indicate greater integration into the network among control participants. Size of voxels = 3×3×3mm.
3.3. Common Brain Areas of Group Differences across the Prefrontal Networks
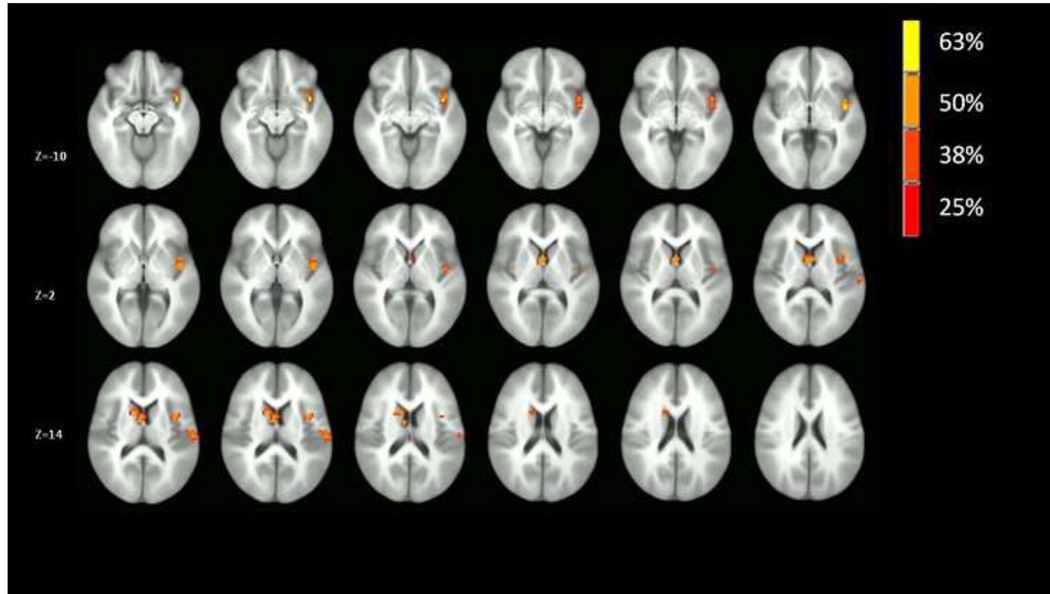
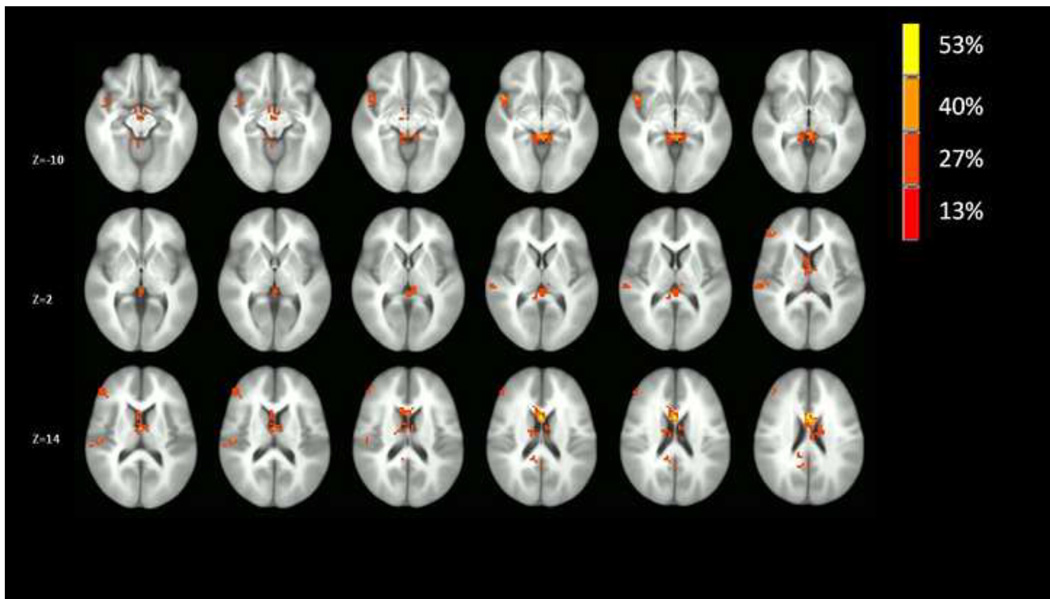
Results of the voxel-wise conjunction analyses are provided in Figures 2–3. As can be seen in Figure 2, the right insular cortex was the predominant site of common group differences in co-activation across the prefrontal networks. As indicated in Figures 2, the right mid-insula and right ventral anterior insula were two sites with distinctly high degrees of common group differences across the prefrontal networks. Further, there were no other regions across the brain that demonstrated a comparable degree of common group differences in co-activation across the prefrontal networks, though network co-activation in the left caudate nucleus did differ between groups in 50% of the prefrontal networks. By contrast, as indicated in Figure 3, there was no comparable commonality of group differences in co-activation across non-prefrontal networks observed for the insular cortex, which suggests a selectivity of group differences in insular cortex engagement with prefrontal networks.
Figure 2.
Conjunction analysis indicating the extent of conjoint co-activation (percentage of the eight prefrontal networks in which the group’s differed on each voxel). The map is thresholded at 25% (2 or more conjoint co-activations out of 8 networks) with a cluster threshold of 17 contiguous voxels (P < .05 corrected) to illustrate the anatomical location of voxels that have common group differences across the networks.
Figure 3.
Voxel-wise conjunction analysis indicating the extent of conjoint co-activation (percentage of the 15 non-prefrontal-related networks in which the group’s differed on each voxel). The map is thresholded at 13% (2 or more activations out of 15 networks) with a cluster threshold of 17 contiguous voxels (P < .05 corrected) to illustrate the anatomical location of voxels that have common group differences across networks.
3.4. Follow-up Between-Group Differences of Functional Connectivity with Right Insular Cortex
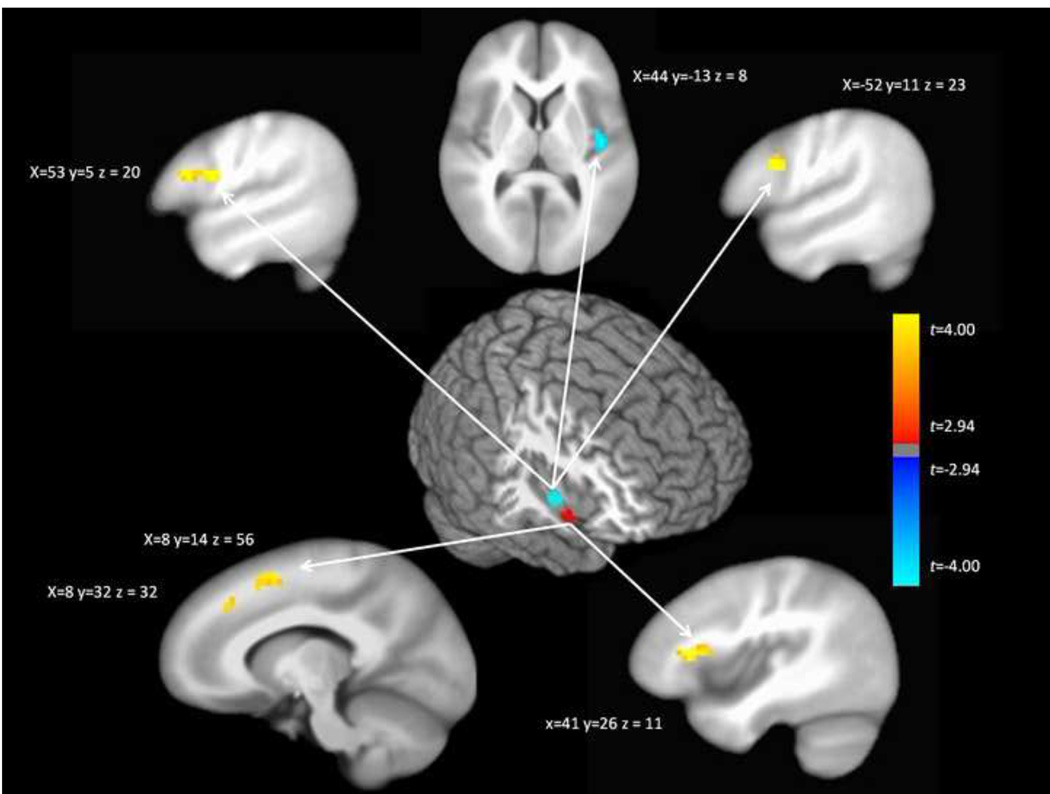
Given the strong evidence for altered functional connectivity of the right ventral anterior insula and right dorsal insula among cocaine dependent participants, we conducted follow-up tests of seed-based functional connectivity comparing connectivity with these two regions between cocaine dependent and control participants. Seeds consisted of 4 mm spherical ROIs centered on the peak areas (right ventral anterior insula – X = 40, Y = 5, Z = −9; right dorsal insula – X = 44, Y = −1, Z = −1) of common group differences across the prefrontal networks (Figures 2 and 3). As indicated in Figure 4, these analyses revealed several sites of altered connectivity with the seed regions among cocaine addicts. Notably, cocaine dependent participants demonstrated greater connectivity of the right ventral anterior insula with the right inferior frontal gyrus and dorsomedial PFC. Cocaine dependent participants also demonstrated greater connectivity of the right dorsal insula with bilateral dorsolateral PFC and weaker connectivity with the dorsal posterior insular cortex.
Figure 4.
Between-group differences in seed maps of connectivity with the right anterior insula (bottom) and right mid/posterior insula (top). The figure illustrates the ROIs in the insula (either ventral anterior insula or dorsal anterior/mid insula) and anatomical sites where functional connectivity with these ROIs differences among cocaine addicts. Warmer colors in the between-group difference maps indicate regions with greater connectivity with the seed region among cocaine addicts; cooler colors indicate regions with weaker connectivity with the seed region among cocaine addicts. The maps are thresholded at P < .05 corrected.
3.5. Supplemental Analyses
Given that the cocaine dependent individuals differed from the control group in age, sex, minority status, and education, we conducted additional conjunction analyses in which we tested the unique effects of age, sex, minority status, and education to test the alternative hypothesis that our observed findings with the insula were actually due to these group differences in demographics. As can be seen in Supplemental Figures 1–4, alternative group differences in these demographic characteristics do not explain the observed findings regarding insular cortex connectivity.
4. Discussion
The present results support the hypothesis that cocaine dependence is related to altered interactions of the insular cortex with prefrontal neural networks. We used resting-state fMRI data and ICA to identify spatially independent prefrontal networks, and supplemented these analyses with seed map connectivity analyses that identify specific individual nodes with altered connectivity with the seed regions. Networks identified during resting-state scans correspond to both task-related patterns of activations (Smith et al., 2009) and structural connectivity pathways (van den Heuvel et al., 2009). Thus, resting-state functional connectivity is theorized to indicate intrinsic functional connectivity pathways (Deco et al., 2011) and is ideally suited for testing hypotheses about altered intrinsic (i.e., not dependent on task demands) connectivity pathways among patient groups. We identified eight independent components of activation with peak loadings in the prefrontal cortex and demonstrated that cocaine dependence was associated with an altered engagement of the insular cortex, predominantly the right insular cortex, with each of the independent prefrontal networks. No other neural region demonstrated similar commonalities in group differences across the networks, and the insular cortex did not demonstrate commonalities in group differences in co-activation for non-prefrontal-related networks. Similarly, the seed map analyses demonstrated stronger connectivity of the ventral anterior insula with the right inferior frontal gyrus and dorsomedial PFC, and of the mid/posterior insula with bilateral dlPFC and dorsal posterior insular cortex, which provides corroborating and extending evidence for altered interactions of the right insular cortex with prefrontal networks in cocaine dependence.
The current results have implications for models of cocaine dependence. Prevailing theories posit that two processes are largely responsible for mediating drug use disorders: heightened motivational/incentive salience of drug cues and decreased cognitive control ability to modulate response tendencies elicited by drug cues (Garavan and Hester, 2007; Goldstein and Volkow, 2002; Robinson and Berridge, 2001, 2008). A more recent theory is that interoception links these two processes, such that changes in motivational states and subjective awareness of these states biases the cognitive control of response tendencies towards drug use (Naqvi and Bechara, 2009, 2010). This model has largely emerged from an influential study demonstrating that damage to the insular cortex, but not to other neural regions, was associated with remarkable ease of smoking cessation (Naqvi et al., 2007). These lesion effect findings are consistent with prior imaging studies demonstrating insular cortex activation among individuals with substance use disorders in drug cue-induced craving studies (Garavan et al., 2000; Kilts et al., 2004). Based on this theoretical model of the importance of interoceptive processes in drug use disorders, it would be expected that the functional connectivity of the insular cortex with prefrontal networks would be altered among cocaine addicts. Our results support this possibility and demonstrate widespread altered integration of the insular cortex with distributed prefrontal networks as well as specific anatomical nodes (e.g., dlPFC, dorsal ACC, etc) believed to mediate cognitive control processes.
Though there was evidence of altered integration of the insular cortex across prefrontal networks among cocaine dependent individuals, there was variability in the direction and the anatomical location of the effect. With the exception of component 8 (‘lateral frontocingulate network’), those prefrontal networks demonstrating significantly altered functional connectivity with the ventral anterior insula (i.e., components 4 ‘medial frontocingulate network’, 5 ‘frontotemporal network’ and 6 ‘dorsolateral prefrontal network’) in individuals with cocaine use disorders exhibited increased insula connectivity (Table 2). Given the role of the ventral anterior insula in salience detection and awareness, increased ventral anterior insula integration into these prefrontal networks could perhaps indicate greater biasing of decision-making processes mediated by these prefrontal networks by heightened awareness or detection of drug cues or internal craving-related bodily states. This interpretation is consistent with the disruption of nicotine dependence in individuals following insula damage (Naqvi et al., 2007). The cocaine dependence-related reduction in anterior insula functional connectivity with the anterior cingulate cortex component (component 8 ‘lateral frontocingulate network’) is consistent with lesser integration of conscious awareness within conflict monitoring processes, possibly interfering with adequate ability to divert attention away from distracting drug cues. The observation of greater or lesser integration of the ventral anterior insula among individuals with cocaine use disorders depending on the specific prefrontal network suggests a nuanced relationship between ventral anterior insula connectivity with prefrontal networks and highlights the importance of defining large-scale networks that facilitates detection of these sorts of nuanced relationships.
By contrast, with the exception of component 7 (‘inferior frontoparietal network’), those prefrontal components demonstrating significantly altered functional connectivity with the posterior insula (i.e., components 1 ‘right frontoparietal network’, 3 ‘left frontoparietal network’, and 5 ‘frontotemporal network’) in individuals with cocaine use disorders exhibited decreased insula connectivity (Table 2). Given the hypothesized role of the posterior insula in coding bodily state changes, these brain patterns may suggests that cocaine use disorders are associated with less integration of bodily state information into these latter networks, and an enhanced role of such information related to the inferior frontoparietal network. A diminished role of somatic states in influencing performance monitoring and attention – functions associated with the frontoparietal and frontotemporal networks - is consistent with observed deficits in error processing (Franken et al., 2007) and the automatic nature of the attentional bias effect for drug use reminders in individuals with cocaine use disorders (Liu et al., 2011).
The seed map analyses corroborate these interpretations by demonstrating stronger connectivity in cocaine dependent individuals of the ventral anterior insula with the right inferior frontal gyrus and dorsomedial PFC, regions critically involved in response inhibition (Aron et al., 2003; Aron et al., 2004) and cognitive conflict/error monitoring (Botvinick et al., 2001; Kerns et al., 2004; Modirrousta and Fellows, 2008). Similarly, stronger connectivity was observed among cocaine addicts of the mid/posterior insula with bilateral dlPFC, regions critically involved in cognitive control (Botvinick et al., 2001; Kerns et al., 2004). These specific interregional differences in functional connectivity associated with cocaine dependence provide inferences as to how insula function becomes more involved with cognitive control processes presumably mediated by the prefrontal cortex. For example, perhaps the anterior insula’s specific heightened connectivity with the inferior frontal gyrus mediates the overall greater integration of the anterior insula into component 4 ‘medial frontocingulate network’. Similarly, perhaps the mid/posterior insula’s specific heightened connectivity with bilateral dlPFC mediates mid/posterior insula’s greater integration into component 3 ‘left frontoparietal network’.
Prior investigations of the insular cortex functional connectivity among healthy populations demonstrated that both the anterior insular cortex (including both ventral and dorsal anterior insula) were connected with cognitive control regions, including dorsal ACC and lateral PFC (Taylor, et al., 2009; Cauda et al., 2010; Deen et al., 2011;). Here, we observed that connectivity of both the anterior and dorsal insula were more tightly connected with dorsal ACC/dmPFC and lateral PFC among cocaine dependent participants, indicating that normative connection strength is actually enhanced among this population. By contrast, whereas ventral and dorsal anterior insula demonstrate positive connections across the insula, here we observed that cocaine dependent participants had decreased connectivity between dorsal insula and posterior insula, suggesting weaker than normal connection strength and further highlighting insula connectivity differences among cocaine dependent populations. This pattern of altered connectivity suggests greater functional segregation within the insular cortex among cocaine dependent individuals. That is, the insular cortex is functionally heterogeneous (Craig, 2002, 2009; Deen et al., 2011; Jonides et al., 1998), and the observed diminished functional connectivity of the anterior/mid insula with the dorsal posterior insula suggests poorer ability to integrate information between different regions of the insula among cocaine addicts. This finding supports the association of cocaine dependence with wide dysfunction of connectivity involving the right insular cortex.
This is the first study, to our knowledge, to explicitly test the hypothesis of altered interactions between the insular cortex and prefrontal networks among cocaine addicts. While we provide support for the emerging view of interoceptive processes in drug use disorders, this study is not without limitations. First, the use of resting-state data in the absence of task-data precludes the ability to link the identified networks with specific cognitive functions. As such, the current results must be replicated with task data to support the current inferences. Second, the groups differed in age, gender, education, and race, and while we used these variables as covariates in our analyses, it is nonetheless important to test whether these results generalize to more specific and homogenous populations. Third, the cocaine addicts in this study had variable years, frequency, and recency of drug use and it cannot be determined with certainty the degree of possible drug intoxication or withdrawal the participant was experiencing during the scan. Thus, it cannot be determined the degree to which the current results are due to effects of cocaine use per se, cocaine dependence vulnerability (i.e., pre-morbid risk factors), or consequences of chronic cocaine use (i.e., postmorbid correlates). Future research is necessary to further explore the implications of these results for models of interoception and the insular cortex in drug use disorders.
Nonetheless, the present study suggests altered integration of the insular cortex into large-scale prefrontal networks among cocaine dependent individuals, with specific anatomical sites of altered functional integration including the ventral anterior insula and dorsal anterior/mid insula. The observation of altered functional connectivity with these large-scale networks suggests altered integration of the awareness, salience detection, and interoceptive functions mediated by the insula into cognitive control and decision-making processes mediated the prefrontal networks among cocaine dependent individuals. Further research is necessary to specifically delineate the behavioral correlates of these altered patterns of functional connectivity as well as their prospective relationships with drug use and treatment response.
Supplementary Material
Supplemental Figure 1. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with sex.
Supplemental Figure 2. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with minority status.
Supplemental Figure 3. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with education.
Supplemental Figure 4. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with age.
Acknowledgements
Portions of this project were supported by Award Number UL1RR029884 and KL2RR029883 from the National Center for Research Resources, and RO1DA019999, R21DA025243, and T32DA022981 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no financial conflicts of interest.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50:1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Struct Funct. 2010;214:645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, 3rd, Nelson B, Bell C, Mueller BA, Specker S, Lim KO. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69:1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer Robert L, Gibbon Miriam, Williams Janet BW. Biometrics Research. New York: New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37:117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu X-M, Li N, Wang C-X, Zhang H, Qian R-B, Xu H-S, Hu X, Zhang D-R. Abnormal Brain Default-Mode Network Functional Connectivity in Drug Addicts. PLoS ONE. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, Lee TW, Sejnowski TJ. Spatially independent activity patterns in functional MRI data during the stroop color-naming task. Proc Natl Acad Sci U S A. 1998;95:803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 2009;47:1666–1677. doi: 10.1016/j.neuroimage.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, Strohle A, Heinz A, Northoff G, Bermpohl F. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. Neuroimage. 2010;50:1606–1617. doi: 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Rockville, MD: Office of Applied Studies; 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. NSDUH Series H-38A, HHS Publication No. SMA 10–4586Findings. [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with sex.
Supplemental Figure 2. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with minority status.
Supplemental Figure 3. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with education.
Supplemental Figure 4. Conjunction analysis indicating the extent of conjoint co-activation in the eight prefrontal networks associated with age.