Abstract
Background:
Tumours arising in younger women appear to be biologically more aggressive and tend to have a poorer outcome. Being relatively resistant to conventional treatments, breast cancer stem cells (CSCs) have been postulated as a possible cause of disease recurrence after treatment. In this study, we used ALDH1 as a CSC marker and determined whether ALDH1 expression correlated with clinical outcome in young women with breast cancer.
Methods:
The expression of ALDH1 was evaluated through immunohistochemistry on microarrayed cores obtained from 141 consecutive patients up to 35 years of age.
Results:
The expression of ALDH1 was observed in 25% (35 of 141) of tumours, in a median of 5% of cells. Younger women were 14 times more likely to have ALDH1-positive tumours (P<0.01, OR 14.4, 95% CI 4.34–48.09). The ALDH1 correlated independently with ER negativity (P=0.01, OR 0.33, 95% CI 0.15–0.77). There was no correlation with disease recurrence or breast cancer-related deaths.
Conclusion:
In younger women, ALDH1 was more highly expressed, and it correlated with ER negativity. It, however, did not predict survival in this study.
Keywords: ALDH1, breast cancer, young women
Breast cancers arising in young women have a comparatively poorer outcome (El Saghir et al, 2006). These tumours are often of high tumour grade, with lymphovascular invasion and nodal involvement, and a significant number are triple negative, lacking in oestrogen receptor (ER), progresterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) expression (Fernandopulle et al, 2006; Bauer et al, 2007). Triple-negative tumours have a propensity for early and rapidly progressive metastatic recurrence and, as a group, respond less favourably to chemotherapy (Dent et al, 2007; Liedtke et al, 2008; Tan et al, 2008).
The relative abundance of breast cancer stem cells (CSCs) in triple-negative tumours may be a possible explanation (Honeth et al, 2008; Idowu et al, 2012). The CSCs are largely quiescent and thus less susceptible to conventional treatments. Aberrant expression of ATP-cassette binding transporters, anti-apoptotic factors and DNA repair enzymes further contribute to chemoresistance (Phillips et al, 2006; Shi and Harris, 2006). Aldehyde dehydrogenase 1 (ALDH1), identified as a breast CSC marker, has been associated with chemoresistance and poor prognosis (Pearce et al, 2005; Ginestier et al, 2007; Tanei et al, 2009). As ALDH1 was reportedly upregulated in triple-negative tumours and tumours arising in African women, which share several similarities with those arising from young women, we hypothesise that a relative abundance of ALDH1-positive CSCs may account for the poor outcome in younger women despite aggressive treatment (Ginestier et al, 2007; Nalwoga et al, 2010; Zhou et al, 2010; Ohi et al, 2011; Idowu et al, 2012). In this study, we used ALDH1 to identify the breast CSC subpopulation and evaluated whether ALDH1 expression correlated with clinical outcome.
Materials and methods
Patient and tumour characteristics
Microarrayed tissue comprising 1 mm cores were collected from 141 consecutive women ⩽35 years old who underwent surgery at the Singapore General Hospital from 1993 to 2009. Two separate cores were obtained from each tumour specimen; necrotic regions were avoided. This study has Ethical Committee approval (2011/433/B). Median patient age was 32 years (range 19 to 35 years). Although the ethnic make-up was reflective of the local population, with Chinese women accounted for the majority (67%), these younger women were 4 times more likely to be Malay as compared with those diagnosed after 35 years of age (P<0.01, OR 3.90, 95% CI 2.08–7.31). The majority of tumours (128 of 141, 91%) were classified as invasive ductal carcinoma of no special type (Table 1). Median pathological tumour size was 22.5 mm (range 5 to 155 mm), and median tumour grade, according to the modified Bloom and Richardson criteria, was 3. In all, 43% of tumours had nodal involvement; 55% were ER positive, 53% PR positive and 21% HER2 positive. All women underwent curative surgery and received adjuvant therapy according to the NCCN guidelines. None received neoadjuvant treatment. Over the follow-up period (median 70 months, range 18 to 221 months), 49 patients developed recurrent disease (13 with local recurrence alone) and 27 died of breast cancer-related causes.
Table 1. Comparison of standard clinicopathological parameters in women >35 years of age and those ⩽35 years of age (n=286).
| Women >35 years of age (n=145) | Women ⩽35 years of age (n=141) | P-value | |
|---|---|---|---|
| Ethnicity |
|
|
<0.01 |
| Chinese | 129 | 95 | |
| Malay | 8 | 20 | |
| Indian | 2 | 7 | |
| Others |
6 |
19 |
|
| Tumour histology |
|
|
0.75 |
| IDC | 130 | 128 | |
| Non-IDC |
15 |
13 |
|
| Median pathological tumour size (mm) |
22.5 (5.0–115.0) |
28.0 (2.0–190.0) |
0.05 |
| Tumour grade |
|
|
<0.01 |
| 1 | 23 | 11 | |
| 2 | 51 | 43 | |
| 3 |
63 |
86 |
|
| Lymphovascular invasion |
|
|
<0.01 |
| 115 | 47 | ||
| Present | 30 | 94 | |
| Absent |
|
|
|
| Nodal status |
|
|
0.25 |
| Positive | 64 | 44 | |
| Negative |
63 |
59 |
|
| ER status |
|
|
<0.01 |
| Positive | 116 | 77 | |
| Negative |
29 |
64 |
|
| PR status |
|
|
0.81 |
| Positive | 72 | 72 | |
| Negative |
73 |
69 |
|
| HER2 status |
|
|
0.90 |
| Positive | 32 | 32 | |
| Negative | 113 | 109 |
Abbreviations: ER=oestrogen receptor; HER2=human epidermal growth factor receptor-2; IDC=invasive ductal carcinoma; Non-IDC=tumour histologies other than invasive ductal carcinoma; PR=progesterone receptor.
Comparison was made with 145 women >35 years old diagnosed during the same period. Median age of this group was 67 years (range 36 to 89 years). Details are shown in Table 1. Similarly, the majority of women were of Chinese ethnicity (129 of 145, 89.0%), Most tumours were classified histologically as being of invasive ductal carcinoma of no special type (130 of 145, 89.6%), Median tumour size was 28 mm (range 2 to 190 mm), and median tumour grade was 2. Compared with tumours arising in younger women, these tumours were more likely to be of lower tumour grade and ER positive (P<0.01 and P<0.01, OR 3.33, 95% CI 1.97–5.62 respectively). However, these tumours were found more likely to have lymphovascular invasion (P<0.01, OR 7.67, 95% CI 4.50–13.07). Recurrence occurred in 33 patients (local recurrence alone in 4) and 32 patients died from breast cancer-related causes.
Immunohistochemistry
The expressions of ALDH1, ER, PR and HER2 were evaluated by immunohistochemistry. Primary antibodies used included rabbit monoclonal ALDHA1 antibody (clone EP1933Y, 1 : 100 dilution, Abcam, Cambridge, UK), ER antibody (clone SP1, 1 : 50 dilution, RM-9101-R7), PR antibody (clone PgR636, 1 : 200 dilution, Dako M3569, Glostrup, Denmark) and HER2 antibody (clone SP3, 1 : 200 dilution, RM-9103-R7). Briefly, 4 μm-thick sections were dewaxed in xylene and rehydrated in graduated ethanol solutions. Sections were subjected to heat-induced antigen retrieval (0.01 ℳ Tris-0.001 M EDTA, pH 9, at 98 °C in a microwave) and then run on the Dako Autostainer Plus. The Dako Envision Detection kit (K5007) was used and slides were counterstained in Mayer's haematoxylin (Dako S3309). Normal liver was used as positive control. The ALDH1 staining was scored with respect to staining intensity (0=no staining, 1=weak staining, 2=moderate staining and 3=strong staining) and the percentage of tumour cells stained. Various thresholds for positive staining have been reported (Ginestier et al, 2007; Chang et al, 2009; Nalwoga et al, 2010; Sullivan et al, 2010). Tumours with positive staining in the cytoplasm in >10% of cells were considered positive in this analysis. For ER and PR, nuclear staining in at least 1% of tumour cells was considered positive; for HER2, strong membranous staining in at least 30% of tumour cells was considered positive.
Statistical methods
Correlation analyses were made using the χ2 test or Fisher's exact test with GraphPad Prism version 4 (GraphPad software Inc., San Diego, CA, USA). Cox proportional hazard regression model was performed with the Stata package release 8.1 (Stata Corporation, College Station, TX, USA). A two-tailed P-value test was used and a P-value of <0.05 was considered statistically significant.
Results
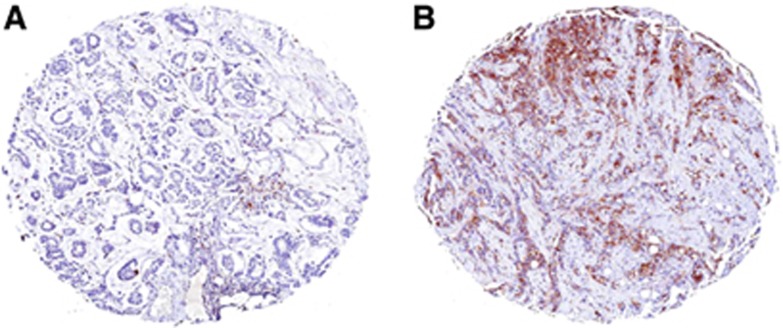
Of the 141 tumours, 35 (25%) stained positive for ALDH1 (Figure 1). Median staining intensity was weak (score 1), and ALDH1 staining was completely absent in 69 tumours (49%). In most tumours, ALDH1 staining was observed only in a median of 5% of tumour cells and only 7 tumours had >50% of cells staining positive for ALDH1. The ALDH1 was observed mainly in the cytoplasm. Scattered foci of staining were observed in the stroma, although nuclear staining was largely absent. Among the 145 women >35 years old, only 3 tumours stained positive for ALDH1. Intensity of staining was weak (score 1) in all cases and 10% to 20% of cells stained positive for ALDH1. Young women were 14 times more likely to have ALDH1-positive tumours (P<0.01, OR 14.4, 95% CI 4.34–48.09).
Figure 1.
Immunohistochemistry of ALDH1 in invasive breast carcinoma. (A) negative staining; (B) positive cytoplasmic staining in >10% of tumour cells.
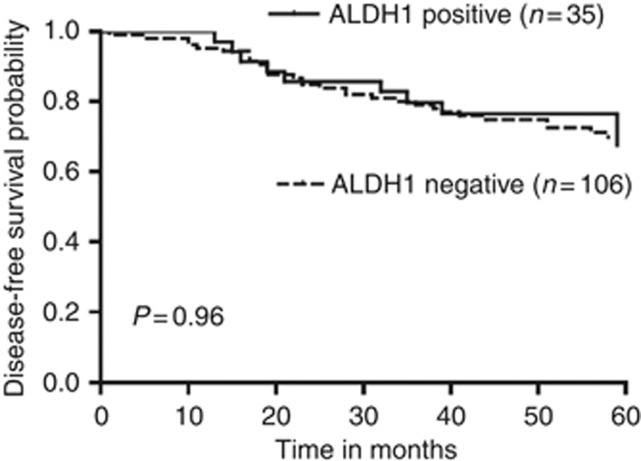
Among the young women, tumours expressing ALDH1 were 3 times more likely to be ER negative (P<0.01, OR 3.03, 95% CI 0.15–0.73) and PR negative (P<0.01, OR 3.52, 95% CI 0.12–0.65; Table 2). The ALDH1-positive tumours tended to be smaller, of high tumour grade and HER2 positive, but these did not reach statistical significance (P=0.11, P=0.24 and P=0.62, respectively). There was no correlation with age, ethnicity, tumour histology, lymphovascular invasion and nodal status (P>0.05). On multivariate analysis, ER negativity was independently correlated with ALDH1 expression (P=0.01, OR 0.33, 95% CI 0.15–0.77; Table 3). The expression of ALDH1 did not correlate with disease recurrence, whether local or distant or breast cancer-related deaths, and neither was there an association with the 5-year disease-free survival (P>0.05; Table 2 and Figure 2).
Table 2. Correlation analyses of ALDH1 expression and standard clinicopathological parameters and outcome (n=141).
| ALDH1 positive (n=35) | ALDH1 negative (n=106) | P-value | |
|---|---|---|---|
| Median age (years) |
33 (23–35) |
32 (19–35) |
0.76 |
| Ethnicity |
|
|
0.71 |
| Chinese | 22 | 73 | |
| Malay | 5 | 15 | |
| Indian | 3 | 4 | |
| Others |
5 |
14 |
|
| Tumour histology |
|
|
0.06 |
| IDC | 32 | 96 | |
| ILC | 0 | 2 | |
| Mucinous carcinoma | 0 | 7 | |
| Medullary carcinoma |
3 |
1 |
|
| Median pathological tumour size (mm) |
20.0 (9.0–100.0) |
26.0 (5.0–155.0) |
0.11 |
| Tumour grade |
|
|
0.24 |
| 1 | 1 | 10 | |
| 2 | 10 | 33 | |
| 3 |
23 |
63 |
|
| Lymphovascular invasion |
|
|
0.49 |
| Present | 10 | 37 | |
| Absent |
25 |
69 |
|
| Nodal status |
|
|
0.54 |
| Positive | 11 | 33 | |
| Negative |
18 |
41 |
|
| ER status |
|
|
<0.01 |
| Positive | 12 | 65 | |
| Negative |
23 |
41 |
|
| PR status |
|
|
<0.01 |
| Positive | 10 | 62 | |
| Negative |
25 |
44 |
|
| HER2 status |
|
|
0.62 |
| Positive | 9 | 23 | |
| Negative |
26 |
83 |
|
| Recurrence |
|
|
0.39 |
| Yes | 11 | 42 | |
| No |
24 |
64 |
|
| Death |
|
|
0.40 |
| Yes | 5 | 22 | |
| No |
30 |
84 |
|
| Median 5-year disease-free survival (months) | 56.0 (13.0–60.0) | 57.0 (10.0–60.0) | 0.96 |
Abbreviations: ALDH1=aldehyde dehydrogenase 1; ER=oestrogen receptor; HER2=human epidermal growth factor receptor-2; IDC=invasive ductal carcinoma; ILC= invasive lobular carcinoma; PR=progesterone receptor.
Table 3. Cox regression analysis stratifying by ALDH1 expression (n=139).
| Parameter | Odds ratio | s.e. | P-value | 95% Confidence interval |
|---|---|---|---|---|
| ER status |
0.33 |
0.14 |
0.01 |
0.15–0.77 |
| HER2 status |
1.13 |
0.54 |
0.79 |
0.45–2.88 |
| Tumour grade |
2.67 |
2.95 |
0.38 |
0.30–23.34 |
| Tumour size | 0.99 | 0.01 | 0.36 | 0.97–1.01 |
Abbreviations: ALDH1=aldehyde dehydrogenase 1; ER=oestrogen receptor; HER2=human epidermal growth factor receptor-2.
Figure 2.
Kaplan and Meier curves of disease-free survival stratifying patients by cytoplasmic ALDH1 expression.
Discussion
In this study, we have found ALDH1 expression to be more common in tumours arising in younger women up to 35 years of age. A recent study also reported similar findings (Mieog et al, 2012). Other studies have not found such a correlation, but variations in age stratifications preclude direct comparisons (Gong et al, 2010; Nalwoga et al, 2010). Preferential upregulation of ALDH1 may account for the biological differences between tumours in younger and older postmenopausal women. Genes involved in stem cell biology were among the genes found to be differentially expressed among young women in a large multicentric genomic analysis that included data from Singapore (Anders et al, 2008). We chose to focus on ALDH1 expression because ALDH1 identifies a highly tumourigenic subpopulation; tumours could be generated with as few as 20 ALDH1-positive CD44+/CD24− cells, whereas a similar effect was not observed even with more than 50 000 ALDH1-negative CD44+/CD24− cells (Ginestier et al, 2007).
In our study, we too observed that ALDH1-positive cells constituted only a small portion of the tumour population (Ginestier et al, 2007). The association with hormone unresponsiveness has been reported previously (Ginestier et al, 2007; Nalwoga et al, 2010; Zhou et al, 2010; Tsang et al, 2012). Although ER- and PR-negative tumours tend to be more aggressive, ALDH1 expression did not correlate with recurrence or death, and failed to predict survival, in our study. A recent report found CSCs, identified using ALDH1 and CD44/CD24 expression, to identify a poor prognostic subgroup among luminal-type cancers (Tsang et al, 2012). Several other studies have reported a poor outcome in ALDH1-rich tumours, but none of these studies stratified patients by age (Ginestier et al, 2007; Tanei et al, 2009; Resetkova et al, 2010; Zhou et al, 2010). The only study that reported an age-dependent effect of ALDH1 expression on prognosis stratified patients by whether they were <65 or >65 years of age (Mieog et al, 2012). It is possible that ALDH1 alone is not sufficient for risk stratification among an already high-risk group such as young women. Furthermore, the antibody used in our study is specific for the ALDH1A1 isoform. Although ALHDH1A1 is the most commonly evaluated, 16 other isoforms have been identified and the clinical significance of these is uncertain (Sladek, 2003; Marcato et al, 2011a).
The hypothesis that disease recurrence arises from CSCs that elude conventional chemotherapeutic and irradiation treatments remains an attractive one. However, much remains to be understood about CSCs. One study reported that high ALDH1 expression in tumour-associated stromal cells was associated with a good outcome in triple-negative tumours (De Brot et al, 2012). The ALDH1 may have other cellular functions that differ according to cancer type, as seen in ovarian cancer where ALDH1 expression was associated with a favourable outcome instead (Chang et al, 2009). Although it is unlikely a coincidence that CSCs are often found to be enriched in poor-risk tumours, it is perhaps oversimplistic to assume that the mere presence of CSCs can account for the more aggressive phenotype and poorer clinical outcome. It is likely that the mechanisms that trigger CSCs re-activation have a greater impact on tumour behaviour and outcome. In fact, CSCs may be the solitary dormant cells in cell cycle arrest (Naumov et al, 2002; Allan et al, 2006). In this quiescent state, no proliferation occurs and no disease is clinically evident. It remains unclear what triggers an exit from cell cycle arrest into the proliferative phase that leads to tumour regrowth. The multitude of genes other than those involved in stem cell biology that were identified in tumours in younger women suggest that the mechanisms involved are far more complex than can be explained by a single factor such as CSCs (Anders et al, 2008).
Conclusion
In conclusion, we observed that ALDH1 expression was more common in younger women and was independently correlated with ER negativity. However, ALDH1 expression did not predict for disease recurrence or death in these women. Further studies to elucidate the extent of CSC involvement in tumours in younger women and to elucidate the mechanisms that regulate dormancy and cell cycling in CSCs will provide us with a better understanding of tumour biology and facilitate the development of novel therapeutic targets.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98. doi: 10.3233/bd-2007-26108. [DOI] [PubMed] [Google Scholar]
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- Chang B, Liu G, Xue F, Rosen DG, Xiao L, Wang X, Liu J. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22:817–823. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brot M, Rocha RM, Soares FA, Gobbi H. Prognostic impact of the cancer stem cell related markers ALDH1 and EZH2 in triple negative and basal-like breast cancers. Pathology. 2012;44:303–312. doi: 10.1097/PAT.0b013e3283534bcb. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, Shamseddine AI. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandopulle SM, Cher-Siangang P, Tan PH. Breast carcinoma in women 35 years and younger: a pathological study. Pathology. 2006;38:219–222. doi: 10.1080/00313020600699268. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Yao H, Liu Q, Chen J, Shi J, Su F, Song E. Markers of tumor-initiating cells predict chemoresistance in breast cancer. PLoS One. 2010;5:e15630. doi: 10.1371/journal.pone.0015630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, Manjili MH. CD44(+)/CD24(-/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364–373. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011a;10:1378–1384. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011b;29:32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- Mieog JS, de Kruijf EM, Bastiaannet E, Kuppen PJ, Sajet A, de Craen AJ, Smit VT, van de Velde CJ, Liefers GJ. Age determines the prognostic role of the cancer stem cell marker aldehyde dehydrogenase-1 in breast cancer. BMC Cancer. 2012;12:42. doi: 10.1186/1471-2407-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer. 2010;102:369–375. doi: 10.1038/sj.bjc.6605488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC, Chambers AF. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai Y, Sagara Y, Tanimoto A. Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology. 2011;59:776–780. doi: 10.1111/j.1365-2559.2011.03884.x. [DOI] [PubMed] [Google Scholar]
- Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA, Bonnet D. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23:752–760. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, Badve S. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11:41–52. doi: 10.1007/s10911-006-9011-7. [DOI] [PubMed] [Google Scholar]
- Sladek NE. Human aldehyde dehydrogenases: potential pathological, pharmacological, and toxicological impact. J Biochem Mol Toxicol. 2003;17:7–23. doi: 10.1002/jbt.10057. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, Xie Y, Scaglioni PP, DiMaio JM, Gazdar AF, Shay JW, Wistuba II, Minna JD. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DS, Marchio C, Jones RL, Savage K, Smith IE, Dowsett M, Reis-Filho JS. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat. 2008;111:27–44. doi: 10.1007/s10549-007-9756-8. [DOI] [PubMed] [Google Scholar]
- Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- Tsang JY, Huang YH, Luo MH, Ni YB, Chan SK, Lui PC, Yu AM, Tan PH, Tse GM. Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;136:407–417. doi: 10.1007/s10549-012-2271-6. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jiang Y, Yan T, Di G, Shen Z, Shao Z, Lu J. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat. 2010;122:795–801. doi: 10.1007/s10549-010-0999-4. [DOI] [PubMed] [Google Scholar]