Abstract
In experimental models of glomerular and nonglomerular renal disease, single nephron filtration rate and proximal tubular reabsorption of fluid decrease or increase in parallel in the same nephron. To assess whether intrinsic adaptations in proximal tubular function, i.e., changes that are independent of the peritubular or humoral milieu, contribute to this phenomenon, segments of rabbit late superficial proximal convoluted tubules (PCT) were studied by in vitro perfusion. PCT were obtained from normal kidneys, from remnant kidneys, and from kidneys embolized with microspheres. Single nephron filtration rates are increased in the remnant and decreased in the embolized kidneys. Whereas the embolized-kidney rabbits were nonazotemic (the contralateral kidney was left in situ), the remnant-kidney animals were uremic. In order to study a nonazotemic model of increased single nephron filtration rate, PCT were also obtained from uninephrectomized rabbits.
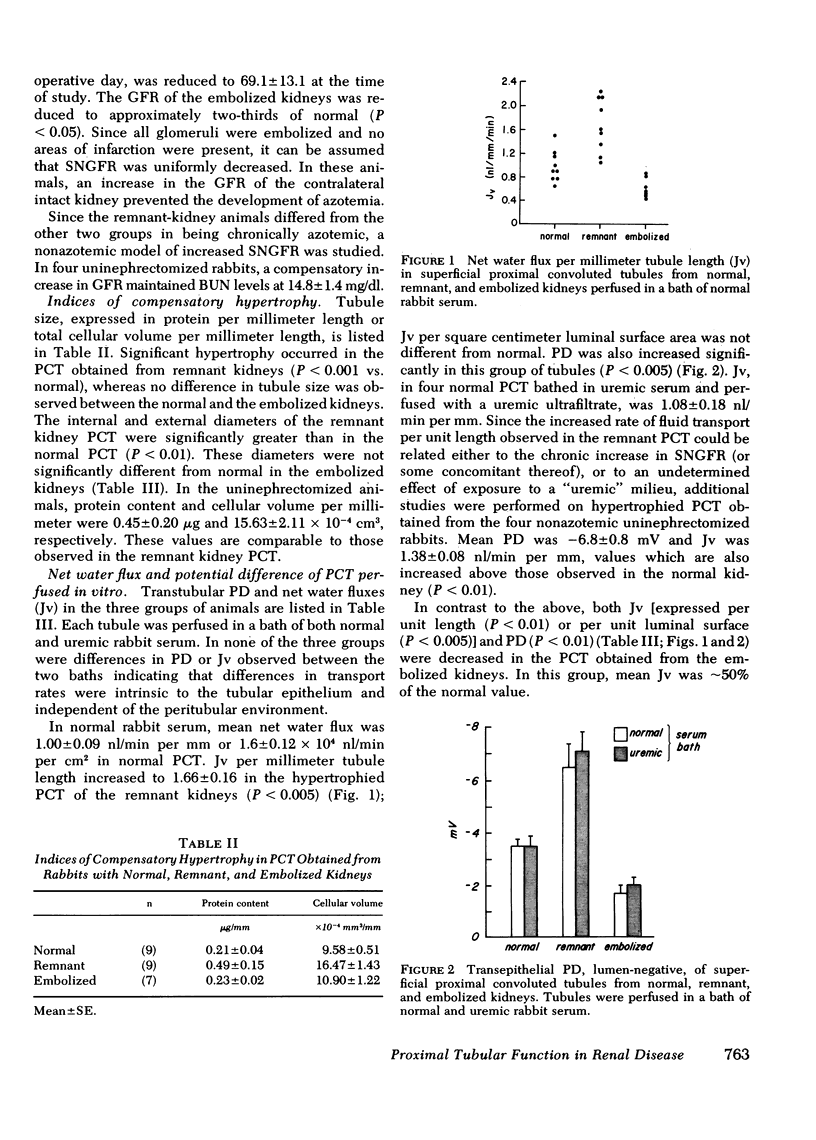
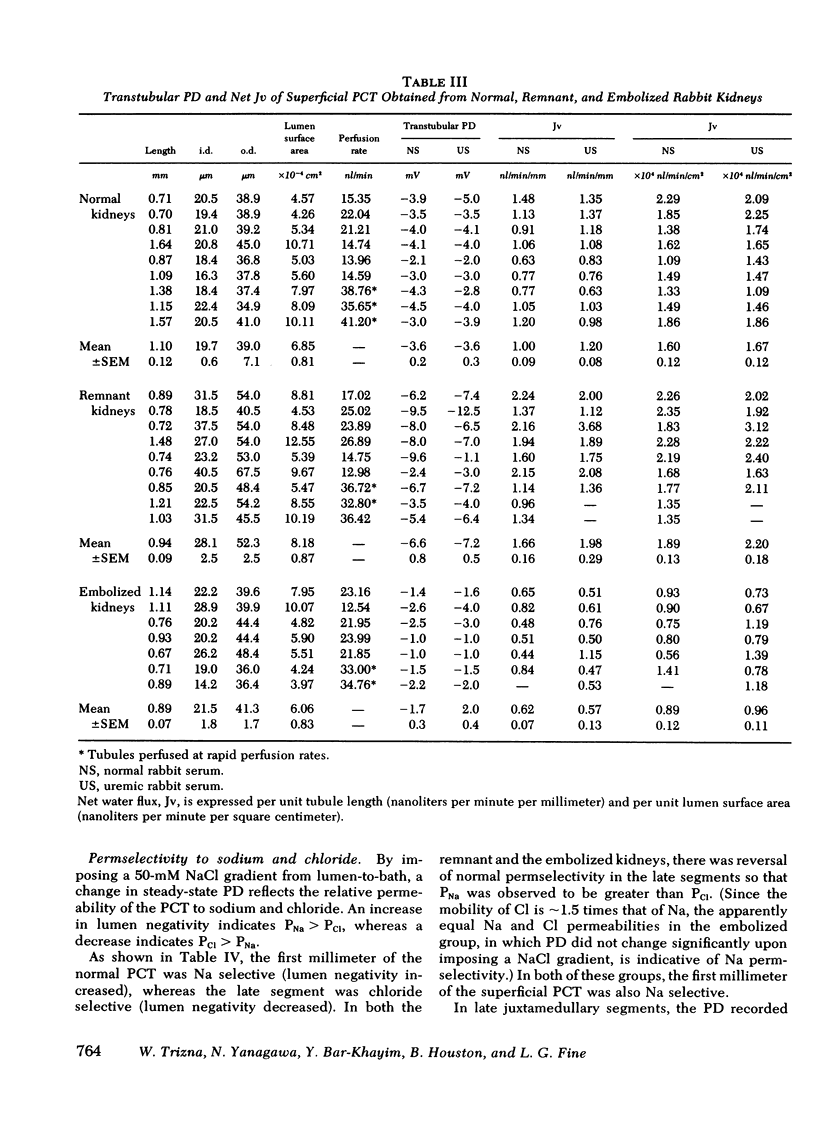
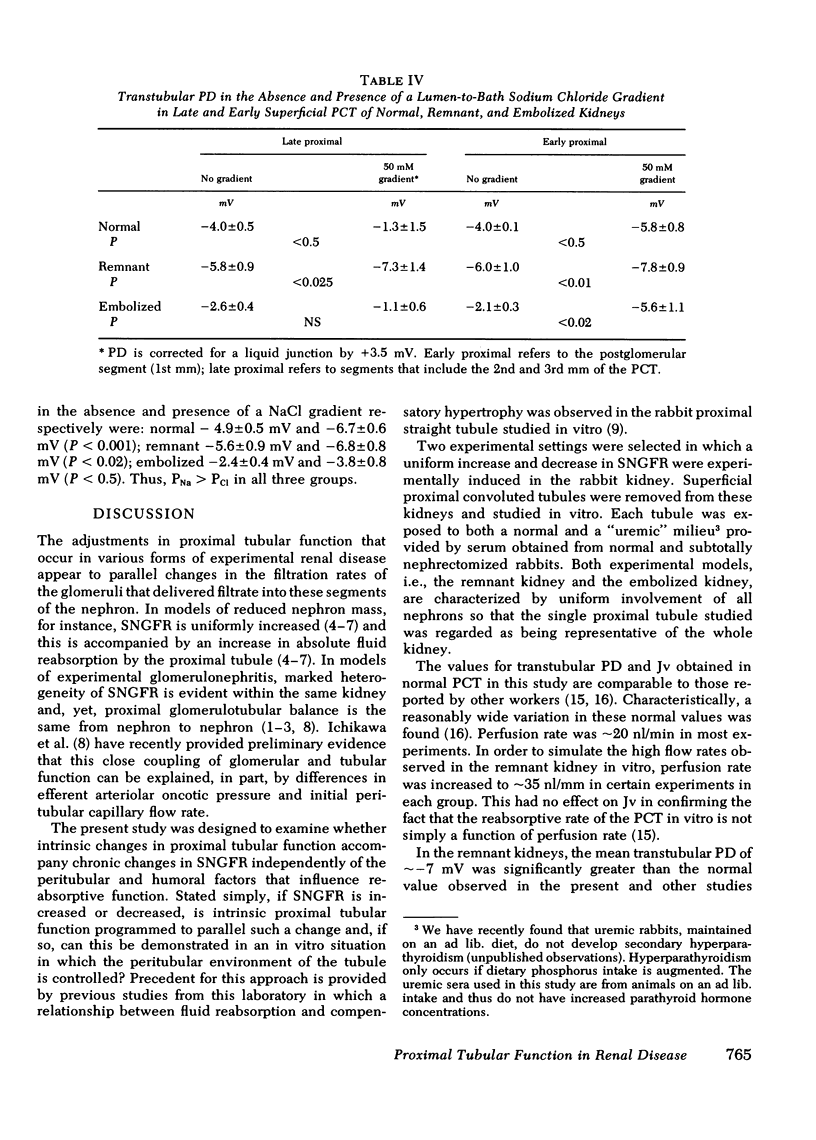
Significant compensatory hypertrophy occurred in the PCT of the remnant kidney. Net fluid reabsorption (Jv) per unit length was increased by ∼60%; Jv per unit luminal surface area was the same as in the normal PCT. Transepithelial potential difference (PD) was significantly greater than normal. This was associated with a reversal of the normal permselective properties (PCl > PNa) of the late superficial PCT so that PNa exceeded PCl. The changes could not be ascribed to some undetermined effect of the uremic state in vivo, since increases in tubule size, Jv per unit length, and PD also occurred in PCT from nonazotemic uninephrectomized rabbits.
In contrast, Jv, per unit length or per unit luminal surface area, was decreased by ∼50% in PCT from embolized kidneys and PD was also reduced. In these tubules, the normal permselective properties were also reversed. Tubule size, however, was not significantly different from normal.
The increases or decreases in Jv that occurred in the different disease models were not dependent on tubular fluid flow rate or the uremic milieu in vitro.
These studies indicate that intrinsic proximal tubular function is modified by the disease state in vivo and that the “memory” of this adaptation is expressed in the in vitro situation. The changes in Jv observed in vitro parallel the increases or decreases in single nephron filtration rates that occur in vivo. Compensatory hypertrophy, with an attendant increase in luminal surface area, could explain the increased Jv per millimeter in the remnant kidneys, but the adaptation observed in the embolized kidneys cannot be ascribed to changes in tubule size.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. E., Wilson C. B., Gottschalk C. W. Pathophysiology of experimental glomerulonephritis in rats. J Clin Invest. 1974 May;53(5):1402–1423. doi: 10.1172/JCI107689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A., Warnock D. G., Rector F. C., Jr Ion selectivity and proximal salt reabsorption. Am J Physiol. 1978 Sep;235(3):F234–F245. doi: 10.1152/ajprenal.1978.235.3.F234. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Prasad J., Chambless S., Klahr S. Response of deep nephrons and the terminal collecting duct to a reduction in renal mass. Am J Physiol. 1979 May;236(5):F454–F464. doi: 10.1152/ajprenal.1979.236.5.F454. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Control of fluid absorption in the renal proximal tubule. J Clin Invest. 1968 Sep;47(9):2016–2024. doi: 10.1172/JCI105888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Electrical potential difference across proximal convoluted tubules. Am J Physiol. 1970 Dec;219(6):1714–1716. doi: 10.1152/ajplegacy.1970.219.6.1714. [DOI] [PubMed] [Google Scholar]
- Cardinal J., Lutz M. D., Burg M. B., Orloff J. Lack of relationship of potential difference to fluid absorption in the proximal renal tubule. Kidney Int. 1975 Feb;7(2):94–102. doi: 10.1038/ki.1975.14. [DOI] [PubMed] [Google Scholar]
- Chonko A. M., Osgood R. W., Nickel A. E., Ferris T. F., Stein J. H. The measurement of nephron filtration rate and absolute reabsorption in the proximal tubule of the rabbit kidney. J Clin Invest. 1975 Jul;56(1):232–235. doi: 10.1172/JCI108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Schlondorff D., Trizna W., Gilbert R. M., Bricker N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978 Jun;61(6):1519–1527. doi: 10.1172/JCI109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Trizna W., Bourgoignie J. J., Bricker N. S. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978 Jun;61(6):1508–1518. doi: 10.1172/JCI109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Yanagawa N., Schultze R. G., Tuck M., Trizna W. Functional profile of the isolated uremic nephron: potassium adaptation in the rabbit cortical collecting tubule. J Clin Invest. 1979 Oct;64(4):1033–1043. doi: 10.1172/JCI109540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Irish J. M., 3rd, Hall D. A. Studies of isolated renal tubules in vitro. Annu Rev Physiol. 1978;40:249–277. doi: 10.1146/annurev.ph.40.030178.001341. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R. Characteristics of volume reabsorption in rabbit superficial and juxtamedullary proximal convoluted tubules. J Clin Invest. 1979 Mar;63(3):410–418. doi: 10.1172/JCI109317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubowitz H., Mazumdar D. C., Kawamura J., Crosson J. T., Weisser F., Rolf D., Bricker N. S. Experimental glomerulonephritis in the rat: structural and functional observations. Kidney Int. 1974 May;5(5):356–364. doi: 10.1038/ki.1974.51. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Passive electrical properties of toad urinary bladder epithelium. Intercellular electrical coupling and transepithelial cellular and shunt conductances. J Gen Physiol. 1974 Jul;64(1):1–25. doi: 10.1085/jgp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A., Marcondes M., Malnic G. Micropuncture study in rats with experimental glomerulonephritis. Kidney Int. 1973 Jan;3(1):14–23. doi: 10.1038/ki.1973.3. [DOI] [PubMed] [Google Scholar]
- Schultze R. G., Weisser F., Bricker N. S. The influence of uremia on fractional sodium reabsorption by the proximal tubule of rats. Kidney Int. 1972 Aug;2(2):59–65. doi: 10.1038/ki.1972.72. [DOI] [PubMed] [Google Scholar]
- Weber H., Lin K. Y., Bricker N. S. Effect of sodium intake on single nephron glomerular filtration rate and sodium reabsorption in experimental uremia. Kidney Int. 1975 Jul;8(1):14–20. doi: 10.1038/ki.1975.71. [DOI] [PubMed] [Google Scholar]
- Wen S. F., Wong N. L., Evanson R. L., Lockhart E. A., Dirks J. H. Micropuncture studies of sodium transport in the remnant kidney of the dog. The effect of graded volume expansion. J Clin Invest. 1973 Feb;52(2):386–387. doi: 10.1172/JCI107195. [DOI] [PMC free article] [PubMed] [Google Scholar]



