Abstract
Cancer chemoprevention involves the chronic administration of a synthetic, natural or biological agent to reduce or delay the occurrence of malignancy. The potential value of this approach has been demonstrated with trials in breast, prostate and colon cancer. The paradigm for developing new chemopreventive agents has changed markedly in the last decade and now involves extensive preclinical mechanistic evaluation of agents before clinical trials are instituted and a focus on defining biomarkers of activity that can be used as early predictors of efficacy. This review will summarise the current status of the field of chemoprevention and highlight potential new developments.
Keywords: Cancer chemoprevention, biomarkers, trials, development
Given the steady increase in global cancer incidence with its associated morbidity and mortality, together with the spiralling healthcare costs of treatment, there is increasing interest in strategies for disease prevention. One approach with enormous potential is chemoprevention, which is defined as the use of natural, synthetic or biological agents to reverse, suppress or prevent either the initial phases of carcinogenesis or the progression of premalignant cells to invasive disease (Sporn, 1976). Interest in this area of research has markedly increased with improved understanding of the biology of carcinogenesis and the identification of potential molecular targets to perturb this process. Interest has been further stimulated by successes in the chemoprevention of breast, prostate and colon cancer, and the fact that there are now 10 FDA-approved (Wu et al, 2011a) agents for the treatment of precancerous lesions or cancer risk reduction.
Over the past 10 years, it has become apparent that the definition of chemoprevention should incorporate the concept of ‘delay', which implies that the preventive effect may last for a finite period. The rate of tumour development is decreased even if the incidence eventually returns to that of the untreated population (Lippman and Hong, 2002), and many years or decades may be added to human lifespan.
Chemoprevention may involve perturbation of a variety of steps in tumour initiation, promotion and progression. Numerous potential mechanisms have been described and attempts have been made to broadly classify agents according to the effects they have on different stages of carcinogenesis (De Flora and Ferguson, 2005; Table 1). However, it is likely that many agents, particularly those that are dietary derived and multi-targeted, will have effects throughout the carcinogenic process. Compounds that inhibit cancer initiation are traditionally termed ‘blocking agents'. They may act by preventing the interaction between chemical carcinogens or endogenous free radicals and DNA, thereby reducing the level of damage and resulting mutations which contribute not only to cancer initiation but also progressive genomic instability and overall neoplastic transformation. Protection may be achieved as a consequence of decreased cellular uptake and metabolic activation of pro-carcinogens and/or enhanced detoxification of reactive electrophiles and free radical scavenging, as well as induction of repair pathways (Yu and Kong, 2007; Valko et al, 2007). Downregulation of chronic inflammatory responses and the production of reactive oxygen and nitrogen species may also contribute to the prevention of cancer initiation. Other protective processes include modulation of DNA methyl transferases to prevent or reverse the hypermethylation-induced inactivation of tumour suppressor genes. Inhibition of histone deacetylases has also been described among a variety of effects of blocking agents on epigenetic mechanisms of carcinogenesis (Hauser and Jung, 2008). Once initiation has occurred, chemopreventive agents may influence the promotion and progression of initiated cells; such compounds are often termed ‘suppressing agents'. The major reported mechanisms contributing to this activity involve the inhibition of signal transduction pathways (for example, by targeting nuclear factor (NF)-κB) to perturb the effects of tumour promoters (Karin 2006), which would otherwise lead to cell proliferation. In some instances, hormones may promote tumour progression, and anti-oestrogens such as tamoxifen can block this effect (Yager and Davidson, 2006). Recent reports suggest interference with cancer cell metabolism and energy homoeostasis via effects on pathways such as AMPK and mTOR signalling may be an attractive goal for chemopreventive agents (Din et al, 2012). Other mechanisms of chemoprevention include the induction of apoptosis and inhibition of angiogenesis (Noonan et al, 2007). Some potential molecular targets for chemopreventive agents are shown in Table 2.
Table 1. Potential mechanisms of chemoprevention.
|
Mechanisms of tumour-blocking agents |
| Scavenging of free radicals |
| Antioxidant activity |
| Induction of phase II drug-metabolising enzymes |
| Inhibition of phase I drug-metabolising enzymes |
| Induction of DNA repair |
| Blockade of carcinogen uptake |
|
Mechanisms of tumour-suppressing agents |
| Alteration in gene expression |
| Inhibition of cell proliferation, clonal expansion |
| Induction of terminal differentiation, senescence |
| Induction of apoptosis in preneoplastic lesions |
| Modulation of signal transduction |
Table 2. Selected molecular targets of potential chemopreventive agents (effects may be tissue and cell specific as well as dose dependent).
| Gene expression | Transcription factors | Protein kinases | Enzymes | Others |
|---|---|---|---|---|
| Chemokines |
NF-κB |
IκBα kinase |
FTPase |
ICAM-1 |
| Cyclin D1 |
AP-1 |
EGFR |
Xanthine oxidase |
VCAM-1 |
| MMP9 |
Egr-1 |
HER2 |
Haemeoxygenase |
ELAM-1 |
| COX2 |
STAT1 |
AKT |
uPA |
TF |
| 5-LOX |
STAT3 |
JAK2 |
GST |
Bcl-2 |
| iNOS |
STAT5 |
TYK2 |
GSH-px |
Bcl-xl |
| IL-12 |
PPAR-γ |
JNK |
|
P53 |
| TNF |
EpRE |
PKC |
|
|
| IL-6 |
CBP |
Src |
|
MDR |
| IL-8 |
β-catenin |
PKA |
|
Telomerase |
| Cyclin D1 |
Abbreviations: AP-1=activator protein 1; CBP=CREB-binding protein; COX2=cyclooxygenase 2; EGFR=epidermal growth factor receptor; Egr-1=early growth response protein 1; ELAM-1=endothelial-leukocyte adhesion molecule 1; EpRE=energy per resource element; GSH=glutathione; GST=glutathione-S-transferase; HER2=human epidermal growth factor receptor 2; ICAM-1=intercellular adhesion molecule 1; IL=interleukin; iNOS=inducible nitric oxide synthase; JAK2=janus kinase 2; JNK=c-Jun N-terminal kinases; MDR=multi drug resistance; MMP9=matrix metallopeptidase 9; NF-κB=nuclear factor-κB; PKA=protein kinase A; PKC=protein kinase C; PPARγ=peroxisome proliferator-activated receptor-γ; STAT=signal transducer and activator of transcription; TF=tissue factor; TNF=tumour necrosis factor; uPA=urokinase-type plasminogen activator; VCAM-1=vascular cell adhesion molecule 1.
Three broad approaches to the clinical use of chemopreventive agents have been described (Kelloff et al, 1995). ‘Primary chemoprevention' involves the administration of agents to the general ‘healthy' population or to those without overt disease but with particular risk factors. Examples may include the administration of agents such as oltipraz, which induce phase I or II enzymes to modify carcinogen metabolism in an exposed population. ‘Secondary chemoprevention' involves the identification of individuals with premalignant lesions and administration of agents to prevent progression to invasive cancer. This would encompass the use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with colorectal adenomas. Definitions of primary and secondary chemoprevention vary and some groups now combine the two scenarios under the term primary chemoprevention. ‘Tertiary chemoprevention' is defined as the administration of agents to prevent recurrence or second primary cancers in individuals who have undergone successful treatment of early disease.
Potential value of chemoprevention
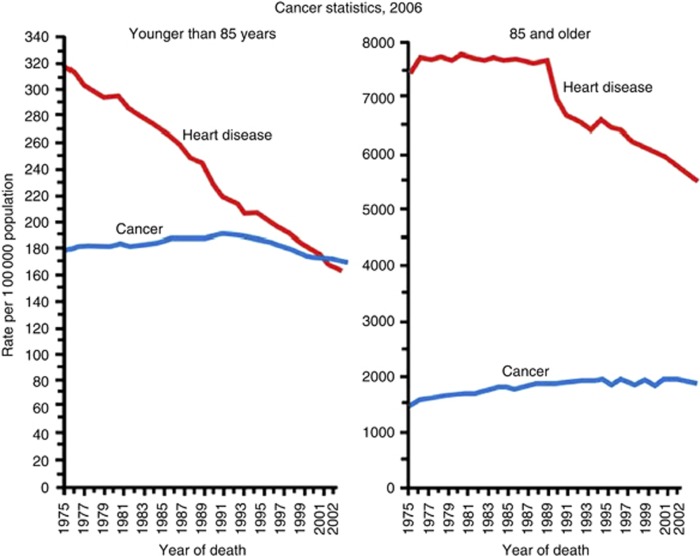
The potential impact that chemoprevention could have on the death rate from cancer is evident from the way this approach has transformed the incidence of cardiovascular disease (Hansson, 2005). The introduction of drugs that suppress cholesterol synthesis, modify platelet aggregation or lower blood pressure has led to a steady fall in heart disease over the past 3 decades. The rate of cardiovascular disease was almost twice that of cancer for those under 85 years of age in the mid-1970s, but fell below that of cancer in 1999 (Figure 1; National Center for Health Statistics, Centers for Disease Control and Prevention, 2005). The cardiovascular community has been aggressive with chemoprevention, although many of the drugs they use can have serious or undesirable side effects. The major reason for successful uptake of preventive interventions has been the demonstration of measurable markers of increased risk of disease and death, such as hypertension and hypercholesterolaemia. It is clearly essential to identify similar measurable risk factors for cancer that will allow chemoprevention to be focused on subgroups of individuals, reducing anxieties about potential side effects and providing a surrogate end point of effective exposure (akin to the reduction in blood pressure), which may predict a reduced risk of disease.
Figure 1.
Death rates* for cancer and heart disease for ages younger than 85, and 85 and older, 1975–2004. *Rates are age-adjusted to the 2000 US standard population. National Center for Health Statistics.
Agent selection for chemoprevention
There has been a major change in philosophy underlying the selection of promising chemopreventive agents in the last decade. Initially, selection was predominantly based on observational studies reporting an association between consumption of pharmaceutical (for example, aspirin) or dietary (for example, retinoids) components in a population, and a reduced incidence or mortality from cancer. Occasionally, early-phase clinical studies would be performed to explore duration of dosing and intermediate biomarkers of efficacy but in the majority of cases (for example, for beta-carotene) large randomised trials were undertaken, exploring the relative rates of cancer over many years in exposed and control populations without such prior information.
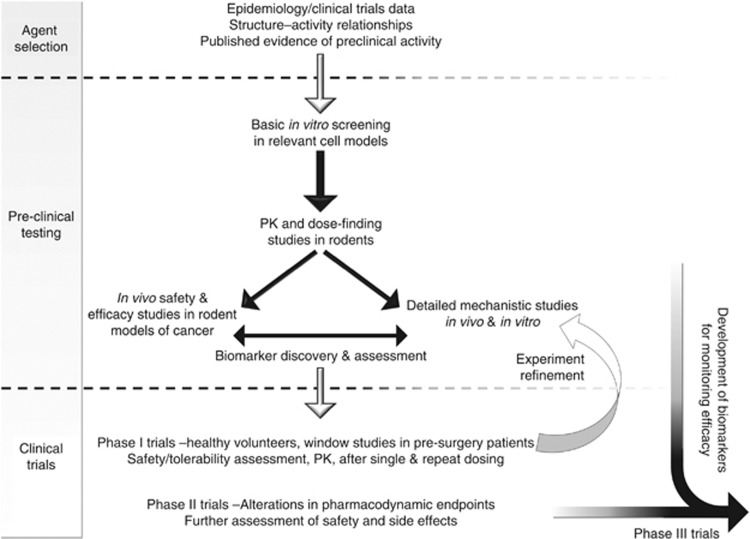
In recent years, there has been a more rigorous approach to the selection of agents for clinical development (Figure 2). Initial selection may still be based on epidemiological data suggesting an effect on cancer incidence, but subsequent extensive preclinical studies, using clinically achievable concentrations in models, which are relevant to human carcinogenesis, are increasingly undertaken before clinical trials begin (Scott et al, 2009). Preclinical testing should comprise a series of investigations, initially utilising in vitro and in vivo mechanistic assays. These can include measures of the effect of the agent under investigation on potentially important processes, such as inhibition of proliferation, modification of angiogenesis and inflammation or induction of apoptosis. Subsequently, in vivo testing may explore the prevention of tumour development as measured by incidence, overall burden or time to occurrence. Historically, animal models involved carcinogenic exposure but, increasingly, transgenic/mutant rodent models (for example, ApcMin mice for colon cancer, TRAMP mice for prostate cancer) are now utilised, given their greater relevance to the complexities of human carcinogenesis (Abate-Shen et al, 2008). Such in vivo models can provide additional information on pharmacokinetics and safety. Target tissue levels can be measured to ensure appropriate delivery, and tissue concentrations producing an effect can be compared with subsequent human levels. This can provide a guide to appropriate dosing and scheduling (Wu et al, 2011a). Increasingly, preclinical testing may be linked to early-phase clinical trials. Potential biomarkers of activity may be developed in the laboratory and evaluated in patients with the hope that they can help determine optimal dose and be used in later phase studies. Eventually, agents are selected for clinical development on the basis of these evaluations and the availability of an appropriate formulation (oral whenever possible) with minimal or no potential toxicity and low cost.
Figure 2.
Stages in the preclinical and clinical development of potential chemoprevention agents..
Trial design
Clinical chemoprevention agent development has utilised a similar model to new drug development in cancer therapy with sequential phase I, II and III studies (Lippman et al, 1994). Phase I studies have a primary aim of determining safety and pharmacokinetics such that a dose and schedule that is well tolerated by participants can be defined. Some phase I studies may incorporate preliminary assessments of potential biomarkers of effect (Lippman and Hong, 2002). Exposure is relatively short (usually up to 3 months). The choice of starting dose and schedule is extremely difficult and may be guided by preclinical studies. Dose conversions can be used, which aim to achieve plasma concentrations in humans, which should be safe and may approximate to levels producing a biological effect in preclinical models (Brown et al, 2010). PK data from phase I studies provide the actual levels that are achieved in humans, and these can be taken back into refined preclinical models to explore possible mechanisms of effect at clinically achievable concentrations. Studies may utilise existing drugs such as aspirin for which there is already extensive human data, and in this situation rapid progress to phase III trials can be contemplated.
Phase II trials, utilising the optimal dose previously defined, may follow with the primary aim of exploring, in relatively few patients, the impact of exposure on a biological end point. When potential biomarkers of effect are available, these can be examined in these patients. These trials may incorporate a placebo (phase IIb) to better define subtle side effects and tolerability, and also to more accurately measure biological effects.
Phase III trials usually involve thousands of participants over a long duration. Typically, there is randomisation between the agent under investigation and a placebo. Modification of a clinically relevant value, which is usually the incidence of malignancy, is the standard end point. Such trials may take many years and have huge costs.
Given the length of development of agents for chemoprevention, recent interest has focussed on phase 0 trials. These employ very low doses of experimental agent and utilise new methodologies and technologies to study pharmacokinetics at a dose that minimises any risk of toxicity (Kummar and Doroshow, 2011). It is hoped that this approach will provide information to help determine a rational dosage regime for future studies and will also lead to early termination of the development of agents that have unfavourable bioavailability, metabolism or distribution.
Biomarkers
The long latency of progression from premalignant lesions to invasive cancer offers the prospect of intervention to prevent disease. It is also a major obstacle for chemoprevention trials using cancer incidence as the primary end point, as it may take decades to obtain significantly different disease rates for an active agent. This puts a huge burden on trial participants, research infrastructure and financial budgets. An essential component to chemopreventive agent development, highlighted by consensus groups in the United States (Crowell, 2005) and Europe (Gerhauser et al, 2006), is a need to identify end points, which occur earlier than cancer, that accurately predict the potential to reduce cancer incidence. Targeting of these end points would aid choice of doses and schedules, and provide an early readout of potential efficacy. Few such easily measurable surrogates have been determined in carcinogenesis. Many approaches to biomarker selection have been taken and include the detection of high-risk adenomas or aberrant crypts in the colon and rectum, mammographic density in the breast, and changes of intraepithelial neoplasia in the head and neck and bladder. The Early Detection Research Network of the National Cancer Institute (United States) has a national programme to identify biomarkers associated with carcinogenesis utilising proteomic assessment of blood and urine (National Cancer Institute, 2008). An alternative, complementary approach that may be particularly useful in the early short-term trials would be to define mechanism-based pharmacodynamic biomarkers that reflect agent activity and correlate with efficacy in preclinical models, and determine their utility in humans.
Clinical trials of chemoprevention
Several large randomised clinical chemoprevention trials have been undertaken – predominantly since the 1980s. There have been important positive results in breast and prostate cancer and familial adenomatous polyposis (FAP) but also several negative trials and four (in lung and colon cancer), which have suggested a harmful effect for the agents under investigation. Many lessons have been learnt from these experiences in trial design, and selection of agents and doses for future trials.
Important positive trials
The first trials to show a significant benefit for chemoprevention were undertaken in breast cancer. The Breast Cancer Prevention Trial (BCPT) included >13 000 women at increased risk of breast cancer (Fisher et al, 1998) and demonstrated a 49% reduction in invasive breast cancer and a 50% decrease in non-invasive disease, with the use of tamoxifen for 5 years compared with placebo. There was, however, a doubling of the risk of endometrial carcinoma and an increased incidence of thromboembolic events. Two other trials have been undertaken – International Breast Cancer Intervention Study (IBIS)-1) that compared tamoxifen against placebo and IBIS-2 that compared tamoxifen vs anastrozole. IBIS-1 (Cuzick et al, 2007) confirmed the protective effect seen in the BCPT but, importantly, has demonstrated that the increased risk of toxicity declined and was equivalent to that of patients on placebo by 10 years (or 5 years after tamoxifen discontinuation). Given concerns about the toxicity of tamoxifen and the effect it had in reducing women's acceptance of its use as a chemopreventive, the Study of Tamoxifen And Raloxifene (STAR) study was initiated to compare these two agents for their effect on prevention and associated toxicities. Encouragingly, raloxifene was as effective as tamoxifen in reducing invasive breast cancer but did not increase the risk of endometrial tumours (Vogel et al, 2006). An update of the STAR trial (Vogel et al, 2010; increased median follow-up from 47 to 81 months) showed that raloxifene became less effective than tamoxifen in reducing invasive cancer but retained its greater safety profile. Both tamoxifen and raloxifene have obtained FDA approval for breast cancer prevention. The aromatase inhibitor, exemestane, has also been shown to have a chemopreventive effect for women with at least one risk factor for disease. A recent analysis showed a 65% relative reduction in the annual incidence of invasive disease (Goss et al, 2011).
Two positive large trials have explored the chemoprevention of prostate cancer, with cancer incidence as the end point. The Prostate Cancer Prevention Trial compared the 5α-reductase inhibitor, finasteride, with placebo given for 7 years in 18 882 men. At the time of analysis, 86.3% of participants had completed 7 years of treatment. There was a 26% reduction in the diagnosis of prostate cancer in the finasteride arm (P<0.001), but the protective effect appeared to be limited to lower grade tumours (Thompson et al, 2003). There was an increase in the number of biopsy cases with higher grade disease (1.8% vs 1.1%), which raised concerns. Subsequent analysis of prostatectomy specimens suggested that this observation was an artefact resulting from the effect of finasteride on prostate size, which affected the sampling in biopsy specimens rather than being a true increase (Lucia et al, 2007). Unfortunately, partly because of the initial findings, the FDA has not approved finasteride for prostate cancer chemoprevention. A subsequent randomised trial, the Reduction by Dutasteride of Prostate Cancer Events, demonstrated that dutasteride (another 5α-reductase inhibitor) reduced the risk of biopsy proven prostate cancer by 23% compared with placebo but, as with finasteride, the major benefit was in the prevention of lower grade malignancies (Andriole et al, 2010).
Preclinical and epidemiological studies have shown huge promise for the role of NSAIDs to reduce colorectal cancer risk. Despite this potential, no prospective trials to date have studied the impact on colorectal cancer as a primary end point. Prospective randomised controlled trials with cardiovascular disease as the primary end point have shown, from secondary analyses, reductions in the development of colorectal cancer and death from malignancy. The first publication of long-term follow-up of participants in five trials showed that daily aspirin at any dose reduced the risk of colorectal cancer by 24% and of associated mortality by 35% after a delay of 8–10 years (Flossman and Rothwell, 2007). Subsequently, analysis (Rothwell et al, 2010) using data from eight randomised trials found that daily aspirin use at any dose was associated with a 21% reduction in all cancer deaths, with the benefit only apparent after 5 years. This work was extended to include an additional 43 randomised trials of daily aspirin. Cancer death was reduced by 15%, with benefits seen within 3 years at high doses (⩾300 mg per day) and after 5 years for lower doses (<300 mg per day). These data suggest that low-dose aspirin may reduce the risk of sporadic colorectal adenomas within a few years but requires 5 years to produce an effect on invasive cancer and cancer death (Rothwell et al, 2012). At high doses, however, it appears that aspirin may reduce cancer death with a direct effect on the growth and spread of established tumours as well as their initiation, as cancer death was reduced within 2–3 years following randomisation. Further analysis of five trials showed that aspirin reduced the risk of cancer with distant metastasis, particularly for adenocarcinomas (Algra and Rothwell, 2012). Some patients were included with localised malignancy, and for those who were assigned aspirin there was a lower risk of developing metastases during subsequent follow-up.
Questions still remain, however, as to whether aspirin should be routinely recommended for cancer prevention. The main concerns are that two large prospective trials, the Women's Health and Physicians' Health Studies (Steering Committee of the Physicians' Health Study Group, 1989; Harris et al, 2003), were negative and the cardiovascular trials occurred before cancer screening and surveillance were routine. Nevertheless, the recent data does suggest that aspirin can reduce cancer incidence and death, and this effect is delayed. Hopefully, a clearer guide to the ratio of benefit to risk will emerge with completion of two ongoing US trials.
For those individuals with hereditary colon cancer (Lynch syndrome), a randomised study has shown a significant benefit for aspirin and has led to a second prospective study, which aims to define optimal dosing (Burn et al, 2011).
Selective COX2 inhibitors were introduced into clinical trials as an alternative to standard NSAIDs, given their reduced propensity to induce gastrointestinal toxicity. The initial study in 83 patients with FAP demonstrated a 28% reduction in adenoma burden (Steinbach et al, 2000). These results led to the accelerated approval of celecoxib for the treatment of FAP. Two subsequent trials of celecoxib confirmed the preventative effect against recurrent adenomas but both studies were associated with two- to three-fold increases in serious cardiovascular events (Solomon et al, 2005). A recent study with the fish oil extract, eicosapentaenoic acid in patients with FAP demonstrated a similar reduction in adenoma burden to that seen with celecoxib but with minimal toxicity (West et al, 2010), and this is being further developed.
Important negative trials
Two of the earliest chemoprevention trials were based on data linking cancer risk reduction and intake of carotenoids. These included smokers, with lung cancer incidence as the end point. The α-tocopherol (vitamin E), β-carotene (ATBC) prevention study enrolled 29 133 men with a randomisation to α-tocopherol, β-carotene, a combination of both or a placebo. The first results were alarming, showing an 18% increased incidence of lung cancers, increased cardiovascular disease and an 8% increased overall mortality for those on β-carotene (The Alpha-Tocopherol). Subsequent analysis revealed the adverse effect to be stronger in men with a modest alcohol intake and smokers of >20 cigarettes daily (Albanes et al, 1996). Interestingly, prostate cancer incidence and death were reduced in the vitamin E arms (by 32% and 41%, respectively). The β-Carotene And Retinol Efficacy Trial included men with occupational asbestos exposure or men and women who were current or former cigarette smokers (18 314 participants) randomised to β-carotene plus retinyl palmitate or placebo. This was closed early because those in the intervention arm had higher lung cancer death rates (17% and 28%, respectively) and higher rates of cardiovascular disease mortality (Omenn et al, 1996). Further investigation of β-carotene for cancer prevention was discontinued as a result.
One of the largest prevention studies was the Selenium and vitamin E Cancer Prevention Trial (Lippman et al, 2009). Men (35 534) were randomised to receive α-tocopherol, selenium, both agents and a placebo. Unfortunately, the trial was closed early after an interim analysis indicated an extremely low likelihood of producing a positive result. A later report showed a significant 17% increase in the risk of prostate cancer among those who received vitamin E (Klein et al, 2011). Detailed analyses were subsequently undertaken on two smaller previous studies that had suggested a protective effect from selenium. In both of these, benefit of selenium was seen but was limited to those with lowest baseline blood selenium levels (Duffield-Lillico et al, 2002). The benefits and risks of nutritional supplementation may thus depend on prior exposure – those with marginal or deficient nutrient intakes may be the group to benefit whereas those whose intake is already adequate or high may experience harm.
An explanation for the detrimental effects seen in the prostate and lung prevention trials may thus be the dose of experimental agent chosen. Selenium and β-carotene are naturally occurring dietary constituents, which are important in normal human physiology. It is plausible that a U-shaped dose–response curve exists where either deficiency or supraphysiological doses may cause harm. The negative results from these large expensive trials have led many to reassess the design of clinical chemoprevention studies and to move towards smaller studies focussing on higher-risk individuals, and to rely on more detailed prior preclinical mechanistic evaluation to provide information that may better guide dose selection.
Conclusions and future developments
Chemoprevention is a relatively new field for research. In recent years, preclinical and clinical development strategies have evolved so that agents are increasingly selected for further development based on mechanisms of action rather than relying on historical epidemiological observations. Many new targets have been defined including the upregulation of Nrf2, NF-κB and various members of the STAT family of transcription factors. New agents that target the cyclin family of cell cycle regulators – cyclin D1, D2 and D3 –are being actively pursued, as these are often abnormally expressed in pre-neoplasia. The approach of Short-term Intermittent Therapy to Eliminate Premalignancy (SITEP) (Wu and Lippman, 2011b) is being investigated and is based on the hypothesis that intermittent therapy may eliminate premalignant cells through selective apoptosis induced by synthetic lethal interactions. It has resulted in efficacy in mouse models of carcinogenesis that result from APC and KRAS mutations, and is being tested in breast cancer chemoprevention, based on the synthetic lethality between the mutated tumour suppressor genes BRCA1 or BRCA 2, and PARP1 (Fong et al, 2009). Increasing interest is focusing on a move from single agent chemoprevention to combination approaches. An important trial combined difluoromethylornithine and sulindac in 375 patients with a history of resected adenomas and demonstrated a 60% reduction in recurrence rates (Meyskens et al, 2008). As with chemotherapy, the hope is that such an approach will produce a synergistic or additive effect and will also allow their lowest active doses to be chosen to reduce toxicity.
Although there have been several major achievements in chemoprevention, there is clearly a huge amount to be done to mirror the successes seen in cardiovascular medicine. It is frustrating that one of the agents with great potential in colorectal cancer, aspirin, still has not been properly assessed in prospective randomised trials in this disease. Hopefully, important information will be obtained on its tolerability in large populations from the recently completed AspECT trial in Barrett's oesophagus, and this will move the field forward. Much more work needs to be undertaken with nutritionally derived agents. It is estimated that $30 billions is spent on dietary supplements each year (Cohen, 2012) and yet no chemoprevention trials with these have yielded positive outcomes. An important lesson obtained from experience with selenium in prostate cancer prevention is the need to understand the role of these agents in populations with different endogenous exposure, leading to varying tissue levels before study entry.
One further development that is aimed at reducing the size, cost and duration of clinical trials, thereby enabling more agents to be examined, is the selection of higher-risk individuals for inclusion in studies. Many approaches have been taken to identify clinico-pathological variables and molecular markers that can predict which patients may have premalignancy. One current approach involves modelling germ line and somatic markers of risk and predictive markers of agent benefit or toxicity such that it may be possible to personalise cancer prevention.
Hopefully, these developments will lead to larger numbers of trials that are built on high quality preclinical research and produce more positive results in the future. It will then be possible for chemoprevention to take an important role in reducing the risk of cancer in society.
References
- Abate-Shen C, Brown PH, Colburn NH, Gerner EW, Green JE, Lipkin M, Nelson WG, Threadgill D. The untapped potential of genetically engineered mouse models in chemoprevention research: opportunities and challenges. Cancer Prev Res. 2008;1:161–166. doi: 10.1158/1940-6207.CAPR-08-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. α-Tocopherol and β-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS, REDUCE Study Group Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- Brown VA, Patel K, Viskaduraki M, Crowell J, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Gerdes A-M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard M-L, Dunlop MG, Ho JWC, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJW, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT, CAPP2 Investigators Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PA. Assessing supplement safety: the FDA's controversial proposal. N Engl J Med. 2012;366:389–391. doi: 10.1056/NEJMp1113325. [DOI] [PubMed] [Google Scholar]
- Crowell JA. The chemopreventive agent development research program in the Division of Cancer prevention of the US National Cancer Institute: an overview. Eur J Cancer. 2005;41:1889–1910. doi: 10.1016/j.ejca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, International Breast Cancer Intervention Study I Investigators Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Din FVN, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Flossman E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JHM, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Gerhauser C, Bartsch H, Crowell J, De Flora S, D′Incali M, Dittrich C, Frank N, Mihich E, Steffen C, Tortora G, Gescher A. Development of novel cancer chemopreventive agents in Europe—neglected Cinderella or rising phoenix? ESF workshop on cancer chemoprevention, DKFZ, Heidelberg, September 18-20, 2005. Eur J Cancer. 2006;42:1338–1343. doi: 10.1016/j.ejca.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H, NCIC CTG MAP.3 Study Investigators Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Harris RE, Chelbowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Women's health initiative. breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the women's health initiative. Cancer Res. 2003;63:6096–6101. [PubMed] [Google Scholar]
- Hauser A-T, Jung M. Targeting epigenetic mechanisms: potential of natural products in cancer chemoprevention. Planta Medica. 2008;74:1593–1601. doi: 10.1055/s-2008-1081347. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Johnson JR, Crowell JA, Boone CW, DeGeorge JJ, Steele VE, Mehta MU, Temeck JW, Schmidt WJ, Burke G, Greenwald P, Temple RJ. Approaches to the development and marketing approval of drugs that prevent cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:1–10. [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummar S, Doroshow JH. Phase 0 trials: expediting the development of chemoprevention agents. Cancer Prev Res. 2011;4:288–292. doi: 10.1158/1940-6207.CAPR-11-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman SM, Benner SE, Hong WK. Cancer chemoprevention. J Clin Oncol. 1994;12:851–873. doi: 10.1200/JCO.1994.12.4.851. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Hong WK. Cancer prevention by delay. Commentary re: J A O'Shaughnessy et al., Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:305–313. [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, III, Grawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Amer Med Ass. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia MS, Epstein JI, Goodman PJ, Darke AK, Reuter VE, Civantos F, Tangen CM, Parnes HL, Lippman SM, La Rosa FG, Kattan MW, Crawford ED, Ford LG, Coltman CA, Jr, Thompson IM. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99:1375–1383. doi: 10.1093/jnci/djm117. [DOI] [PubMed] [Google Scholar]
- Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics, Centers for Disease Control and Prevention . US Mortality Data, 1960 to 2004, National Center for Health Statistics. Centers for Disease Control and Prevention US Government publication; 2005. [Google Scholar]
- National Cancer Institute . The Early Detection Research Network: investing in translational research on biomarkers of early cancer and cancer risk. Fourth Report. U.S. Department of Health and Human Services. National Institutes of Health: Bethesda, MD; 2008. [Google Scholar]
- Noonan DM, Benelli R, Albini A. Angiogenesis and cancer prevention: a vision. Recent Results in Cancer Res. 2007;174:219–224. doi: 10.1007/978-3-540-37696-5_19. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Hammar S. Effects of a combination of β carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes GR, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JFF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res. 2009;2:525–530. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- Steering Committee of the Physicians' Health Study Research Group Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B, Godio L, Patterson S, Rodriguez-Bigas MA, Jester SL, King KL, Schumacher M, Abbruzzese J, DuBois RN, Hittelman WN, Zimmerman S, Sherman JW, Kelloff G. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Goodman PJ, Tangen CM, Scott Lucia M, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N, National Surgical Adjuvant Breast and Bowel Project Update of the National Surgical Adjuvant breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NJ, Clark SK, Phillips RKS, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- Wu X, Lippman SM. An intermittent approach for cancer chemoprevention. Nat Rev Cancer. 2011b;11:879–885. doi: 10.1038/nrc3167. [DOI] [PubMed] [Google Scholar]
- Wu X, Patterson S, Hawk E. Chemoprevention—history and general principles. Best Pract Res Clin Gastroenterol. 2011a;25:445–459. doi: 10.1016/j.bpg.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Yu S, Kong A-NT. Targeting carcinogen metabolism by dietary cancer preventive compounds. Curr Cancer Drug Targets. 2007;7:416–424. doi: 10.2174/156800907781386669. [DOI] [PubMed] [Google Scholar]