Abstract
Neurons producing melanocortin receptor agonist, α-MSH derived from proopiomelanocortin, and antagonist, agouti-related protein, are known to be sensitive to metabolic stress such as food deprivation and glucoprivation. However, how these neurons respond to emotional/psychological stress remained to be elucidated. We report here that acute emotional stressors, i.e. restraint and forced swim, evoked mRNA expression of c-fos, a neuronal activation marker, in a high percentage of proopiomelanocortin neurons (up to 53% for restraint stress and 62% for forced swim), with marked variations along the rostro-caudal axis of the arcuate nucleus. In contrast, only a small population of agouti-related protein neurons in this brain region was activated. These neuronal activation patterns were correlated with behavioral reactions. Both stressors suppressed feeding and induced anxiety-like behavior in the elevated plus-maze test, as reflected by a reduction in the percentage of entries and time spent in the open arms. Central pretreatment with SHU9119, a melanocortin receptor antagonist, dose dependently attenuated the anorectic and anxiogenic effects elicited by acute restraint or forced swim. These results indicate that the melancortinergic pathway can be rapidly recruited by acute emotional stress, and that activation of melanocortin signaling is involved in mediating stress-induced anorexia and anxiety.
The central melanocortin system consists of the agonist, α-MSH, a proopiomelanocortin (POMC) cleavage product, the endogenous antagonist agouti-related protein (AgRP), and their common melanocortin receptors, melanocortin-3 receptor (MC3R) and melanocortin-4 receptor (MC4R). This system is well studied for its functions in the control of eating behavior and body weight. α-MSH and AgRP have opposite effects on food intake and body weight. α-MSH inhibits appetite and decreases body weight gain, whereas AgRP stimulates eating and increases body weight gain (1–3).
Emerging evidence suggests functional interactions between the endogenous melanocortin system and the stress system. Anatomical studies demonstrate that α-MSH and AgRP neurons innervate a variety of brain regions that have been well characterized in stress reactions (4). When administered centrally, α-MSH and its analogs mimic stress-induced behavioral responses such as excessive grooming (5, 6), decreased exploration in a novel and fearful environment (7), and reduced food intake (8, 9). However, the physiological role of endogenous melanocortin signaling in behavioral responses to stress remains to be elucidated.
Stressors can be grouped into two major categories, i.e. psychological/emotional stressors and physiological stressors, based upon their “processive” and “systemic” natures, with the former defined as limbic sensitive and the latter as limbic insensitive (10–12). Although these two types of challenges converge to activate a common response system, e.g. hypothalamo-pituitary-adrenal axis, they involve distinct neuronal circuits, and lead to different behavioral and physiological outcomes (10, 12). It is well known that POMC and AgRP neurons located in the arcuate nucleus respond inversely to metabolic stress, such as food deprivation and glucoprivation (13–16). By contrast, little is known about how POMC and AgRP neuronal pathways respond to psychological/emotional stress.
Previous studies have classified restraint and forced swim as psychological/emotional stressors based upon the expression patterns of immediate early genes in the brain after stress administration (10–12, 17, 18). These two stressors were used in the present study to determine the role of melanocortin signaling in stress responses. This is based upon the following considerations: 1) they share the ability to induce c-fos mRNA expression in the arcuate nucleus, in which POMC and AgRP cell bodies are located (4, 19); and 2) they permit opposite motoric activities, with limited movement for restraint stress and free bodily exertion for forced swim stress, resulting in distinct coping with stressful situations. Using these two emotional stress models, we compared the responsiveness of POMC and AgRP neurons, and determined the behavioral consequences, i.e. feeding activity and anxiety-like responses. Furthermore, pharmacological studies were designed to determine the involvement of central melanocortin signaling in emotional stress-induced changes in feeding and anxiety-like behavior.
Methods and Materials
Animals
Adult male Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA), weighing 250–300 g, were housed in groups of two under conditions of constant temperature and humidity on a 12-h light, 12-h dark cycle, with lights on at 0700 h. Food and water were available ad libitum. Animals were allowed to acclimate to these housing conditions for 1 wk before experiments began. All animal procedures were conducted in accordance with National Institutes of Health guidelines, and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Surgery
Rats were anesthetized with rabbit cocktail (43 mg/kg ketamine, 9 mg/kg xylazine, and 1.4 mg/kg acepromazine in saline) and fixed in a stereotaxic apparatus for cannula implantation. Using the stereotaxic coordinates, 0.8-mm posterior, 1.5-mm lateral to midline, and 2.5-mm ventral to bregma, a permanent 22-gauge stainless steel guide cannula (C313G; Plastics One, Roanoke, VA) was implanted into the lateral ventricle. The guide cannula was secured in place using dental cement and three stainless steel mounting screws anchored to the skull. A 28-gauge stainless steel dummy cannula, extending 1 mm beyond the guide, was used to seal the guide cannula when not in use. After surgery the animals were housed individually to avoid damage to guide and dummy cannula.
Microinjection
After intracerebroventricular (icv) cannulation, animals were allowed to recover for 7 d. During this period, they were handled daily to minimize stress caused by the microinjection procedure.
Melanocortin receptor antagonist SHU9119 (Peninsula Laboratories, Inc., San Carlos, CA) was freshly dissolved in 0.9% sterile saline before use. All icv injections were performed on conscious, unrestrained, freely moving rats in their home cages. Injections were performed over 1 min using a 33-gauge stainless steel injector connected to a 10-μl syringe (Hamilton Company, NV), which was operated by an infusion pump set to dispense 2 μl solution per minute. The injector was inserted and extended 1 mm beyond the tip of the guide cannula. Drug solutions or vehicle was infused in a volume of 2 μl delivered over 1 min. An additional minute was allowed for diffusion and prevention of backflow through the needle track before the injector was withdrawn.
Acute stress procedures
Animals were randomly assigned to either the control group or one of two acute stress conditions. All stress procedures were performed in a separate procedure room. Animals were transported to this room and habituated for 2 h on the day before the experiment, then returned to the housing room. On the experimental day, animals were first moved to the procedure room for 2 h without any disturbance. All acute stress procedures were performed in the light cycle.
Restraint stress
Rats were confined in white flexible plastic wrappers (19 × 25 cm) enclosed in a 20.5 × 7.5 × 7.5-cm clear Plexiglas open frame for 30 min. Holes at both ends of the open frame allowed for free air circulation.
Forced swim stress
Rats were placed in a water tank (45 cm high by 30 cm in diameter; filled with water at a depth of 35 cm) and forced to swim for 10 min. Animals were then dried and returned to the home cage.
Experimental protocol
Experiment 1: expression of c-fos mRNA in POMC and AgRP neurons in the arcuate nucleus after acute restraint and forced swim stress
Three groups of animals (n = 5 per group) were used. Two groups of animals were subjected to 30-min restraint stress or 10-min forced swim stress. Thirty minutes after the onset of stress, the animals were killed by decapitation. The nonstressed control animals were kept in the home cage without disturbance until being decapitated. Trunk blood was collected into heparin-treated tubes. Plasma was separated by centrifugation (3000 rpm for 15 min) for RIAs of corticosterone and leptin. Brains were removed, frozen in an isopentane-dry ice bath adjusted to −35 to −40 C, and stored at −80 C. Brain sections (14 μm) were cut on a Leica cryostat (Leica Microsystems GmbH, Wetzlar, Germany) through the hypothalamus and amygdala, thaw mounted onto polylysine-coated slides, and stored at −80 C until processing for in situ hybridization.
Experiment 2: effects of blockade of melanocortin signaling on the feeding response to acute restraint and forced swim stress
In our pilot studies, we used a fasting-induced feeding paradigm to determine the effect of acute restraint and forced swim stress on food intake. We found that 30-min acute restraint and 10-min forced swim stress were able to suppress food intake in fasted animals. The present study confirmed the anorectic effects of these two stressors in nonfasted rats by measuring spontaneous food intake in the dark cycle.
A total of 63 rats was used to investigate the effect of melanocortin signaling on feeding responses to acute emotional stressors. The rats were injected icv with either vehicle (saline), 0.05 nmol SHU9119, or 0.5 nmol SHU9119, and then subjected to either no stress, 30-min restraint, or 10-min forced swim stress. Before the experiment rats were weighed and counterbalanced into different treatment groups. icv injections were performed at 1 h before the dark cycle (1800 h), with stressors applied 30 min after injection. A preweighed chow hopper was placed in the home cage of each rat at the onset of the dark cycle (1900 h). Food intake was measured by weighing the remaining pellets and the spillage for 0.5, 2, and 24 h. A red light was provided during the measurement of food consumption in the dark cycle. To minimize disruption of food accessibility, two sets of containers were used to provide preweighed food to each animal. Food intake was calculated by subtracting the weight of remaining food from the initial weight.
Experiment 3: effects of blockade of melanocortin signaling on anxiety-like behavior in response to acute restraint and forced swim stress
A total of 59 rats was used to investigate the effect of melanocortin signaling on anxiety-like behavior in response to acute emotional stress. Animals received icv injection of either vehicle (saline), 0.05 nmol SHU9119, or 0.5 nmol SHU9119. Thirty minutes after icv injection, the animals were subjected to either no stress (control), 30-min restraint, or 10-min forced swim stress. Rats were tested in an elevated plus-maze test 30 min after the onset of restraint and forced swim. The elevated plus-maze was made of black-painted Plexiglas, with four arms (45-cm long and 12-cm wide) arranged in the shape of a “plus” sign and elevated to the height of 70 cm from the floor. Two arms have no side or end walls (open arms). The other two arms have side walls and end walls (45-cm high) but are open on top (closed arms). The open and closed arms intersect, having a central 12- × 12-cm square platform giving access to all arms. The rats were placed in the central square facing the corner between a closed arm and an open arm, and allowed to explore the elevated plus-maze for 5 min. Their activity on the elevated plus-maze was recorded by an Etho-Vision video tracking system (Noldus Information Technology Inc., Leesburg, VA). After each test the maze was thoroughly cleaned with 20% alcohol to eliminate the odor and trace of the previously tested animal. The time spent on the open and closed arms and the numbers of entries made into each arm were measured. Entry was defined as all four paws being positioned within one arm. The degree of anxiety was assessed by calculating the percentage of open arm entries (entries into the open arms/total entries into all arms) and percentage of open arm time (time spent in the open arms/total time spent in all arms).
Verification of cannula placement
Histological verification of the icv cannula was performed at the end of the experiments. Animals were deeply anesthetized and decapitated. The brain was then removed and frozen in isopentane (−35 C). Brain sections at 40 μm were cut in the coronal plane with a cryostat. The sections were stained with cresyl violet, and the location of the cannula tract was verified.
In situ hybridization for c-fos mRNA
Antisense cRNA probes for c-fos mRNA (783 mer) were labeled with 35S-UTP and 35S-CTP using a procedure reported in our previous studies (9, 19). Briefly, the linearized plasmid was incubated at 37 C for 2 h in a 25-μl reaction mixture consisting of transcription buffer (Invitrogen Corp., Carlsbad, CA), 75 μCi 35S-UTP, 75 μCi 35S-CTP, 150 μm ATP, 150 μm GTP, 10 mm dithiothreitol, 20 U ribonuclease (RNase) inhibitor, and 6 U T7 RNA polymerase. The radioactively labeled cRNA probe was separated from free nucleotides using a Microspin column (Bio-Rad Laboratories, Hercules, CA). In situ hybridization on brain tissue sections were performed as described previously (9, 19). Briefly, brain sections were fixed in 4% paraformaldehyde for 1 h and rinsed twice in 2× saline sodium citrate (SSC) [300 mm NaCl, 30 mm Na citrate (pH 7.2)]. Sections were then acetylated in 0.1 m triethanolamine (pH 8.0), with 0.25% acetic anhydride for 10 min, dehydrated through a graded series of ethanol (50–100%, 30 sec each), and subsequently air dried. 35S-Labeled cRNA probes were diluted to 3 × 106 cpm/75 μl in 50% hybridization buffer [50% formamide, 10% dextran sulfate, 3× SSC, 50 mm sodium phosphate buffer (pH 7.4), 1× Denhardt’s solution, 0.1 mg/ml yeast tRNA, and 30 mm dithiothreitol]. Diluted probes (75 μl) were placed on each slide, and the sections were coverslipped. Brain slides were placed in plastic trays moistened with 50% formamide and incubated at 55 C for 18 h. The following day, coverslips were lifted with 2× SSC, and slides were rinsed three times in 2× SSC, then incubated in RNase A (200 μg/ml) for 1 h at 37 C. Slides were then washed in SSC with increasing stringency, i.e. 2×, 1×, 0.5×, and 0.1× SSC (5 min each at room temperature). Finally, the sections were placed in 0.1× SSC at 70 C for 1 h, then rinsed in distilled water and dehydrated in a graded series of alcohols (50–100%, 30 sec each). Brain sections were exposed to x-ray film (BioMax MR; Eastman Kodak, Rochester, NY) for 5 d. Signal specificity was ensured by hybridization with sense-strand probes and pretreatment of brain sections with RNase A (200 μg/ml at 37 C for 60 min).
Analysis of induction and distribution of c-fos mRNA
A series of brain sections, 98 μm apart and anatomically matched, from each animal were used for quantification of c-fos mRNA levels. c-fos mRNA expression was analyzed by computer-assisted optical densitometry. Digital images of brain sections were captured from x-ray films in the linear range of the gray levels using a CCD camera (Model XC-77; Sony, Tokyo, Japan). The integrated optical density of in situ hybridization signals in various brain regions was determined using a MCID system (Imaging Research, Inc., Ontario, Canada). Optical density measures were defined as being 3.5 sd above background and were multiplied by the target area (number of pixels above background), yielding integrated optical density. Measurements were taken bilaterally using a standardized sampling box for each brain region to that ensure equivalent areas were analyzed between animals. c-fos mRNA levels were quantified bilaterally in the arcuate nucleus (12 sections), the paraventricular nucleus (PVN) of the hypothalamus (four sections), ventromedial hypothalamus (eight sections), dorsomedial hypothalamus (12 sections), central amygdala (14 sections), medial amygdala (14 sections), and basolateral amygdala (14 sections).
Double-labeling in situ hybridization
To examine the extent of colocalization of c-fos mRNA with POMC or AgRP mRNA, double-labeling in situ hybridization was performed as described in our earlier studies (9). cRNA probes directed against POMC or AgRP mRNA were generated and labeled with digoxigenin-UTP (Roche Diagnostics, Mannheim, Germany), and the cRNA probe complementary to c-fos was labeled with 35S-CTP and 35S-UTP using a standard transcription method. Brain sections were hybridized with a mixture of c-fos and POMC cRNA probes or a mixture of c-fos and AgRP cRNA probes for 18 h. The following day brain sections were washed with SSC, treated with RNase A (200 μg/ml) for 1 h at 37 C. After the final wash in 0.1× SSC at 68 C for 1 h, sections were then processed for immunohistochemistry to visualize the digoxigenin-labeled mRNA. Briefly, brain sections were treated with a blocking solution [0.1 m phosphate buffer containing 0.5% Triton X-100, and 0.25% carrageenan (pH 7.5)] for 4 h, and then incubated overnight with Fab fragments from an antidigoxigenin antibody from sheep, conjugated with horse-radish peroxidase (Roche Diagnostics), diluted 1:15,000. After rinsing in both 0.1 m phosphate buffer and 0.1 m Tris buffer (30 min each), sections were incubated with a color reaction buffer (0.45% nitroblue tetrazolium chloride, 0.35% 5-bromo-4-chloro-3-indoylphosphate 4-toluidine salt, 5% polyvinyl alcohol, and 0.24% levamisole). The color reaction was completed in 2 h for POMC mRNA and 10 h for AgRP mRNA. Sections were then rinsed in water and incubated with 0.1 m glycine buffer (pH 2.2), containing 0.5% Triton X-100 for 10 min. Finally, brain sections were fixed in 25% glutaraldehyde for 2 h. After rinsing in water and dehydrating in a graded series of ethanol (50–100%), brain section slides were dipped in liquid emulsion (Eastman Kodak), air dried, and stored in a dark box at 4 C. After 4-wk exposure, brain section slides were developed and fixed. After rinsing in water, the slides were dehydrated using a graded series of ethanol and coverslipped in a xylene-based mounting medium (Permount; Fisher Scientific, Houston, TX).
The distribution of cells that was positive for c-fos, POMC, or AgRP mRNAs was determined using an Olympus microscope (Olympus Corp., Japan). Digoxigenin-labeled mRNAs (POMC and AgRP) were visualized under a bright field as a blue-purple precipitate. 35S-labeled mRNA (c-fos) was visualized using a dark-field condenser as silver grain clusters. Sections through the arcuate nucleus were analyzed to determine the extent of colocalization of c-fos with POMC or AgRP. For the combinations of c-fos with POMC or AgRP, nine sections along the rostro-caudal axis, corresponding to Bregma −2.1 to −4.2 mm, of the arcuate nucleus per rat, and five rats for each condition were analyzed. Cell counts were determined at ×20 magnification bilaterally within the entire arcuate nucleus that was defined using Nissl counterstained slides. The total number of cells for c-fos, POMC, or AgRP was counted. A digoxigenin-labeled cell was considered to be double labeled if the density of silver grains directly overlaying the cell body was 3 times more than the background level.
Image processing
Film autoradiogram images were captured with a Sony CCD video camera set above a Northern Lights box using a MCID system (Imaging Research). Bright-field and dark-field microscopic images at high magnification to show double labeling were captured on a DP controller digital camera attached to an Olympus microscope. Images were processed using Adobe Photoshop software (Adobe Systems, Inc., San Jose, CA).
Plasma corticosterone and leptin analysis
Plasma corticosterone was assayed using a highly specific corticosterone antibody (Chemicon International, Inc., Temecula, CA). Briefly, 10-μl duplicate samples of plasma were heated at 70 C for 30 min to denature corticosterone-binding protein, and incubated overnight with corticosterone antibody and [3H]corticosterone (PerkinElmer, Boston, MA). Free and bound corticosterone was separated by incubation with charcoal for 15 min. Plasma leptin was measured with a RIA kit (Linco Research, St. Charles, MO). The sensitivity for this assay was 0.5 ng/ml.
Data analysis
Results are expressed as mean ± sem. One-way ANOVA with repeated measures was used to compare the rostro-caudal distribution of the proportions of arcuate POMC and AgRP neurons double labeled for c-fos mRNA across the different treatment groups. The effect of the melanocortin antagonist SHU9119 on food intake and anxiety-like behavior in response to acute stress was analyzed using two-way ANOVA. Post hoc comparisons were performed using Bonferroni/Dunn. P < 0.05 was considered statistically significant.
Results
c-fos mRNA expression patterns in response to acute restraint and forced swim stress
c-fos has been widely used as a marker of neuronal activation in response to stress. To determine neuronal activation in response to acute restraint and forced swim stress, we determined the expression of c-fos mRNA in the brain focusing on the arcuate nucleus, in which POMC and AgRP neurons are located. As shown in Fig. 1, whereas control rats showed negligible c-fos mRNA expression in a variety of brain regions, robust c-fos mRNA expression was induced in the hypothalamus after the exposure to 30-min restraint or 10-min forced swim stress (Table 1). The highest level of c-fos mRNA expression was observed in the PVN in response to either acute stressor. Robust stimulation of neurons in the arcuate nucleus was observed after each of the stressors. c-fos mRNA was detected throughout the rostrocaudal extent of the arcuate nucleus. In addition, c-fos mRNA induction was observed in the dorsomedial and ventromedial hypothalamus.
Fig. 1.
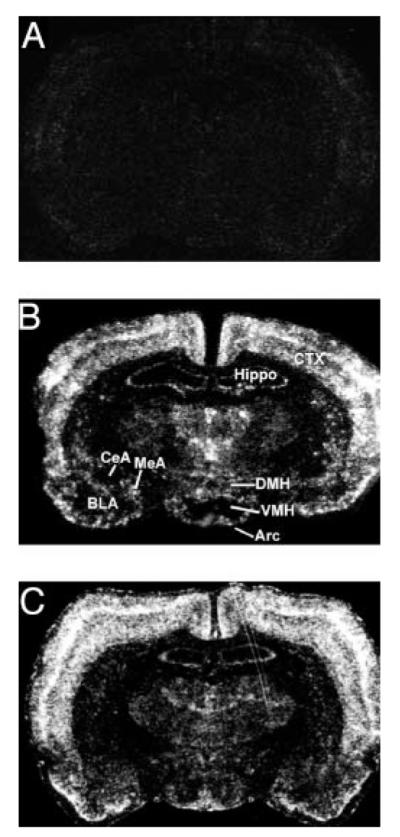
Images of film autoradiograms (×2) showing c-fos mRNA expression in the brain under nonstressful condition (A), 30 min after the onset of restraint (B), or forced swim stress (C). Arc, Arcuate nucleus; BLA, basolateral amygdala; CeA, central amygdala; CTX, cortex; DMH, dorsomedial hypothalamus; Hippo, hippocampus; MeA, medial amygdala; VMH, ventromedial hypothalamus.
TABLE 1.
c-fos mRNA expression in the subregions of the hypothalamus and amygdala after acute restraint and forced swim stress
| Brain area | Treatment |
||
|---|---|---|---|
| Control | Restraint | Forced swim | |
| Arc | 83 ± 47 | 1204 ± 238a | 3529 ± 767a |
| PVN | 255 ± 206 | 13398 ± 3793a | 13481 ± 4584a |
| VMH | 5 ± 3 | 314 ± 79a | 625 ± 288b |
| DMH | 19 ± 6 | 1560 ± 417a | 1325 ± 422a |
| MeA | 32 ± 18 | 841 ± 316b | 2525 ± 1193b |
| CeA | 25 ± 16 | 126 ± 60 | 262 ± 132 |
| BLA | 35 ± 26 | 57 ± 25 | 261 ± 186b |
The levels of c-fos mRNA were represented by the integrated optical density measurements of film autoradiograms, expressed as mean±SEM (n = 5 per group). Arc, Arcuate nucleus; BLA, basolateral amygdala; CeA, central amygdala; DMH, dorsomedial hypothalamus; MeA, medial amygdala; VMH, ventromedial hypothalamus.
P ≤ 0.01, compared with the nonstressed control group.
P < 0.05.
Levels of c-fos mRNA expression were also quantified for subdivisions of the amygdala, one of the major extrahypothalamic targets of POMC and AgRP projections (4). Within this region, distribution of c-fos mRNA expression was heterogeneous, with the highest level of c-fos mRNA expression in the medial amygdala after either restraint or forced swim stress (Table 1). Much lower levels of c-fos mRNA were seen in the central amygdala and basolateral amygdala. The preference of activation of neurons in the medial amygdala over the central amygdala is consistent with the previous reports indicating that the medial amygdala is more sensitive to emotional stress (11, 12, 18).
Effects of acute emotional stress on plasma corticosterone and leptin levels
Acute restraint and forced swim provoked a pronounced surge of corticosterone. Circulating corticosterone levels were increased 30 min after the initiation of restraint and forced swim stress compared with naive controls (plasma corticosterone concentrations: nonstressed control 0.01 ± 0 μg/dl; restraint stress 24.5 ± 1.72 μg/dl; forced swim stress 31.9 ± 2.33 μg/dl). ANOVA analysis showed a significant effect of stress administration [F (2, 12) = 107.67; P < 0.0001]. Post hoc analyses indicated that both restraint and forced swim stress significantly increased corticosterone levels in plasma (P < 0.0001 for restraint stress and forced swim), compared with the nonstressed control group. In addition, leptin levels were measured in plasma collected from the same animals. However, neither restraint nor forced swim significantly altered leptin levels in plasma, which was measured at 30 min after the onset of stress [F (2, 12) = 1.444; P = 0.2741] (plasma leptin concentrations: control 2.04 ± 0.21 ng/ml; restraint stress 1.64 ± 0.56 ng/ml; forced swim stress 1.43 ± 0.34 ng/ml).
Activation of POMC vs. AgRP neurons by acute restraint and forced swim stress
POMC and AgRP cells constitute two distinct cell populations in the arcuate nucleus. To reveal the detailed profiles of the distribution of activated POMC and AgRP neurons along the rostro-caudal axis of the arcuate nucleus, we analyzed the number of singly labeled POMC/AgRP cells, c-fos-positive cells, and POMC/AgRP cells double-labeled for c-fos mRNA from nine coronal levels of the nucleus. It should be noted that there was no attempt to count absolute numbers of cells within the entire arcuate nucleus. The cell counting data at different coronal planes obtained from each rat were used to calculate the relative percentage of colocalization of c-fos mRNA with POMC or AgRP mRNA for a rostrocaudal comparison.
POMC neurons were distributed in the lateral sector of the arcuate nucleus (Fig. 2). c-fos mRNA was rarely observed in POMC neurons in nonstressed control rats. In response to acute restraint and forced swim stress, c-fos mRNA was induced in a high percentage of POMC neurons (up to 53% for restraint, up to 62% for forced swim) 30 min after the onset of stress (Figs. 2 and 3). Although the total number of POMC neurons exhibited no marked rostro-caudal variations, the number of c-fos-positive POMC neurons after either stressor showed evident rostro-caudal heterogeneity (Fig. 3, B and C). A “bell-shaped” distribution pattern of double-labeled POMC neurons with the peak in the middle level of arcuate nucleus was observed (Fig. 3D). Forced swim stress induced higher percentages of double-labeled POMC neurons at the levels from Bregma −2.6 to −2.8 mm than those in animals subjected to restraint stress (P<0.01 at Bregma−2.6; P<0.05 at Bregma −2.8).
Fig. 2.
Microscopic images showing induction of c-fos mRNA expression in POMC neurons in the arcuate nucleus under the basal condition (control, A and A’) or after acute restraint (B and B’) or forced swim (C and C’) stress. A–C, Bright-field microscopic images (×50) showing the distribution of POMC-expressing neurons (detected by digoxigenin-labeled riboprobe, dark purple cells). A’–C’, High magnification of dark-field microscopic images (×100) showing that POMC neurons (dark purple cells) coexpress c-fos mRNA (detected by 35S-labeled riboprobe, clusters of white grains). Black arrowheads indicate cells not double labeled for c-fos mRNA and POMC mRNA. Yellow arrowheads indicate cells double labeled for c-fos mRNA and POMC mRNA.
Fig. 3.
Number of POMC neurons and POMC neurons double labeled for c-fos mRNA along the rostro-caudal axis of the arcuate nucleus under basal condition (control), or after restraint or forced swim stress. Cell counts were made on nine sections per animal corresponding to nine coronal planes from Bregma −2.1 to Bregma −4.2 mm. Sections for cell counting were anatomically matched between animals within the same treatment group as well as between different treatments. A, Control. B, Restraint stress. C, Forced swim. D, Distribution of the percentages of POMC neurons positive for c-fos mRNA along the rostral-caudal axis of the arcuate nucleus under basal condition (naive) or after restraint and forced swim. Data are expressed as mean ± SEM (n = 5 per group). *, P < 0.05, **, P < 0.01, restraint vs. forced swim stress.
To compare the pattern of neuronal activation between POMCand AgRP neurons, adjacent brain sections were used to evaluate the distribution and proportion of AgRP neurons double labeled for c-fos mRNA. AgRP neurons in the arcuate nucleus were concentrated in the medial sector of the nucleus (Fig. 4). The distribution of AgRP neurons at the coronal planes from Bregma −2.1 to Bregma −4.2 mm exhibited a rostro-caudal difference with a peak at the level of Bregma −3.3 mm (Fig. 5). In contrast to the high percentages of POMC neurons double labeled for c-fos mRNA, especially in the middle portion of the arcuate nucleus, only moderate proportions of AgRP neurons were found to coexpress c-fos mRNA after restraint and forced swim stress, ranging from 18–24% (mean values) for restraint stress and 18–23% (mean values) for forced swim (Fig. 5). The c-fos-positive AgRP neurons in the arcuate nucleus were distributed uniformly without evident rostro-caudal differences.
Fig. 4.
Microscopic images showing induction of c-fos mRNA expression in AgRP neurons in the arcuate nucleus under basal condition (control A and A’) or after acute restraint (B and B’) and forced swim (C and C’) stress. A–C, Bright-field microscopic images (×50) showing the distribution of AgRP-expressing neurons (detected by digoxigenin-labeled riboprobe, dark purple cells). A’–C’, High magnification of dark-field microscopic images showing AgRP neurons (dark purple cells) double labeled for c-fos mRNA (detected by 35S-labeled riboprobe, clusters of white grains) (×100). Black arrowheads indicate cells not double labeled for c-fos mRNA and AgRP mRNA. Yellow arrowheads indicate cells double labeled for c-fos mRNA and AgRP mRNA.
Fig. 5.
Number of AgRP neurons and AgRP neurons double labeled for c-fos mRNA along the rostro-caudal axis of the arcuate nucleus under basal condition (control) or after restraint and forced swim stress. Cell counts were made on sections corresponding to nine coronal planes from Bregma −2.1 to Bregma −4.2 mm. Brain sections for cell counting were adjacent to those used for POMC cell counting in Fig. 3. A, Control. B, Restraint stress. C, Forced swim. D, Distribution of the percentages of AgRP neurons positive for c-fos mRNA along the rostro-caudal axis of the arcuate nucleus under basal condition (control) or after restraint and forced swim stress. Data are expressed as mean ± SEM (n = 5 per group).
Effect of the central melanocortinergic pathway on behavioral responses to acute restraint and forced swim stress
The c-fos colocalization data described above indicated a preponderant recruitment of the arcuate POMC neuronal pathway over the AgRP neuronal pathway by two acute emotional stressors. Next, we investigated the corresponding behavioral responses to stress and determined whether endogenous central melanocortinergic pathway contributes to behavioral consequences of acute emotional stress.
Melanocortin signaling and acute emotional stress-induced anorexia
One behavioral consequence of emotional stress is anorexia (24–25). We examined the effect of acute restraint and forced swim stress on spontaneous food intake. ANOVA revealed a significant effect on food intake for treatments and time [F (8, 108) = 6.112; P < 0.001]. After acute restraint (30 min) or forced swim stress (10 min), food intake was significantly reduced over the 30 min (P < 0.01 for both stressors) and 2-h (P < 0.01 for both stressors) periods. To examine the role of central melanocortin signaling in stress-induced short-term anorexia, the melanocortin receptor antagonist SHU9119 was icv injected 30 min before the application of acute stress. SHU9119 at either 0.05 or 0.5 nmol administered alone had no significant effect on 30-min and 2-h food intake compared with the vehicle treatment in nonstressed rats (Fig. 6). However, food intake by 24 h was significantly increased after icv injection of 0.5 nmol SHU9119 (vehicle: 7.4 ± 0.33 g/100 g body weight; SHU9119–0.5 nmol: 10.6 ± 0.55 g/100 g body weight; P < 0.01). The pretreatment with SHU9119 was able to attenuate the suppression of food intake over 30 min and 2 h induced by either restraint [F (2, 18) = 3.736; P < 0.05] or forced swim stress [F (2, 16) = 6.652, P < 0.01] in a dose-dependent manner (Fig. 6). Post hoc analyses indicated that SHU9119 at 0.5 nmol significantly prevented the reduction of food intake caused by either stressor, both at 30 min and 2-h time points (30-min food intake: P < 0.01 for restraint stress, P < 0.05 for forced swim stress; 2-h food intake: P < 0.05 for restraint stress, P < 0.01 for forced swim stress). The lower dose of SHU9119 also significantly suppressed food intake over the 2-h period after forced swim stress (P < 0.01, Fig. 6B), though it had no discernable impact on restraint stress-induced anorexia.
Fig. 6.
Effect of acute restraint and forced swim stress on feeding behavior. Rats were injected icv with 0, 0.05, or 0.5 nmol SHU9119 30 min before exposure to either no stress (control), restraint for 30 min, or forced swim stress for 10 min. icv injections were performed at 1 h before the dark cycle, with stressors applied 30 min after icv injection. The treatment groups were as follows: vehicle followed by no stress (n = 12), 0.05 nmol SHU9119 followed by no stress (n = 5), 0.5 nmol SHU9119 followed by no stress (n = 6), vehicle followed by restraint stress (n = 9), 0.05 nmol SHU9119 followed by restraint stress (n = 6), 0.5 nmol SHU9119 followed by restraint stress (n = 6), vehicle followed by forced swim stress (n = 7), 0.05 nmol SHU9119 followed by forced swim stress (n = 6), and 0.5 nmol SHU9119 followed by forced swim stress (n = 6). Food was provided 30 min after the onset of either stressor. Food intake was measured over 30 min (A) and 120 min (B) and adjusted by body weight. Data are expressed as mean ± SEM. **, P < 0.01, compared with the nonstressed group. #, P < 0.05, ##, P < 0.01, compared with the vehicle-treated condition combined with the same stress exposure.
Melanocortin signaling and acute emotional stress-induced anxiety-like behavior
Another behavioral consequence of emotional stress is anxiety-like activity (20–23). The elevated plus-maze test is widely used to evaluate the relative anxiety status of rodents. In this test the percentage of open arm entries and time spent in the open arms has been validated as a measure of anxiety (26). The effect of exposure to acute restraint or forced swim stress, combined with treatment with the melanocortin receptor antagonist SHU9119, on the percentage of open arm entries and time spent in the open arms was evaluated. Exposure to acute restraint stress or forced swim stress markedly and significantly reduced the percentage of open arm entries (P < 0.001 for each stressor) and the percentage of time spent in the open arms (P < 0.01 for each stressor), compared with the non-stressed control group (Fig. 7), indicating anxiogenic effects of these two stressors. Although central administration of SHU9119 alone did not significantly alter the anxiety level in nonstressed rats, it dose dependently attenuated the reduction in the percentage of open arm entries induced by restraint and forced swim stress [F (5, 29) = 4.713; P < 0.01] and the percentage of time spent in the open arms [F (5, 29) = 3.504; P < 0.05], compared with the vehicle treatment. Post hoc analyses indicated that pretreatment with the dose of 0.5 nmol SHU9119 significantly attenuated the acute stress-induced reduction of the percentage of open arm entries (P < 0.01 for both stressors) and of time spent in the open arms (P < 0.05 for the restraint stress condition; P < 0.01 for the forced swim stress condition).
Fig. 7.
Effect of acute restraint and forced swim stress on anxiety-like behavior in the elevated plus-maze test. Rats were injected icv with 0, 0.05, or 0.5 nmol SHU9119 30 min before exposure to either no stress (control), restraint for 30 min, or forced swim stress for 10 min. The treatment groups were as follows: vehicle followed by no stress (n = 10), 0.5 nmol SHU9119 followed by no stress (n = 5), vehicle followed by restraint stress (n = 13), 0.05 nmol SHU9119 followed by restraint stress (n = 5), 0.5 nmol SHU9119 followed by restraint stress (n = 5), vehicle followed by forced swim stress (n = 8), 0.05 nmol SHU9119 followed by forced swim stress (n = 5), and 0.5 nmol SHU9119 followed by forced swim stress (n = 5). Elevated plus-maze tests were performed for 5 min after acute stress. The number of entries into the open arms and closed arms and time spent in the open arms and closed arms was measured. Entries into the open arms/total entries into all arms [OTR(e)] (A) and time spent in the open arms/total time spent [OTR(t)] in all arms (B) were calculated. Data are expressed as mean ± SEM. **, P < 0.01; ***, P < 0.001, compared with the nonstressed control group. #, P < 0.05; ##, P < 0.01 compared with the vehicle-treated condition combined with the same stress exposure. +, P < 0.05; ++, P < 0.01 compared with 0.05 nmol SHU9119.
Discussion
The present study provided a detailed analysis of the responsiveness of POMC and AgRP neurons along the rostro-caudal extent of the arcuate nucleus to acute emotional stress. We showed that the POMC pathway is predominantly recruited by acute restraint and forced swim stress. The functional consequence of activation of the POMC pathway was further determined by investigating the effect of pharmacologically blocking central melanocortin signaling on acute stress-induced anorexia and anxiety-like behavior. We demonstrated that pretreatment with the melanocortin receptor antagonist SHU9119 prevented acute emotional stress-induced anorectic and anxiogenic-like effects, suggesting the involvement of melanocortin signaling in mediating behavioral responses to emotional stressors.
Activation of POMC vs. AgRP neurons by acute emotional stress
c-fos, as an immediate early gene, has been used extensively as a marker for neuronal activation to delineate individual neurons as well as neural circuits that are responsive to stressful stimuli (27–32). By analyzing c-fos mRNA induction in POMC neurons vs. AgRP neurons in the arcuate nucleus on adjacent brain sections, we revealed that a much higher percentage of POMC neurons was activated by either restraint or forced swim stress, in comparison with AgRP neurons in response to the same stressors. Although the total number of AgRP neurons is almost twice as much as POMC neurons; however, this does not translate to a doubled density of AgRP terminals in various brain regions (4). On the other hand, the abundance of POMC mRNA appears to be much higher than that of AgRP mRNA. Therefore, the percentage rather than absolute numbers of activated POMC and AgRP cells may better represent their relative responsiveness to acute emotional stress.
Although forced swim stress and restraint showed different stimulation intensity in the arcuate nucleus, the overall rostro-caudal distribution patterns of activated POMC and AgRP neurons in response to two stressors are similar. The similar neuronal activation patterns produced by restraint and forced swim may relate to their common emotional aspect, as opposed to activity. Moreover, it seems that POMC neurons in the middle level of the arcuate nucleus are most sensitive to emotional stress, as reflected by the highest percentage of POMC neurons positive for c-fos mRNA. This finding is consistent with previous reports on functional diversity of POMC neurons at the rostro-caudal extent of arcuate nucleus (33, 34).
The predominant recruitment of the arcuate POMC neuronal pathway by emotional stress is in contrast to that induced by metabolic stressors, e.g. food deprivation and glucoprivation, in which AgRP neurons are primarily activated. It has been reported that 71% of neuropeptide Y (NPY)/AgRP neurons in the arcuate nucleus are c-fos positive after 24-h fasting (35). In addition, the electrical activity of AgRP/NPY neurons is enhanced during fasting (36). This is consistent with the postulated association of c-fos induction with intracellular excitatory events. On the other hand, central glucoprivation induced by administration of 2-deoxy-d-glusose evokes c-fos mRNA expression in arcuate NPY/AgRP neurons (37). Under the circumstances that food and/or glucose are deprived, increased AgRP/NPY neuronal activity is believed to contribute to an increase in feeding (38). Interestingly, a moderate percentage of AgRP neurons was also activated by acute emotional stress. Whether the activated AgRP/NPY pathway under stress conditions represents a mechanism to compensate for stress-induced anorexia or underlie stress coping is not known.
Although the mechanisms underlying the predominant activation of POMC by acute emotional stress remain to be elucidated, previous studies have identified leptin as a potent stimulator of POMC neuronal activity. Administration of leptin induces c-fos mRNA expression in POMC neurons (39). In addition, electrophysiological studies demonstrate that leptin increases the frequency of action potentials in POMC neurons (40). However, it is unlikely that leptin is responsible for POMC neuronal activation during acute emotional stress because leptin levels were not increased by either restraint or forced swim stress.
Involvement of melanocortin signaling in behavioral responses to acute emotional stress
The functional role of predominant activation of POMC neurons was investigated by examining the effects of melanocortin signaling on behavioral responses to acute emotional stress. A large body of evidence shows that exposure to emotional stress exerts anorectic effects in rodents and humans (41). Given that central administration of POMC-derived melanocortin receptor agonist α-MSH and its analog MTII elicits potent inhibition of food intake (9, 42–47), the predominant activation of the central melanocortinergic pathway may underlie the mechanism of emotional stress-induced anorexia. To test this we blocked central melanocortin receptors MC3R and MC4R using SHU9119 before exposure to stress. Acute restraint and forced swim stress-induced anorectic effects were prevented by pretreatment with SHU9119. SHU9119 binds to both MC3R and MC4R, the two primary central melanocortin receptors (44). However, the reversal of stress-induced anorexia by SHU9119 is believed to be mediated by MC4R because disruption of MC4R eliminates feeding responses to melanocortins and its analogs (48). Furthermore, the feeding effect mediated by MC4R has been restricted to specific brain regions (49). The PVN is likely to be the key neuroanatomical substrate for MC4R functions in the control of food intake (49). The activated POMC neurons and subsequent α-MSH release in the PVN may mediate stress-induced anorexia. Future studies will address whether blocking MC4R specifically in the PVN can reverse stress-induced anorexia.
Another behavioral consequence of emotional stress is the induction of anxiogenic-like behavior. We show that acute restraint and forced swim stress induce anxiety-like behavior as indicated by decreased percentages of open arm entries and time spent in the open arms in the elevated plus-maze test. This stress behavioral reaction temporally coincides with POMC neuronal activation. It has been reported that enhancing central melanocortin signaling by administering exogenous α-MSH and its analogs mimics stress-induced anxiogenic effects. For instance, icv injection of α-MSH and MTII reduces time spent in the open arms in the elevated plus-maze test (50), and the number of licking periods in Vogel test (51). Our observations that the blockade of central melanocortin receptors with SHU9119 attenuated restraint and forced swim stress-induced anxiety-like behavior support the hypothesis that anxiogenic-like behavioral responses to emotional stress are mediated via activation of the central melanocortinergic pathway.
One candidate brain area that may act as a relay for enhanced arcuate POMC neuronal activity and anxiety-like behavior induced by emotional stress is the medial amygdala. This nucleus is a recipient of α-MSH/POMC projections and expresses high levels of melancortin receptors (4, 52, 53). There exists an additional POMC neuronal cell population located in the nucleus of solitary tract. However, α-MSH/POMC fibers in the medial amygdala are likely derived from POMC neurons in the arcuate nucleus instead of the nucleus of solitary tract. This assumption is based upon the finding that arcuate lesions almost completely eliminate α-MSH immunoreactivity in the amygdala (54). Evidence suggests that the medial amygdala is a critical area for emotional processing (55–57). It has been shown that the medial amygdala is particularly sensitive to emotional/psychological stress (11, 12, 18), and pharmacological manipulations of this anatomical division alter the anxiety state (58, 59).
Emotional stress can trigger anorexia and anxiety in humans (20–25). However, the neurobiological basis of this comorbidity between anorexia and anxiety is poorly understood. The results of this study provide evidence suggesting that increased central melanocortin signaling may be involved in the mechanisms underlying emotional stress-induced anorectic and anxiogenic effects.
Acknowledgments
The authors thank Honghong Yao for early contributions to this work.
This work was supported by American Heart Association Scientist Development Award AHA0530345N (to X.-Y.L.) and National Institutes of Health Grants 1R01MH073844 and 1R01MH076929 (to X.-Y.L.).
Abbreviations
- AgRP
Agouti-related protein
- icv
intracerebroventricular
- MC3R
melanocortin-3 receptor
- MC4R
melanocortin-4 receptor
- NPY
neuropeptide Y
- POMC
proopiomelanocortin
- PVN
paraventricular nucleus
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Endocrinology is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the endocrine community.
References
- 1.Seeley RJ, Drazen DL, Clegg DJ. The critical role of the melanocortin system in the control of energy balance. Annu Rev Nutr. 2004;24:133–149. doi: 10.1146/annurev.nutr.24.012003.132428. [DOI] [PubMed] [Google Scholar]
- 2.Lu XY. Role of central melanocortin signaling in eating disorders. Psychopharmacol Bull. 2001;35:45–65. [PubMed] [Google Scholar]
- 3.MacNeil DJ, Howard AD, Guan X, Fong TM, Nargund RP, Bednarek MA, Goulet MT, Weinberg DH, Strack AM, Marsh DJ, Chen HY, Shen CP, Chen AS, Rosenblum CI, MacNeil T, Tota M, MacIntyre ED, VanderPloeg LH. The role of melanocortins in body weight regulation: opportunities for the treatment of obesity. Eur J Pharmacol. 2002;440:141–157. doi: 10.1016/s0014-2999(02)01425-5. [DOI] [PubMed] [Google Scholar]
- 4.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adan RA, Szklarczyk AW, Oosterom J, Brakkee JH, Nijenhuis WA, Schaaper WM, Meloen RH, Gispen WH. Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. Eur J Pharmacol. 1999;378:249–258. doi: 10.1016/s0014-2999(99)00465-3. [DOI] [PubMed] [Google Scholar]
- 6.DeBarioglio SR, Lezcano N, Celis ME. αMSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides. 1991;12:203–205. doi: 10.1016/0196-9781(91)90189-v. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of α-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 8.Vergoni AV, Bertolini A. Role of melanocortins in the central control of feeding. Eur J Pharmacol. 2000;405:25–32. doi: 10.1016/s0014-2999(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 9.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 11.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governinghypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 12.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner CG, McMahon CD, Marks DL, Daniel JA, Steele B, Sartin JL. A role for agouti-related protein in appetite regulation in a species with continuous nutrient delivery. Neuroendocrinology. 2004;80:210–218. doi: 10.1159/000082735. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 15.Sergeyev V, Broberger C, Gorbatyuk O, Hokfelt T. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport. 2000;11:117–121. doi: 10.1097/00001756-200001170-00023. [DOI] [PubMed] [Google Scholar]
- 16.Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-D-glucose-induced neuropeptide Y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology. 2003;144:75–83. doi: 10.1210/en.2002-220659. [DOI] [PubMed] [Google Scholar]
- 17.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 18.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- 20.Dierker LC, Merikangas KR. Familial psychiatric illness and posttraumatic stress disorder: findings from a family study of substance abuse and anxiety disorders. J Clin Psychiatry. 2001;62:715–720. doi: 10.4088/jcp.v62n0909. [DOI] [PubMed] [Google Scholar]
- 21.Jetty PV, Charney DS, Goddard AW. Neurobiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:75–97. doi: 10.1016/s0193-953x(05)70207-0. [DOI] [PubMed] [Google Scholar]
- 22.Pynoos RS, Steinberg AM, Piacentini JC. A developmental psychopathology model of childhood traumatic stress and intersection with anxiety disorders. Biol Psychiatry. 1999;46:1542–1554. doi: 10.1016/s0006-3223(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 23.Rickels K, Rynn M. Overview and clinical presentation of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:1–17. doi: 10.1016/s0193-953x(05)70203-3. [DOI] [PubMed] [Google Scholar]
- 24.Brambilla F. Social stress in anorexia nervosa: a review of immunoendocrine relationships. Physiol Behav. 2001;73:365–369. doi: 10.1016/s0031-9384(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 25.Holden RJ, Pakula IS. Tumor necrosis factor-α: is there a continuum of liability between stress, anxiety states and anorexia nervosa? Med Hypotheses. 1999;52:155–162. doi: 10.1054/mehy.1997.0641. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 27.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 28.Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury GM, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–182. doi: 10.1016/s0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 30.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 31.Morinobu S, Nibuya M, Duman RS. Chronic antidepressant treatment down-regulates the induction of c-fos mRNA in response to acute stress in rat frontal cortex. Neuropsychopharmacology. 1995;12:221–228. doi: 10.1016/0893-133X(94)00067-A. [DOI] [PubMed] [Google Scholar]
- 32.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 33.Wiemann JN, Clifton DK, Steiner RA. Pubertal changes in gonadotropin-releasing hormone and proopiomelanocortin gene expression in the brain of the male rat. Endocrinology. 1989;124:1760–1767. doi: 10.1210/endo-124-4-1760. [DOI] [PubMed] [Google Scholar]
- 34.Chowen-Breed JA, Clifton DK, Steiner RA. Regional specificity of testosterone regulation of proopiomelanocortin gene expression in the arcuate nucleus of the male rat brain. Endocrinology. 1989;124:2875–2881. doi: 10.1210/endo-124-6-2875. [DOI] [PubMed] [Google Scholar]
- 35.Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 37.Minami S, Kamegai J, Sugihara H, Suzuki N, Higuchi H, Wakabayashi I. Central glucoprivation evoked by administration of 2-deoxy-D-glucose induces expression of the c-fos gene in a subpopulation of neuropeptide Y neurons in the rat hypothalamus. Brain Res Mol Brain Res. 1995;33:305–310. doi: 10.1016/0169-328x(95)00151-h. [DOI] [PubMed] [Google Scholar]
- 38.Flier JS. AgRP in energy balance: will the real AgRP please stand up? Cell Metab. 2006;3:83–85. doi: 10.1016/j.cmet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 40.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 41.Smith GP. Animal models of human eating disorders. Ann NY Acad Sci. 1989;575:63–72. doi: 10.1111/j.1749-6632.1989.tb53233.x. [DOI] [PubMed] [Google Scholar]
- 42.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular α-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- 43.Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR, Smith DM, Ghatei MA, Bloom SR. Hypothalamic localization of the feeding effect of agouti-related peptide and α-melanocyte-stimulating hormone. Diabetes. 2000;49:177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- 44.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 45.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 46.Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol. 1998;274(1 Pt 2):R248–R254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- 47.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 50.Rao TL, Kokare DM, Sarkar S, Khisti RT, Chopde CT, Subhedar N. GABAergic agents prevent α-melanocyte stimulating hormone induced anxiety and anorexia in rats. Pharmacol Biochem Behav. 2003;76:417–423. doi: 10.1016/j.pbb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Chaki S, Ogawa S, Toda Y, Funakoshi T, Okuyama S. Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. Eur J Pharmacol. 2003;474:95–101. doi: 10.1016/s0014-2999(03)02033-8. [DOI] [PubMed] [Google Scholar]
- 52.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 53.Jacobowitz DM, O’Donohue TL. α-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Donohue TL, Miller RL, Jacobowitz DM. Identification, characterization and stereotaxic mapping of intraneuronal α-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Res. 1979;176:101–123. doi: 10.1016/0006-8993(79)90873-4. [DOI] [PubMed] [Google Scholar]
- 55.Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16:328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- 56.LeDoux JE. The emotional brain: the mysterious underpinnings of emotional life. Simon & Schuster; New York: 1996. [Google Scholar]
- 57.Ebner K, Rupniak NM, Saria A, Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci USA. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duxon MS, Kennett GA, Lightowler S, Blackburn TP, Fone KC. Activation of 5-HT2B receptors in the medial amygdala causes anxiolysis in the social interaction test in the rat. Neuropharmacology. 1997;36:601–608. doi: 10.1016/s0028-3908(97)00042-7. [DOI] [PubMed] [Google Scholar]
- 59.Forestiero D, Manfrim CM, Guimaraes FS, de Oliveira RM. Anxiolytic-like effects induced by nitric oxide synthase inhibitors microinjected into the medial amygdala of rats. Psychopharmacology (Berl) 2006;184:166–172. doi: 10.1007/s00213-005-0270-6. [DOI] [PubMed] [Google Scholar]








