Abstract
Adaptation to a sustained stimulus is an important phenomenon in psychophysical experiments. When studying the response to an experimental task, the investigator has to account for the change in perceived stimulus intensity with repeated stimulus application and, if the stimulus is sustained, for the change in intensity during the presentation. An example of a sustained stimulus is the cold pressor task (CPT). The task has been used both as an experimental pain task and to study cardiovascular physiology. In functional imaging research, the CPT has been used to evaluate cognitive processing of a noxious stimulus. Investigators typically model the stimulus in a block design as a categorical (on-off) stimulus and do not account for a temporal change in stimulus perception. If the perceived stimulus changes over time, the results may be misleading.
Therefore, we characterized the time course of cold pain in human volunteers and developed a model of the temporal characteristics of perceived cold pain. Fifteen healthy participants underwent cold pain testing by immersing their right foot into a container filled with ice water (2 °C) for 30 seconds alternating with a 30 seconds immersion into a container filled with tepid water 32°C (control). Participants rated the pain intensity using an electronic slide algometer.
Using a mixed general linear model (effectively a polynomial regression model), we determined that pain ratings follow a crescendo-decrescendo pattern that can be described well using a quadratic model. We conclude that the time course of quantitative perception differs fundamentally from the time course of stimulus presentation. This may be important when looking for the physiological correlates of perception as opposed to the presence of a stimulus per se.
Keywords: Pain, Rating, Cold, Sensory Adaptation, Imaging
Introduction
In this report, we present our work on modeling the subjective experience of cold pain. More specifically, we estimate the time course of perceived pain intensity in response to repeated thirty second immersion of the right foot into ice water. This research question is important to the study of pain psychophysics and has particular relevance to the growing field of pain imaging.
When analyzing data from a pain imaging experiment, pain intensity is often assumed to remain constant; it is treated as a binomial variable coded one during the stimulus and zero otherwise [4, 14]. While this assumption may be valid for stimuli of very short duration, it may not hold true for most sustained stimuli. With respect to cold pain, the original work by Wolf and Hardy on the cold pressor task (CPT) [16] demonstrates that the sensation changes over time; their report suggests that we can expect a gradual rise of the cold pain and, after approximately one minute, a gradual decrease of pain. If the CPT is applied repeatedly as in functional magnetic imaging (fMRI) studies, the temporal characteristics may be entirely different. Additional temporal variation may be seen when studying different individuals.
Rarely are investigators interested in brain processes that may happen at the same time but independently of an experimental stimulus. Rather, we wish to understand the perceptual response evoked by that stimulus. Thus, in an fMRI experiment, we must evaluate the subjective time course of any sensory modality, its onset and possible washout if we wish to accurately describe the cognitive response.
Based on these considerations, we set out to study the time course of pain rating both to guide our interpretation of observed brain activation in response to cold pain and, in a more general context, to enhance our understanding of the temporal characteristics of a sustained noxious stimulus.
Materials and Methods
Overall Study Design
After obtaining informed consent and exclusion of any medical condition that would interfere with normal sensation or pain perception, fifteen human volunteers had their right foot repeatedly immersed into ice water alternating with immersion into tepid water. After receiving standard verbal instructions about the purpose and methods of the study, participants rated cold pain intensity continuously using an electronic slide algometer. As additional descriptive information that has been well validated both in the experimental and clinical context, we obtained heat pain threshold and tolerance at the forearm. To ensure participants had a clear understanding of the study procedure they underwent a short test trial of two ice water immersions.
Each participant underwent two sessions, during which the participants’ foot was immersed into cold water for thirty seconds alternating with tepid water for thirty seconds. Each cold/tepid cycle was repeated three times, i.e. four cold and tepid immersions. The duration of ice water immersions was based on the practical consideration of choosing an immersion time long enough to produce a sustained painful sensation in all participants while avoiding a dropout of subjects if the stimulus duration was excessive. Based on preliminary studies, a reasonable duration appeared to be thirty seconds (30s). The foot, as opposed to the hand, was immersed into ice water as this task would be performed with greater ease in brain imaging experiments, a potential application of the CPT.
Slide Algometer
This device consisted of a slide potentiometer attached to a small box, which was mounted to an intravenous (IV) pole. Analog electrical signals from the potentiometer were digitized with a USB data acquisition interface and logged at 5 Hz using a standard laptop computer. The pain scale was displayed on a computer screen as a ten-inch horizontal bar ranging from no pain (left most bar position) to most pain imaginable (fully extended bar).
Ice Water Immersion Design and Timing
Ice and tepid water were prepared using two 8 high by 10 wide by 20 long inch plastic containers. The container was filled with either a mixture of ice cubes and cold water to a depth of five inches or with tepid water alone. Temperature of both containers was measured using a thermometer after several times during the experiment and maintained at 2 to 4 degrees Celsius (cold) and 25 to 28 degrees Celsius (tepid).
Pain Threshold and Tolerance Ratings
Contact heat pain thresholds were obtained for each participant using a small 3 by 3 inch Peltier probe (Medoc Thermal Sensory Analyzer TSA-II, Ramat Yishai, Israel) applied to the skin of the forearm. Detection of first pain sensation (pain threshold) and pain limit (pain tolerance) were assessed on the ventral forearm using an ascending method of limits with a 1 deg C/sec rate of rise (heat) fall (cold). To obtain consistent measurements, psychophysics tests were repeated three times. At the end of the study participants were asked to rate the overall unpleasantness associated with the immersion of their foot into ice water. The rating objective of pain intensity and unpleasantness was explained to participants prior to the rating.
Statistical Analysis
Because we were interested in the time-course of pain intensity within a person rather than individual differences in pain perception, we normalized the pain ratings to a zero to one pain rating scale. This was accomplished by assigning the maximum rating value of each session to the value 1 and the minimum to 0. Other values were scaled accordingly. Since the participant’s foot had to be moved manually from one bath to another at the end of each immersion, there was some small variation of the duration of the entire immersion cycle. To avoid some overlap in the data between immersion cycles, we truncated the data of the tepid phase of the immersion cycle to a time point where the rating for all participants had returned to a non-painful (< 1.5/10) rating for the remainder of the tepid cycle.
Before we describe the statistical models, we first describe the data structure and introduce some notations. We divided the data, recorded at a 5Hz sampling rate, into two parts; ratings obtained during the cold condition and ratings obtained during the tepid condition and analyzed them separately. We denote the ratings from the i th individual, j th session, k th immersion and l th time point at the time tijkl as Yijkl, where i = 1,….,15, j = 1,2, k = 1,…,4, and l = 1,2,…150. We denote the peak ratings of the i th individual, j th session and k th immersion as Zijk and the time that the peak rating occurred as Tijk, where i = 1,….,15, j=1,2, and k = 1,…,4. Therefore, tijk and Tijk represent the time interval between immersion start and the time that a particular rating was obtained. So, tijk and Tijk are always in [0, 30] during the cold condition. During the tepid condition, because of the necessary data truncation (see above), tijk and Tijk are in [0,22].
We first determined whether the peak ratings varied across subsequent immersions. The analysis was carried out using the following mixed effect model [5]:
| (1) |
where α0 and αk (k = 1,…,4) are the fixed population level of peak rating and the error, εijk and the random effect βi (i = 1,…,15) are identically distributed N(0, σ2) and N(0, σβ2), respectively. We then tested the null hypothesis H0 : α1 = α2 = α3 = α4 and calculated the corresponding p-value using the likelihood ratio test.
We then determined whether the time at which the peak ratings were obtained within each immersion were the same. To account for the correlation of the peaking ratings from the same individual, we employed the following mixed effect model:
| (2) |
where α0 and αk (k = 1,…,4) are the fixed population level of peak ratings, and the errors εijk and the random effects βi (i = 1,…,15) are identically distributed, N(0, σ2) and N(0, σβ2) respectively. The null hypothesis here is H0 : α1 = α2 = α3 = α4 and the corresponding p-value was calculated using the likelihood ratio test.
The main analysis focused on the temporal course of the cold ratings. We first evaluated if ratings remained the same during the ice water immersion and, if ratings changed, we planned to estimate how the ratings changed over time. For this purpose, we divided the data into two parts; ratings obtained during the cold condition and ratings obtained during the tepid condition. We evaluated several nested general linear models; the nominal (boxcar) model typically used in an fMRI block design, which assigns a value of one to the cold phase and a value of zero to the tepid phase, the mean model that assumes that the rating data remain constant, the linear model that assumes that the rating data follow a straight line, the quadratic model that assumes that the rating data follow a single inflection curve and the cubic model that assumes that the data follow a sigmoid curve. We evaluated each model in terms of their accuracy or ability to explain the observed data. We used adjusted R-squared values. These represent the proportion of variance in the data that could be explained by each model with the correction of parameters used. However, this form of model comparison may still neglect the over fitting problem introduced by using models with more free parameters. To preclude the possibility of over fitting, we performed a proper model comparison using the cross validation accuracy. This involves optimising the parameters of each model using a training dataset and then examining its accuracy (using the MSD between the actual and predicted pain ratings) on an independent test data set. This sort of procedure accounts properly for differences in model complexity. Furthermore, the cross validation accuracy is a useful proxy that predicts pain ratings in future or independent experiments.
To assess how well this model can be used to predict the pain ratings for future experiments by other investigators using the cross validation, we randomly selected a subset of samples as the training set and another subset of samples as the testing set. For the training set, we first constructed the general linear model and obtained the corresponding coefficients for each person, and immersion, separately. Then the coefficients in the final model were just the mean of each coefficient across all the linear models. The mean squared difference between the actual and predicted pain ratings was used to quantify how good the model fit. This process was repeated 100 times and 5 samples were randomly chosen as the training set and 1 sample was randomly chosen as the testing set with each repetition. Finally, we constructed the coefficients for those models using all 15 samples.
Heat pain threshold and tolerance and overall unpleasantness and intensity ratings were analyzed with simple univariate statistics (t-test, ANOVA). All the statistical analyses were carried out using the statistical software packages R (http://www.r-project.org/) and SAS (ver 9.2, Cary, IN, USA).
Results
Subject Demographics
Fifteen participants entered the study. We had seven female and eight male participants; four African American, two Asian and nine Caucasian participants with an age range from 22 to 41 years.
Pain Threshold and Tolerance
Average heat pain threshold and tolerance ratings, obtained with the Medoc TSA-II, were 45.2 (± 3.29) °C and 48.8 (± 2.27) °C, average overall intensity and unpleasantness ratings, on a scale ranging from 0 to 1, were 0.8 (± 0.299) and 0.74 (± 0.253). We found no significant race or gender differences in these measures (unpleasantness by race, p = 0.339; intensity by race, p = 0.632; intensity by gender, p = 0.450; unpleasantness by gender, p = 0.169).
Change of Peak Pain Rating with Repeated Immersions
We observed that the peak ratings varied across the immersions while the time that these peak ratings were obtained seemed to vary little across the immersions. The analysis from the mixed model confirmed this observation. p-values under the null hypothesis of no effects were 9.59 × 10−5 and 0.013 respectively
Model of Perceived Cold Pain over Time
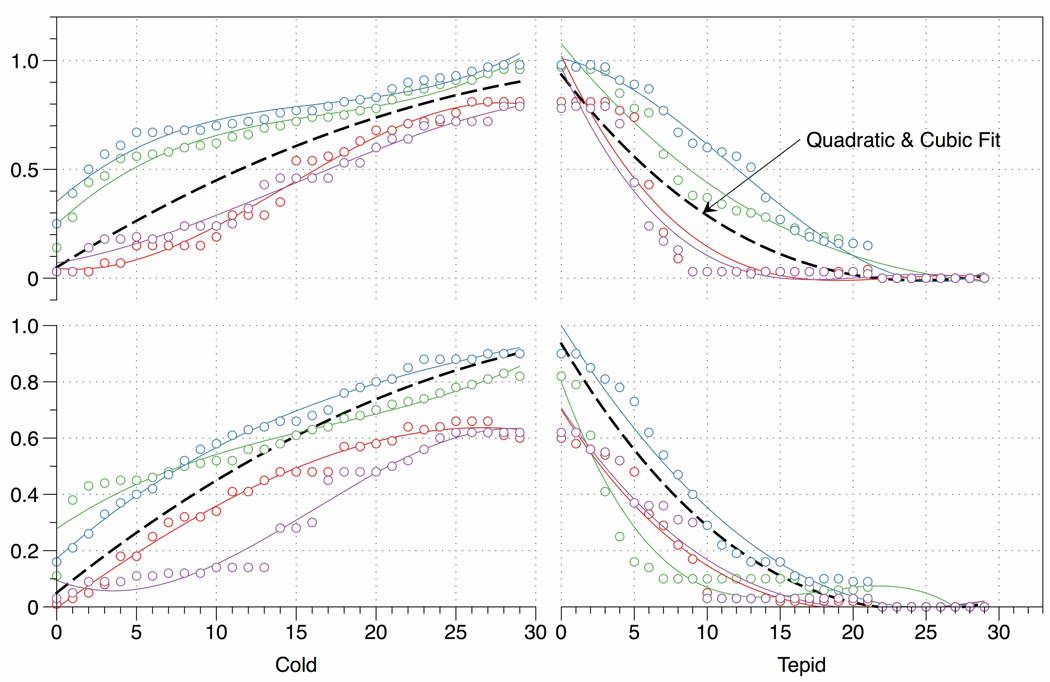
To determine which model can be used to characterize the ratings, we first describe the fit of the model as the mean squared difference (MSD) in table 1. Clearly, the nominal model currently used in many fMRI block design experiments fits poorly with a MSD of 0.4243 for the cold/tepid cycle. Some improvement (MSD = 0.0896) is seen in the mean or intercept only model, even though this average error of approximately 9 % remains relatively large. Advancing from the intercept only (Yijk = α0 + εijk) to the linear model (Yijk = α0 + α1 tijk + εijk), we can formally test the model fit and the significance of the extra parameter α1. We show that the simple linear model performed quite well with the adjusted R-squared value of 0.857. However, the quadratic or cubic model can better characterize the pain ratings because the adjusted R-squared values were much improved for these two models comparing with the simple linear model (table 1). The model fit is illustrated in figure 1 using sample data from two randomly selected participants.
Table 1.
Model Fit (adjusted R squared value)
| Adjusted R2-values | |||
|---|---|---|---|
| Model | Cold & Tepid | Cold | Tepid |
| Linear | 0.857 (52.9%) | 0.882 (63.3%) | 0.825 (42.5%) |
| Quadratic | 0.945 (86.3%) | 0.958 (89.2%) | 0.933 (83.3%) |
| Cubic | 0.968 (95.4%) | 0.978 (97.5%) | 0.958 (93.3%) |
The adjusted R-squared values. The numbers in parentheses are the portion of models with the adjusted R-squared value greater than 0.90.
Figure 1. Rating Data.
Graphical representation of actual ratings from two randomly selected subjects and the rating model based on sample data from all participants. Both upper and lower panel contain actual rating data from one subject. Different colors are used for each of the four immersions per subjects. The predicted ratings based on our model are displayed in black color.
We then assessed the performance of these models when they were used to predict the pain ratings for future experiments. For both cold and the tepid immersion phase, we found that the linear, quadratic and cubic models gave the much smaller MSD between the actual and predicted pain ratings than the constant model and the threshold model that assumes the pain ratings are always 1 in the cold water and 0 in the tepid water (table 2). Finally, we constructed the coefficients for all these models using all 15 samples (table 3).
Table 2.
Model Fit (actual versus predicted ratings)
| Model | Cold | Tepid |
|---|---|---|
| Nominal | 0.4699 (0.1235) | 0.6488 (0.1016) |
| Mean | 0.2850 (0.0530) | 0.3209 (0.0353) |
| Linear | 0.1601 (0.0611) | 0.1839 (0.0489) |
| Quadratic | 0.1707 (0.0625) | 0.1663 (0.0438) |
| Cubic | 0.1671 (0.0580) | 0.1620 (0.0504) |
The mean and standard derivation (in parentheses) of squared difference between the actual and predicted pain ratings across 100 repetitions for several models.
Table 3.
Model Coefficients
| Model | Cold | Tepid | ||||||
|---|---|---|---|---|---|---|---|---|
| α 0 | α1 | α2 | α3 | α0 | α1 | α2 | α3 | |
| Threshold | 1 | 0 | ||||||
| Constant | 0.541 | 0.361 | ||||||
| Linear | 0.090 | 0.030 | 0.852 | −0.044 | ||||
| Quadratic | 5.88×10−3 | 4.64×10−2 | −5.48×10−4 | 1.02 | −8.86×10−2 | 2.00×10−3 | ||
| Cubic | 5.97×10−3 | 4.64×10−2 | −5.45×10−4 | −6.69×10−8 | 0.967 | −6.22×10−2 | −9.57×10−4 | 8.87×10−5 |
The estimated coefficients of each model.
Summarizing our model selection process, we found that pain perception gradually increases during the cold immersion with a decreasing slope at the end of the cold exposure. This changing slope (adaptation phase) is best predicted with a quadratic model. The cubic model adds an additional parameter but, for applied purposes, does not reduce the prediction error in a meaningful way. After discontinuation of the cold stimulus we observed a continuous decrease of pain ratings into the non-painful range that is also best characterized by the quadratic model. The model of a linear increase/decrease provided the most significant improvement over the null (constant term) model. Even though, the cubic model provides a measurable improvement over the quadratic model, this improvement appears to be of little importance in the applied context of pain ratings. This is an example of potential over fitting, which our cross-validation procedure protects against. Therefore our final, quadratic models are:
Discussion
Pain researchers have well documented that perceived pain intensity is not a constant sensation. Two mechanisms have been extensively studied; temporal summation, a mechanism responsible for the increase in pain perception over time and sensory adaptation, the mechanism ascribed to decrease in neuronal inputs of a sustained stimulus. Temporal summation has been studied for a variety of experimental pain tasks [1, 6, 12, 13, 15]. Sensory adaptation, a term based on work on cardiovascular physiology by Landgren [9] to a painful stimulus is apparent in studies such as Wolf and Hardy’s original work [16] on CPT where the duration of the pain stimulus allows sensory adaptation to become greater than temporal summation and thus reduce pain perception. However, as with all thermal pain stimuli, the exact temperature transfer to the skin may also affect the stimulus perception. For ethical reasons, Wolf and Hardy used each other as research subjects when evaluating responses to continuous immersions of their hand into water at temperatures ranging from 2 to 15 degree Celsius for up to six minutes. Most investigators in the more recent literature markedly limit either intensity or duration of the stimulus. We limited our investigation to thirty seconds, a stimulus duration that, in preliminary pilot trials had shown to result in a stimulus that was consistently described as profoundly painful. Interestingly, a change in the slope during the ice water immersion as evidence of sensory adaptation was noted even though our immersion time was short by comparison to Wolf and Hardy’s experiment.
We found the quadratic model to be most useful for future independent experiments. An example of the potential utility of this finding is its use in the analysis of BOLD fMRI data. In these experiments, repeated whole brain images are acquired in sequence over an acquisition session lasting several minutes while a participant is repeatedly exposed to the stimulus. [2, 3, 7, 8, 10, 11]. The stimulus needs to be long enough to evoke the desired effect (pain) but short enough to avoid habituation. The BOLD signal intensity of images acquired during stimulus presentation are averaged and compared to the average BOLD signal intensity obtained during the control condition. Stimulus repetition and data averaging are necessary to compensate for the low signal to noise ratio of the MRI signal. Thus, in BOLD fMRI brain imaging studies participants are exposed to a long sequence of presumably identical stimuli.
The important findings of our investigation with respect to its application in neuroimaging are that (a) the stimulus is not perceived as constant over time as currently assumed in many analytical models (b) the time-course of the subjective CPT stimulus intensity does not change significantly with repeated application, i.e. there is neither habituation or sensitization and (c) pain ratings in response to the CPT can be predicted with high accuracy in future studies.
In summary, in this study we develop a model function that characterizes the time-course of cold pain perception and demonstrate the utility of this model in the study of human brain imaging. We conclude that the time course of a sustained stimulus is an important variable when studying a dynamic cognitive process.
Acknowledgments
This work was supported by the National Center for Research Resources, Grants K-23 RR021874-01 A1 and M01 RR-00032, NIH NCRR, Bethesda, Maryland, United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arendt-Nielsen L, Frokjaer JB, Staahl C, Graven-Nielsen T, Huggins JP, Smart TS, Drewes AM. Effects of gabapentin on experimental somatic pain and temporal summation. Reg Anesth Pain Med. 2007;32:382–388. doi: 10.1016/j.rapm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Craig MR, Kristal AR, Cheney CL, Shattuck AL. The prevalence and impact of 'atypical' days in 4-day food records. J Am Diet Assoc. 2000;100:421–427. doi: 10.1016/S0002-8223(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 3.Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Event-related fMRI of pain: entering a new era in imaging pain. Neuroreport. 1998;9:3019–3023. doi: 10.1097/00001756-199809140-00018. [DOI] [PubMed] [Google Scholar]
- 4.Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- 5.Gelman A. Analysis of Variance: Why It Is More Important than Ever. Ann Stat. 2005;33:1–31. [Google Scholar]
- 6.Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. Pain. 2006;122:295–305. doi: 10.1016/j.pain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Ingvar M. Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1347–1358. doi: 10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupers R, Kehlet H. Brain imaging of clinical pain states: a critical review and strategies for future studies. Lancet Neurol. 2006;5:1033–1044. doi: 10.1016/S1474-4422(06)70624-X. [DOI] [PubMed] [Google Scholar]
- 9.Landgren S. On the excitation mechanism of the carotid baroreceptors. Acta Physiol. Scand. 1952;26:1–34. doi: 10.1111/j.1748-1716.1952.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 10.Maihofner C, Kaltenhauser M, Neundorfer B, Lang E. Temporo-spatial analysis of cortical activation by phasic innocuous and noxious cold stimuli--a magnetoencephalographic study. Pain. 2002;100:281–290. doi: 10.1016/S0304-3959(02)00276-2. [DOI] [PubMed] [Google Scholar]
- 11.Minoshima S, Casey KL. Cerebral Responses to Warmth and Heat and Cold Pain Measured by Positron Emission Tomography. Curr Rev Pain. 1999;3:316–320. doi: 10.1007/s11916-999-0048-3. [DOI] [PubMed] [Google Scholar]
- 12.Nie H, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal summation of pain evoked by mechanical pressure stimulation. Eur J Pain. 2009;13:592–599. doi: 10.1016/j.ejpain.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Obermann M, Pleger B, de Greiff A, Stude P, Kaube H, Diener HC, Katsarava Z. Temporal summation of trigeminal pain in human anterior cingulate cortex. Neuroimage. 2009;46:193–200. doi: 10.1016/j.neuroimage.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Seifert F, Maihofner C. Representation of cold allodynia in the human brain--a functional MRI study. Neuroimage. 2007;35:1168–1180. doi: 10.1016/j.neuroimage.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf S, Hardy JD. Studies on Pain. Observations on Pain Due to Local Cooling and on Factors Involved in the "Cold Pressor" Effect. J Clin Invest. 1941;20:521–533. doi: 10.1172/JCI101245. [DOI] [PMC free article] [PubMed] [Google Scholar]