Abstract
Prevalence of tender points (TP), widespread pain and fibromyalgia, as well as the relationship between TP, widespread pain and mobility was examined in 585 community-dwelling older adults (mean age 78.2 years, 63.4% female). Pain was based on location (none, single site, multisite, widespread). Mobility was measured by the Short Physical Performance Battery (SPPB), gait speed, and self-reported (S–R) mobility difficulty. Tender point count and health characteristics (i.e. BMI, chronic conditions, analgesic use, number of medications, depression, and blocks walked per week) were assessed.
Results
Several participants had 3 or more TP (22.1%) although prevalence of criteria-based fibromyalgia was low (0.3%). Mobility was more limited in persons with higher tender point counts. After adjustment for pain and other risk factors, higher tender point count was associated with poorer SPPB performance (score<10, aOR=1.09 per TP, 95%CI, 1.01–1.17), and slow gait speed (<0.784m/sec, aOR=1.14 per TP, 95%CI, 1.05–1.24), but not with S–R mobility difficulty. S–R mobility difficulty was associated with more disseminated pain (multisite pain, aOR=2.01, 95%CI, 1.21–3.34; widespread pain, aOR=2.47, 95%CI, 1.09–5.62). These findings portray a significant mobility burden related to tender point count and multisite and widespread pain in the older population. Future studies using longitudinal methods are warranted.
Keywords: Aging, Pain, Fibromyalgia, Mobility limitations
INTRODUCTION
Pain in the older community-dwelling population is a common phenomenon49. With advancing age, overall prevalence of chronic pain increases49, and there is a more marked increase in the extent to which pain interferes with daily activities51. Back pain, pain in the lower extremities, and widespread pain are associated with more severe mobility difficulty among older women with disabilities31–33. In population-based studies of older people, impaired physical performance is associated with pain in the back, knee, and foot6,32,34.
A very disabling type of pain, widespread pain35 is a characteristic feature of fibromyalgia57. Prevalence of fibromyalgia may vary by using clinical diagnosis, survey criteria, or the widely used classification criteria of the American College of Rheumatology (ACR)28,58. In the general population, prevalence of fibromyalgia varies between 0.7%–4.4% of adults41,55,57. Most population-based studies have focused on younger and middle aged adults52,56, with only one study reporting prevalence in older adults of approximately 7% of older women and 1% of older men57. Alternatively, in elderly populations, osteoarthritis affecting multiple joints may be an important contributor to the high prevalence of widespread pain11.
The ACR criteria to classify fibromyalgia include widespread pain and the presence of 11 or more out of 18 tender points on manual exam58. Although it has been suggested that tender points are physical findings that are not important as patient symptoms28, the number of tender points correlated positively with severity of pain in fibromyalgia patients41. Fibromyalgia patients often experience limitations in daily physical activities such as walking23, and frequently show impairments on physical function assessments36. In population-based samples, tender point count correlated with specific and non-specific pain8 and distress (i.e. depression, fatigue, difficulty sleeping)9. It is unknown to what degree tender points are associated with mobility problems in the older population. The present study has 2 aims: 1) to determine prevalence of tender points, widespread pain, and fibromyalgia in the older population, and; 2) to determine whether tender point count and widespread pain are associated with poorer mobility performance and S–R mobility difficulty in older community-dwelling adults.
MATERIALS AND METHODS
Participants
The MOBILIZE Boston Study (MBS) is a population-based study of mobility and falls in older individuals living in the community. The sampling frame was the older population living within a 5-mile radius of the Hebrew Rehabilitation Center in Boston which included nearly all of Boston as well as sections of 5 surrounding communities. Further details on the study methods and recruitment were published elsewhere34,46. The present study focused on the baseline assessment of the first 600 persons enrolled in this cohort. To be eligible for the study, people had to: 1) be aged 70 years or older; 2) understand and communicate in English; 3) be able to walk 20 feet without personal assistance (may use walker or cane). Exclusion criteria were 1) presence of a terminal disease (e.g. receiving hospice services, metastatic cancer), or 2) severe cognitive impairment (score <18 on the Mini-Mental State Examination (MMSE)15. Primary reasons for ineligibility were language and living in a nursing home; fewer than 10% of ineligible persons were excluded because of mobility problems34. Spouses of eligible participants could join the study if they were within 6 months of their 65th birthday, or older, and met all other eligibility criteria. However, for purposes of generalizability, we included only those participants aged 70 years or older, which resulted in a sample size of 585. All participants were administered a 2-part baseline assessment that included a home interview by a trained research assistant and a nurse exam at the study clinic at the Hebrew Rehabilitation Center. Participants provided written informed consent for participation. All study procedures were approved by the Institutional Review Board of the Hebrew Rehabilitation Center.
Pain Measurement and Categorization
Presence of musculoskeletal pain was measured by the McGill Pain Map (MPM)37. Participants were shown a homunculus of the front and back of a human figure and asked to identify places on the figures "where you have pain today that you have had for more than a week or two". This method was validated previously in an older population13. Pain was categorized as 1) no site of pain, 2) single site of pain, 3) multisite pain, not fulfilling criteria for widespread pain, and 4) widespread pain. Using the participants' responses on the MPM (excluding head, face and abdominal sites), we classified widespread pain according to the ACR guideline which states, "Pain is considered widespread when all of the following are present: pain in the left side of the body, pain in the right side of the body, pain above the waist, and pain below the waist. In addition, axial skeletal pain (cervical spine or anterior chest or thoracic spine or low back) must be present58." In a second method of pain assessment during the home interview, widespread pain was assessed using a single item in which participants were asked, "during the past year have you had pain, aching, or discomfort all over for 3 months or longer?”. Both pain assessments were conducted in all participants during the home interview.
Tender Point Count
Tender points (TP), widely distributed throughout the musculoskeletal system, were identified according to the ACR criteria for fibromyalgia58. They were identified by applying pressure with the thumb pad of the tester's dominant hand perpendicular to each survey site (total of 18 sites) according to a designated order. Each site was pressed for a total of 4 seconds, and the force was increased by 1 kg per second until 4 kg of pressure was achieved. The participant was asked whether there was pain when the pressure was applied to each tender point. For the MBS, clinical research nurses were trained by an experienced rheumatologist and used a calibrated digital scale in their training to become familiar with applying 4kg of thumb pressure. Careful quality control methods including training and inter-rater reliability assessments were published previously34. Total number of tender points was calculated and persons were classified as having no TP, 1 or 2 TP, or ≥3 TP. For the fibromyalgia classification, the highest category was further divided by TP>10.
Diagnosis of Fibromyalgia
The presence of fibromyalgia was determined by the ACR criteria58: 1) at least 3 months of widespread pain in combination with 2) tenderness in ≥11 of the 18 specific tender point sites. The presence of widespread pain used in our classification of fibromyalgia was determined using either of the following: 1) by the McGill Pain Map; and 2) by the single question during the home interview as described above.
Mobility Measures
Short Physical Performance Battery (SPPB)
Mobility performance was assessed using the SPPB, a battery of 3 tests measuring balance, walking, and chair stands18. Balance was assessed in 3, 10-second stands: standing with feet touching side-by-side, semi-tandem stand with the side of one heel touching the side of the big toe of the other foot, and full tandem (heel-to-toe) stand. Standing balance was scored from 1 to 4, based on performance of each test for up to 10 seconds. Walking speed was assessed by the best of 2 trials of a timed 4-meter usual pace walk. The walk was scored from 1–4 using population-based norms: score of 1: <0.46 m/s; score of 2: 0.47–0.64 m/s; score of 3: 0.65–0.83 m/s; score of 4: ≥0.83 m/s17. Chair stand time was determined as the time required to stand up from a chair 5 times as fast as possible with the arms folded in front of the chest. Established scoring cutpoints for the repeat chair stands (in seconds) were as follows: score of 1: >16.7 seconds; score of 2: 16.6–13.7 seconds; score of 3: 13.6–11.2 seconds; score of 4: ≤11.1 seconds17. The SPPB score, the sum of the 3 test scores, predicts disability and hospitalization in older adults17,39. In this study, we used a score of 9 or lower on the SPPB to indicate poor overall mobility performance.17 The second mobility outcome, slow gait, was defined as the lowest quartile (gait speed <0.784 m/s).
Self-reported mobility difficulty
Mobility difficulty, our third mobility outcome, was assessed during the in-home interview. S–R mobility difficulty was a dichotomous variable based on report of any difficulty walking a quarter of a mile (2–3 blocks) or climbing 10 steps without help from another person19,45. This measure of mobility difficulty has been used extensively in epidemiologic cohort studies of older adults and has been found to be more predictive of mortality than standard measures of activities of daily living5.
Demographic and Health Characteristics
Demographic characteristics included age, sex, race, and education (high school education or higher). Body mass index (BMI) was calculated by measured weight in kilograms divided by height in squared meters.
Comorbidity
Chronic diseases were assessed during the home interview, in which participants were asked if a physician had told them they had heart disease (myocardial infarction, atrial fibrillation, pacemaker, angina, or congestive heart failure), diabetes, spinal stenosis/disc disease, gout, asthma/lung disease, and stroke. In addition, people were asked to report any other disease ever reported by a physician. Knee and hand osteoarthritis were assessed during the clinical nurse exam using established clinical criteria1,2. In a review of medication containers, interviewers recorded the names, dose, and frequency of use of all medications and supplements taken in the 2 weeks prior to the home visit. Daily use of analgesic medication (including over-the-counter and prescriptions analgesics) and total number of medications were determined from the medication data. Also, presence of moderate or severe depression was ascertained using the Center for Epidemiologic Studies Depression scale (CES-D) to apply DSM-IV criteria12,42. Blocks walked per week. Participants were asked how many blocks they walked in the previous week, a validated measure of activity in older adults38.
Statistics
Tender point categories were evaluated according to participant characteristics and health indicators using descriptive statistics and Chi-square tests. Multivariable logistic regression models were performed to separately examine the relationships between tender point count and mobility outcomes, then between pain categories and each of the 3 mobility outcomes, impaired mobility (SPPB<10), slow walking speed (<0.784 m/sec) and S–R mobility difficulty. Models were adjusted for age, sex, and race. Subsequently, to determine whether pain or tender point count had greater impact on mobility, both predictors were included in the same multivariable model. The correlation between tender point count and the pain category variable was Spearman’s rho=0.25 and thus they were not collinear according to standard guidelines for epidemiologic analyses14. In a final model, we performed additional adjustments, adding education, BMI, self reported chronic conditions (heart disease, diabetes, spinal stenosis/disc disease, gout, asthma, and stroke), daily use of analgesic medication, number of medications, depression, and number of blocks walked per week. Adjusted odds ratios (OR) and 95% confidence intervals (CI) are presented. We did not adjust for osteoarthritis in the main models because it was assessed by clinical criteria that included pain in the definition. Additional analyses were performed using the same covariates as in the fourth model, but instead of the pain categorization variable, number of pain sites was included. Data were analyzed using SPSS version 15 (Chicago, IL, USA).
RESULTS
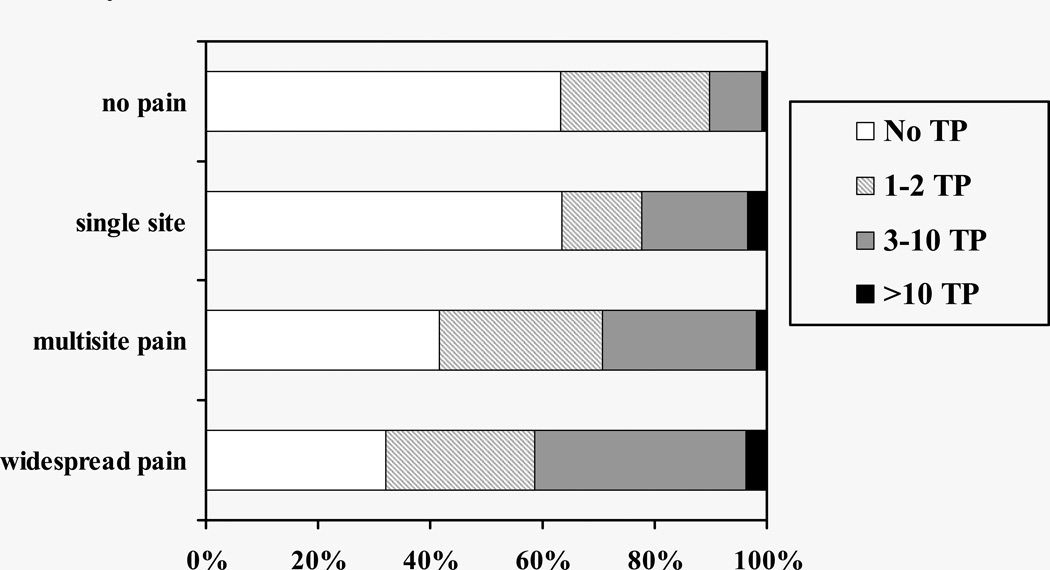
Average age of the 585 participants was 78.2 years, with a range of 70–97 years. Tender points were present in 47.7% of participants, and 22.1% had 3 or more tender points. There were 11 persons (2%) who had 11 or more tender points. Characteristics of participants according to tender point count categories are presented in Table 1. The majority of participants (61.4%) reported one or more areas of pain on the McGill Pain Map; 14.5% had a single pain site, 37.8% had multisite pain (≥2 sites) that did not fulfill criteria for widespread pain, 9.1% had widespread pain. From the people that reported pain all over during the home interview, 38.9% also showed widespread pain on the McGill Pain Map. Conversely, persons that did not report pain all over during the home interview, 6.9% had widespread pain according to the McGill Pain Map. Mean tender point count of persons reporting no pain or widespread pain on the McGill Pain Map was 1.0 (SD=2.1) and 2.8 (SD=3.2), respectively. The strong graded relationship between tender point count and more disseminated pain is shown in Figure 1. Over 40% of persons with widespread pain (41.5%) had 3 or more tender points compared to 10.2% of those with no pain. Only 2 women out of 585 participants (0.3%) met the ACR criteria for fibromyalgia based on pain assessed by the McGill Pain Map, and 3 women fulfilled criteria using the single item of self-reported ‘pain all over’. Only one woman fulfilled both types of fibromyalgia criteria. No men met criteria for fibromyalgia. Thus, 3.8% of persons with widespread pain according to the McGill Pain Map also met the tender point criteria for fibromyalgia. In addition, 3 participants reported that a physician had told them they had fibromyalgia, even though they did not meet the criteria for the disease during the present clinical assessment. Many more women than men had 3 or more tender points (28.3% and 11.2%, respectively). Women also had significantly higher rates of pain across categories (Table 2).
Table 1.
Participant Characteristics according to Tender Point (TP) Categories, MOBILIZE Boston Study, 2005–2007
| No TP (n=306) |
1 or 2 TP (n=150) |
≥3 TP (n=129) |
P value* | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age | ||||
| 70–74 y | 95 (31.0) | 46 (30.7) | 41 (31.8) | |
| 75–84 y | 172 (56.2) | 87 (58.0) | 64 (49.6) | |
| 85 y | 39 (12.7) | 17 (11.3) | 24 (18.6) | .391 |
| Gender | ||||
| female | 156 (51.0) | 110 (73.3) | 105 (81.4) | |
| male | 150 (49.0) | 40 (26.7) | 24 (18.6) | <.001 |
| Race | ||||
| white | 236 (77.1) | 121 (81.2) | 98 (76.0) | |
| black | 54 (17.6) | 22 (14.8) | 23 (17.8) | |
| other | 16 (5.2) | 6 (4.0) | 8 (6.2) | .825 |
| Body Mass Index | ||||
| <25 | 109 (36.3) | 45 (30.6) | 37 (29.8) | |
| 25–29 | 131 (43.7) | 59 (40.1) | 46 (37.1) | |
| ≥ 30 | 60 (20.0) | 43 (29.3) | 41 (33.1) | .044 |
| Education | ||||
| ≤12 y | 98 (32.0) | 53 (35.3) | 59 (45.7) | |
| >12 y | 208 (68.0) | 97 (64.7) | 70 (54.3) | .024 |
| Depression | 14 (4.6) | 16 (10.7) | 14 (10.9) | .018 |
| Heart disease | 118 (38.6) | 68 (45.3) | 54 (41.9) | .376 |
| Diabetes | 60 (19.6) | 21 (14.0) | 26 (20.2) | .286 |
| Spinal stenosis/disc disease | 47 (15.4) | 35 (23.3) | 31 (24.0) | .039 |
| Gout | 35 (11.4) | 20 (13.3) | 9 (7.0) | .219 |
| Asthma/lung disease | 39 (12.7) | 22 (14.7) | 34 (26.4) | .002 |
| Stroke | 37 (12.1) | 12 (8.0) | 13 (10.1) | .401 |
| Daily use of analgesic medication | 95 (31.0) | 45 (30.0) | 54 (41.9) | .058 |
| No. of medications | ||||
| <7 | 129 (42.2) | 49 (32.7) | 38 (29.5) | |
| ≥7 | 177 (57.8) | 101 (67.3) | 91 (70.5) | .020 |
| Blocks walked per week | ||||
| ≤2 blocks | 44 (14.4) | 26 (17.3) | 35 (27.1) | |
| ≤1 mile† | 86 (28.2) | 52 (34.7) | 47 (36.4) | |
| ≤4 miles | 76 (24.9) | 38 (25.3) | 23 (17.8) | |
| >4 miles | 99 (32.5) | 34 (22.7) | 24 (18.6) | .002 |
Chi-square test for between-group differences.
12 blocks per mile, block groups are mutually exclusive.
Figure 1.
Prevalence of Tender Points (TP) According to Pain Categories, MOBILIZE Boston Study 2005–2007
Table 2.
Prevalence of Tender Points, Chronic Pain, Self-reported ‘Pain all over’, and Fibromyalgia in Women and Men aged 70 and older, MOBILIZE Boston Study.
| Women (n=371) |
Men (n=214) |
Total (n=585) |
||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | P value† | |
| Tender point categories‡ | ||||
| 0 | 156 (42.0) | 150 (70.1) | 306 (52.3) | |
| 1–2 | 110 (29.6) | 40 (18.7) | 150 (25.6) | |
| 3–10 | 96 (25.9) | 22 (10.3) | 118 (20.2) | |
| ≥11 | 9 (2.4) | 2 (0.9) | 11 (1.9) | <.001 |
| Pain groups‡ | ||||
| no pain | 131 (35.3) | 95 (44.4) | 226 (38.6) | |
| single site | 50 (13.5) | 35 (16.4) | 85 (14.5) | |
| multisite | 154 (41.5) | 67 (31.3) | 221 (37.8) | |
| widespread | 36 (9.7) | 17 (7.9) | 53 (9.1) | .049 |
| Self-reported ‘pain all over’ | 26 (7.0) | 10 (4.7) | 36 (6.2) | .258 |
| Fibromyalgia* | ||||
| McGill | 2 (0.5) | 0 (0) | 2 (0.3) | n/a |
| Self-report of “pain all over” | 3 (0.8) | 0 (0) | 3 (0.5) | n/a |
Each set of categories totals to 100% of the cohort.
Fibromyalgia is determined by the presence of 1) 11 or more tender points and 2) widespread pain according to McGill pain map or self-report.
Chi square test of the difference between men and women.
The prevalence of poorer mobility performance (SPPB<10) was 39.3% and self-reported mobility difficulty was 34.4%. In separate models, both tender point count and pain categories were independently associated with poorer mobility performance and self-reported mobility difficulty (Models 1 and 2, Table 3). When adjusted for one another, tender points remained associated with mobility performance but the relationship between pain categories and performance diminished (Model 3). Adjusted for each other, both tender points and pain were associated with self-reported mobility difficulty. However, after further multivariable adjustment for chronic conditions and medications, depression and amount of walking, the relationship between tender points and mobility performance persisted (adjusted OR=1.09 for each TP, 95%CI, 1.01–1.17; Model 4). In other words, an older person with 6 tender points, would have a 70% greater likelihood of showing poorer mobility performance compared to a person with no tender points even after adjusting for pain and comorbid conditions. Similarly, persons with higher numbers of tender points were more likely to have a slow gait (adjusted OR=1.14, 95%CI, 1.05–1.24). With respect to self-reported mobility difficulty, having more tender points was associated with difficulty walking or climbing stairs, but this association diminished after adjusting for potential confounders (Table 3).
Table 3.
Adjusted Odds Ratios for Mobility problems based on SPPB performance, Gait Speed, and Self-reported Mobility Difficulty according to Tender Point Count and Pain Categories (according to the McGill Pain Map) in Older Adults, MOBILIZE Boston Study.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| SPPB <10 | ||||||||
| Tender point count | 1.16 | 1.08–1.24 | - | 1.14 | 1.06–1.22 | 1.09 | 1.01–1.17 | |
| McGill pain | ||||||||
| no pain | - | 1.0 | 1.0 | 1.0 | ||||
| single site | - | 1.13 | 0.65–1.95 | 1.09 | 0.62–1.92 | 1.25 | 0.67–2.31 | |
| multisite | - | 1.56 | 1.05–2.33 | 1.39 | 0.92–2.11 | 1.07 | 0.67–1.70 | |
| widespread | - | 2.27 | 1.20–4.29 | 1.85 | 0.96–3.57 | 1.23 | 0.58–2.62 | |
| Gait speed | ||||||||
| Tender point count | 1.18 | 1.10–1.26 | - | 1.17 | 1.09–1.25 | 1.14 | 1.05–1.24 | |
| McGill pain | ||||||||
| no pain | - | 1.0 | 1.0 | 1.0 | ||||
| single site | - | 1.00 | 0.52–1.91 | 0.94 | 0.48–1.84 | 1.14 | 0.55–2.39 | |
| multisite | - | 1.36 | 0.86–2.15 | 1.19 | 0.74–1.91 | 0.86 | 0.50–1.48 | |
| widespread | - | 2.07 | 1.03–4.14 | 1.67 | 0.81–3.45 | 0.99 | 0.42–2.30 | |
| Mobility difficulty | ||||||||
| Tender point count | 1.11 | 1.04–1.18 | - | 1.08 | 1.01–1.15 | 1.01 | 0.94–1.10 | |
| McGill pain | ||||||||
| no pain | - | 1.0 | 1.0 | 1.0 | ||||
| single site | - | 1.12 | 0.62–2.01 | 1.08 | 0.59–1.98 | 1.53 | 0.74–3.14 | |
| multisite | - | 2.43 | 1.61–3.67 | 2.34 | 1.53–3.59 | 2.01 | 1.21–3.34 | |
| widespread | - | 3.77 | 1.05–7.17 | 3.52 | 1.82–6.81 | 2.47 | 1.09–5.62 | |
Model 1 includes tender point count, adjusted for age, gender, and race. Model 2 includes pain categories, adjusted for age, gender, race. Model 3 included both tender point count and pain categories, adjusted for age, gender, and race. Model 4 includes all variables from model 3 and education, Body Mass Index (BMI), self reported chronic conditions: heart disease, diabetes, spinal stenosis/disc disease, gout, asthma, and stroke; daily use of analgesic medication, number of medications, depression, and blocks walked per week.
CI=confidence interval; OR=odds ratio; SPPB=Short Physical Performance Battery
Although after adjusting for tender point count, pain was no longer associated with poorer SPPB performance or with slow gait, multisite and widespread pain were each associated with self-reported mobility difficulty (Table 3). Additional adjustment for knee and hand osteoarthritis (to model 4) did not change the relationships between tender point count and mobility measures. Additional analyses adjusting for total number of pain sites rather than pain categories did not alter the findings.
DISCUSSION
To our knowledge, this is the first study to report on population-based prevalence of tender point counts and fibromyalgia in the older population. The concept of tender point count has been the subject of much controversy throughout the years21,28. Criticisms include that assessment of tender points is cumbersome21,28 and that using a tender point count to diagnose fibromyalgia is arbitrary and exclusionary21. Differences in prevalence rates of fibromyalgia in population-based studies may partially be explained by the use of criteria that either do or do not include tender point count7,9,54,57. In the present cohort of older individuals, fibromyalgia according to ACR criteria was not common. In contrast, Wolfe and colleagues found a much higher prevalence of fibromyalgia particularly among older women (about 7%)57. Of note, although both studies used a pain map approach for pain assessment, the prevalence of widespread pain in the Wolfe study was approximately twice that of our Boston study (20% vs. 9.7% in women, and 12% vs. 7.9% in men, respectively). Nonetheless, it was the low prevalence of the tender point criteria in the MBS population that accounted for the substantial differences in prevalence of fibromyalgia between the 2 studies. As discussed in the Wolfe paper, the detection of tender points may vary depending on the pressure applied by the tester, and this could lead to differences in fibromyalgia prevalence. We employed a series of quality control techniques to promote reliability of the tender point exam34. However, it is possible that the pressure applied was not identical to that used in the Wolfe study.
The fibromyalgia criterion of 11 or more tender points was uncommon across pain categories in the MBS cohort. However, the much lower cutpoint of 3 or more tender points showed a strong graded relationship with more disseminated pain. For these reasons, a tender point count of ≥11 may be too stringent a criterion to determine the presence of fibromyalgia in older people, a statement that has been made before regarding adults in general21. In practice, clinicians may diagnose fibromyalgia based on the presence of widespread pain and symptoms such as fatigue, mental difficulties, and muscle weakness29 even in the absence of 11 tender points. However, others have suggested that older people have lower pressure pain thresholds33. This issue could be investigated in future research efforts.
Osteoarthritis is among the leading causes of mobility difficulty in the older population10,16. Generalized osteoarthritis is not well understood but may contribute to multisite and widespread pain in older persons44. Clinicians may not consider tender point assessment when an elderly patient presents with clinical signs of osteoarthritis. However, when pain presentation is not well correlated with evidence of osteoarthritis, tender point assessment could provide additional clinical information.
In the current study, many participants (especially women) had positive findings on the tender point exam and those participants were not likely to report mobility difficulty. Yet, they were more likely to have poorer mobility performance measured by low SPPB score and slow gait speed. This is an important finding since mobility problems in older adults are predictive of increased dependence and adverse events such as falls, hospitalizations, and nursing home placement17,18,20,39. Both gait speed and the Short Physical Performance Battery provide useful clinical information about risks for hospitalization and other adverse consequences in geriatric patients50. The relationship between tender point count and poorer mobility performance was independent of pain assessment, suggesting that the tender point exam offers additional information about pain conditions in older adults. It may reflect a level of severity of a pain condition that is not detected by standard pain assessment questions in older persons. Alternatively, tender point pain may be a subclinical indicator of changes in joint and muscle structure and function that are reflected in poorer mobility performance, possibly due to deconditioning or pathophysiological changes associated with chronic pain conditions48.
Current findings also show that more disseminated pain is associated with self-reported mobility difficulty and not with SPPB performance or gait speed. When walking or stair-climbing is experienced as painful, it is likely to be reported as difficult. This finding is in concordance with results from a cohort study including well-functioning older community-dwelling adults aged 70–7953. In this study, individuals with low back pain reported difficulty in daily physical function (e.g. walking a distance of 1 mile), but did not demonstrate poorer performance on the SPPB. Similar findings have been reported for older women with disabilities31,35. Hence, self-reported pain associated with self-reported mobility difficulty, and tender point count associated with physical performance (e.g. gait speed) suggest different pathophysiologic conditions and therefore offer different information on pain and mobility status.
A better understanding of pain characteristics (e.g. tender point count, pain location, pain severity) associated with mobility problems (self-reported or by performance testing) could guide better pain management and the development of programs to address aspects of mobility. A number of pharmacologic approaches are used in treatment of fibromyalgia30 that have rarely, if ever, been tested in geriatric populations. Clinical trials with fibromyalgia patients have found that physical training leads to enhanced physical function and reduced pain27. Therefore, older adults with multiple tender points (≥3) may benefit from physical training as well. However, reduction in chronic pain and tender point count have not been reported consistently in clinical exercise trials with fibromyalgia patients4,27. A discrepancy in findings may be attributed to the variety of exercise interventions; most studies did not apply an intervention that fulfilled the exercise recommendations of the American College of Sports Medicine (ACSM) (i.e. 30 minutes of moderate intensity exercise on most days of the week3,26. Future studies can investigate whether an exercise intervention that meets ACSM criteria improves function and reduces pain in older persons with multisite or widespread pain or with an elevated tender point count with or without fibromyalgia.
There are some limitations of this study that should be mentioned. Firstly, the cross-sectional design of the present study is unsuited to establish temporal relationships between tender point count, widespread pain, and mobility. Future longitudinal studies can determine whether increased number of tender points and widespread pain actually lead to new or worsening mobility problems. Secondly, persons with severe mobility problems were excluded from participation. Although they represented a small percent of those who were ineligible, it is possible that there was a disproportionately higher prevalence of pain disorders and possibly fibromyalgia among this group. Thus the prevalence of fibromyalgia may be higher than we report here. Thirdly, potential reversible causes of widespread pain (e.g., vitamin D deficiency and hypercalcemia)22 were not assessed and therefore cannot be excluded. Finally, although many comorbid medical conditions were taken into account, they were based on self-report which may induce errors and not all pain conditions common in fibromyalgia patients, for instance migraine and irritable bowel syndrome25,47 were assessed.
In conclusion, presence of fibromyalgia based on the ACR criteria was uncommon in this population of older adults in the Boston area. Nonetheless, multiple tender points and multisite and widespread pain were much more common. Both the manual tender point exam and the McGill Pain Map provide important information about pain conditions with implications for mobility in older individuals. Further research is needed to determine optimal pain evaluation methods and subsequent approaches to manage pain and its functional consequences in older adults.
PERSPECTIVE.
Higher tender point count, multisite pain and widespread pain are common in community-dwelling older adults and associated with mobility problems. Both the manual tender point exam and the McGill Pain Map may provide important yet different information about risks for mobility disability in older individuals.
ACKNOWLEDGMENTS
The authors acknowledge the MOBILIZE Boston research team and study participants for the contribution of their time, effort, and dedication. This research was supported by the National Institute on Aging: Research Nursing Home Program Project Grant# P01AG004390 and Rubicon 446–07–002 (Netherlands Organization for Scientific Research, NWO).
Footnotes
Institution where data were gathered: Hebrew Rehabilitation Center, 1200 Centre St Roslindale, MA 02131, USA
REFERENCES
- 1.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, III, Mankin H, McShane DJ, Medsger T, Jr, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, Brown C, Cooke TD, Daniel W, Gray R, Greenwald R, Hochberg M, Howell D, Ike R, Kapila P, Kaplan D, Koopman W, Longley S, Mcshane DJ, Medsger T, Michel B, Murphy W, Osial T, Ramsey-Goldman R, Rothschild B, Stark K, Wolfe F. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33:1601–1610. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Bircan C, Karasel SA, Akgün B, El O, Alper S. Effects of muscle strengthening versus aerobic exercise program in fibromyalgia. Rheumatol Int. 2008;28:527–532. doi: 10.1007/s00296-007-0484-5. [DOI] [PubMed] [Google Scholar]
- 5.Brock DB, Lemke JH, Branch LG, Evans DA, Berkman LF. Mortality and physical functioning in epidemiologic studies of three older populations. J Aging Soc Policy. 1994;6:21–37. doi: 10.1300/j031v06n03_04. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Devine A, Dick IM, Dhaliwal SS, Prince RL. Prevalence of lower extremity pain and its association with functionality and quality of life in elderly women in Australia. J Rheumatol. 2003;30:2689–2693. [PubMed] [Google Scholar]
- 7.Cöster L, Kendall S, Gerdle B, Henriksson C, Henriksson KG, Bengtsson A. Chronic widespread musculoskeletal pain - A comparison of those who meet criteria for fibromyalgia and those who do not. Eur J Pain. 2008;12:600–610. doi: 10.1016/j.ejpain.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Croft P, Burt J, Schollum J, Thomas E, Macfarlane G, Silman A. More pain, more tender points: is fibromyalgia just one end of a continuous spectrum? Ann Rheum Dis. 1996;55:482–485. doi: 10.1136/ard.55.7.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ. 1994;309:696–699. doi: 10.1136/bmj.309.6956.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MA, Ettinger WH, Neuhaus JM, Mallon KP. Knee osteoarthritis and physical functioning: evidence from the NHANES I Epidemiologic Followup Study. J Rheumatol. 1991;18:591–598. [PubMed] [Google Scholar]
- 11.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 365:965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 12.Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: Review and revision (CESD and CESD--R) In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. Mahwah NJ: Lawrence Erlbaum Assoc Inc; 2004. pp. 363–377. [Google Scholar]
- 13.Escalante A, Lichtenstein M, Lawrence V, Roberson M, Hazuda HP. Where does it hurt? Stability of recordings of pain location using the McGill Pain Map. J Rheumatol. 1996;23:1788–1793. [PubMed] [Google Scholar]
- 14.Field A. Discovering Statistics Using SPSS. 2nd edition. London: SAGE publications; 2005. [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State – Practical method for grading cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik JM, LaCroix AZ, Abbott RD, Berkman LF, Satterfield S, Evans DA, Wallace RB. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–857. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 21.Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol. 2007;34:914–922. [PubMed] [Google Scholar]
- 22.Helliwell PS, Ibrahim GH, Karim Z, Sokoll K, Johnson H. Unexplained musculoskeletal pain in people of South Asian ethnic group referred to a rheumatology clinic - relationship to biochemical osteomalacia, persistence over time and response to treatment with calcium and vitamin D. Clin Exp Rheumatol. 2006;24:424–427. [PubMed] [Google Scholar]
- 23.Henriksson C, Gundmark I, Bengtsson A, Ek AC. Living with fibromyalgia. Consequences for everyday life. Clin J Pain. 1992;8:138–144. doi: 10.1097/00002508-199206000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–498. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 25.Ifergane G, Buskila D, Simiseshvely N, Zeev K, Cohen H. Prevalence of fibromyalgia syndrome in migraine patients. Cephalalgia. 2006;26:451–456. doi: 10.1111/j.1468-2982.2005.01060.x. [DOI] [PubMed] [Google Scholar]
- 26.Jones KD, Adams D, Winters-Stone K, Burckhardt CS. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005) Health Qual Life Outcomes. 2006;4:67. doi: 10.1186/1477-7525-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones KD, Burckhardt CS, Deodhar AA, Perrin NA, Hanson GC, Bennett RM. A six-month randomized controlled trial of exercise and pyridostigmine in the treatment of fibromyalgia. Arthritis Rheum. 2008;58:612–622. doi: 10.1002/art.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis: a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum. 2006;54:169–176. doi: 10.1002/art.21533. [DOI] [PubMed] [Google Scholar]
- 29.Klippel JH. Primer on Rheumatic Diseases. 12th edition. Arthritis Foundation; 2001. [Google Scholar]
- 30.Lawson K. Emerging pharmacological therapies for fibromyalgia. Curr Opin Investig Drugs. 2006;7:631–636. [PubMed] [Google Scholar]
- 31.Leveille SG, Bean J, Ngo L, McMullen W, Guralnik JM. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2007;128:69–77. doi: 10.1016/j.pain.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leveille SG, Guralnik JM, Ferrucci L, Hirsch R, Simonsick E, Hochberg MC. Foot pain and disability in older women. Am J Epidemiol. 1998;148:657–665. doi: 10.1093/aje/148.7.657. [DOI] [PubMed] [Google Scholar]
- 33.Leveille SG, Guralnik JM, Hochberg M, Hirsch R, Ferrucci L, Langlois J, Rantanen T, Ling S. Low back pain and disability in older women: independent association with difficulty but not inability to perform daily activities. J Gerontol A Biol Sci Med Sci. 1999;54:M487–M493. doi: 10.1093/gerona/54.10.m487. [DOI] [PubMed] [Google Scholar]
- 34.Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, Kang HG, Samelson EJ, Gagnon M, Freeman M, Lipsitz LA. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leveille SG, Ling S, Hochberg MC, Resnick HE, Bandeen-Roche KJ, Won A, Guralnik JM. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135:1038–1046. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 36.Mannerkorpi K, Svantesson U, Broberg C. Relationships between performance-based tests and patients' ratings of activity limitations, self-efficacy, and pain in fibromyalgia. Arch Phys Med Rehabil. 2006;87:259–264. doi: 10.1016/j.apmr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Melzack R. The McGill pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–229. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 38.Paffenbarger RS, Jr, Hyde RT, Hsieh CC, Wing AL. Physical activity, other life-style patterns, cardiovascular disease and longevity. Acta Med Scand. 1986;711(Suppl):85–91. doi: 10.1111/j.0954-6820.1986.tb08936.x. [DOI] [PubMed] [Google Scholar]
- 39.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 40.Pickering G, Jourdan D, Eschalier A, Dubray C. Impact of age, gender and cognitive functioning on pain perception. Gerontology. 2002;48:112–118. doi: 10.1159/000048937. [DOI] [PubMed] [Google Scholar]
- 41.Prescott E, Kjøller M, Jacobsen S, Bülow PM, Danneskiold-Samsøe B, Kamper-Jørgensen F. Fibromyalgia in the adult Danish population: I. A prevalence study. Scand J Rheumatol. 1993;22:233–237. doi: 10.3109/03009749309095129. [DOI] [PubMed] [Google Scholar]
- 42.Radloff L. The CES-D Scale: A self report depression scale for research in the general population. App Psych Meas. 1977;1:385–401. [Google Scholar]
- 43.Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60:793–797. doi: 10.1093/gerona/60.6.793. [DOI] [PubMed] [Google Scholar]
- 44.Rogers J, Shepstone L, Dieppe P. Is osteoarthritis a systemic disorder of bone? Arthritis Rheum. 2004;50:452–457. doi: 10.1002/art.20136. [DOI] [PubMed] [Google Scholar]
- 45.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 46.Samelson EJ, Kelsey JL, Kiel DP, Roman AM, Cupples LA, Freeman MB, Jones RN, Hannan MT, Leveille SG, Gagnon MM, Lipsitz LA. Issues in Conducting Epidemiologic Research in Elders: Lessons from the MOBILIZE Boston Study. Am J Epidemiol. 2008;168:1444–1451. doi: 10.1093/aje/kwn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweinhardt P, Sauro KM, Bushnell MC. Fibromyalgia: A disorder of the brain? Neuroscientist. 2008;14:415–421. doi: 10.1177/1073858407312521. [DOI] [PubMed] [Google Scholar]
- 48.Smeets RJ, Wittink H. The deconditioning paradigm for chronic low back pain unmasked? Pain. 2007;130:201–202. doi: 10.1016/j.pain.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis Rheum. 2007;57:656–665. doi: 10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- 50.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 51.Thomas E, Mottram S, Peat G, Wilkie R, Croft P. The effect of age on the onset of pain interference in a general population of older adults: prospective findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2007;129:21–27. doi: 10.1016/j.pain.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Thompson D, Lettich L, Takeshita J. Fibromyalgia: an overview. Curr Psychiatry Rep. 2003;5:211–217. doi: 10.1007/s11920-003-0045-x. [DOI] [PubMed] [Google Scholar]
- 53.Weiner DK, Haggerty CL, Kritchevsky SB, Harris T, Simonsick EM, Nevitt M, Newman A. Health, Aging, and Body Composition Research Group: How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4:311–320. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 54.Weir PT, Harlan GA, Nkoy FL, Jones SS, Hegmann KT, Gren LH, Lyon JL. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol. 2006;12:124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
- 55.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol. 1999;26:1577–1585. [PubMed] [Google Scholar]
- 56.Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, Russell IJ, Yunus MB. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis Rheum. 1997;40:1560–1570. doi: 10.1002/art.1780400904. [DOI] [PubMed] [Google Scholar]
- 57.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 58.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]