Abstract
Over the next twenty years the number of Americans diagnosed with dementia is expected to more than double (CDC 2007). It is, therefore, an important public health initiative to understand what factors contribute to the longevity of a healthy mind. Both default mode network (DMN) function and increased aerobic fitness have been associated with better cognitive performance and reduced incidence of Alzheimer’s disease among older adults. Here we examine the association between aerobic fitness, functional connectivity in the DMN, and cognitive performance. Results showed significant age-related deficits in functional connectivity in both local and distributed DMN pathways. However, in a group of healthy elderly adults, almost half of the age-related disconnections showed increased functional connectivity as a function of aerobic fitness level. Finally, we examine the hypothesis that functional connectivity in the DMN is one source of variance in the relationship between aerobic fitness and cognition. Results demonstrate instances of both specific and global DMN connectivity mediating the relationship between fitness and cognition. We provide the first evidence for functional connectivity as a source of variance in the association between aerobic fitness and cognition, and discuss results in the context of neurobiological theories of cognitive aging and disease.
Keywords: cognitive aging, fMRI, functional connectivity, aerobic exercise, executive function, spatial memory
1. Introduction
Normal aging is associated with a decline in cognitive functions, such as processing speed, episodic memory, and executive-control skills such as inhibition, planning, and working memory (Park, Polk, Mikels, Taylor, & Marshuetz, 2001). Functional magnetic resonance imaging (fMRI) studies have also revealed that the aging brain is characterized by increased variability in brain activation patterns which are often hard to explain by unified theories of cognitive aging (Dolcos, Rice, & Cabeza, 2002; Reuter-Lorenz & Lustig, 2005; Greenwood, 2007). In turn researchers have started to characterize functional brain networks comprised of regionally separate but temporally connected brain regions (DeLuca, Beckmann, DeStefano, Mathews, & Smith, 2006; Vincent et al., 2007), which could help uncover fundamental patterns of systems-level brain changes associated with cognitive aging (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). One functional brain network, the default mode network (DMN), has received much attention for its capacity to predict individual differences in clinical pathologies such as schizophrenia (Garrity et al., 2007; Harrison, Yucel, Pujol, & Pantelis, 2007), autism (Castelli, Frith, Happe, & Frith, 2002; Kennedy, Redcay, & Courchesne, 2006) and Alzheimer’s Disease and MCI (Lustig et al., 2003; Greicius, Srivastava, Reiss, & Menon, 2004; Celone et al., 2006; Zhou et al., 2008), as well as cognitive performance in healthy college-age and elderly adults (Hampson, Driesen, Skudlarski, Gore, & Constable, 2006; Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; for reviews on the DMN, see Gusnard & Raichle, 2001, and Buckner, Andrews-Hanna, & Schacter, 2008).
Brain regions prototypically associated with the DMN include the posterior cingulate cortex/retrosplenial cortex (PCC/rsp); the bilateral lateral inferior parietal cortex; the ventral anterior cingulate cortex; the frontal medial cortex, and bilateral hippocampal, and parahippocampal cortices (Andrews-Hanna et al., 2007; Buckner et al., 2008; Gusnard & Raichle, 2001). Other regions consistently demonstrated in the DMN, shown with both model-and data- driven analyses as well as structural covariance, include the posterolateral boundary of the superior frontal gyrus and the posteromedial middle frontal gyrus, and bilateral lateral middle temporal gyri (e.g., Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Fair et al., 2008; Margulies et al., 2007; Morris, et al., 1999; Kobayashi et al., 2007;Seeley et al., 2009).
As a functional network the DMN was first recognized for its characteristic deactivation in the presence of a wide variety of external processing demands, leading to the hypothesis that the DMN represents the brain’s state during a “default mode” and while reliably deactivated relative to regions implementing external processing demands, remains functionally coherent throughout both rest and task epochs (Gusnard & Raichle, 2001; Greicius et al., 2004; Buckner et al., 2009). Recent research is consistent with these views, and has shown that the DMN is also present in anaesthetized monkeys (Vincent et al., 2007) and in humans in states of unawareness (sleep, coma, and deep anesthesia) (Boly et al., 2008), suggesting the DMN represents a network of brain regions intrinsically linked to the most basic aspects of brain function. In turn, examining individual variation in the capability to elicit the DMN presents a rich line of research to elucidate the effects of a disrupted network. It is important to note that other functional networks, such as dorsal attention and primary motor networks, have also shown intrinsic functional connectivity in the resting state (e.g., Vincent et al., 2007; Biswal et al., 2005). Yet here we focus on the DMN because of its demonstrated disruption in healthy cognitive aging (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008), and its association with a positive diagnosis of AD (Greicius et al., 2004; Lustig et al., 2003; Supekar et al., 2008; Zhou et al., 2008). In the context of healthy aging, specific network disruptions between the PCC/rsp and the frontal medial cortex are preferentially associated with aging and cognition (Andrews-Hanna et al., 2007), such that disrupted connectivity is most apparent along an anterior-posterior anatomical dimension and is a source of variance in cognitive performance. In the same study an exploratory whole-brain connectivity analysis demonstrated significant age-related dysfunctional connectivity between the PCC/rsp and the medial temporal and hippocampal/parahippocampal cortices (Andrews-Hanna et al., 2007). However, the cognitive relevance of these connectivity differences was not reported.
Age-related cognitive decline and increases in variability of brain activation have also been studied as a function of individual differences, such as healthy lifestyle factors, that are associated with increased cognitive and cortical function. Research shows that increased aerobic fitness is structurally and functionally neuroprotective in healthy older adults, and may even delay the onset, or reduce the rate of decline, of Alzheimer and vascular dementias (Van Praag, Christie, Sejnowski, & Gage, 1999; Colcombe & Kramer, 2003; Colcombe et al., 2004, 2006; Cotman, Berchtold, & Christie, 2007; Kramer & Erickson, 2007; Pereira et al., 2007). For example, Kramer et al. (1999) examined the influence of fitness training on cognitive function in a sample of 124 older adults in a randomized clinical trial. Subjects were randomized to either a walking or toning and stretching group and participated in fitness training three times a week for six months. Aerobic training (walking) selectively improved performance in a variety of tasks that tap aspects of executive control (i.e. task switching, stopping, and flanker tasks). Building on this study, in a meta-analysis Colcombe and Kramer (2003) found that exercise interventions significantly improved measures of processing speed, visuospatial processing, controlled processing, and executive control processes. Consistent with Kramer et al. (1999), cognitive gains were largest for tasks of executive control (see Kramer & Erickson, 2007 for a recent review of this literature).
Rodent studies provide further support for the prophylactic effects of exercise on cognition and the brain (Christie et al., 2008; Clark et al., 2009; Farmer et al., 2004; Molteni et al., 2004; Neeper, Gomez-Pinilla, Choi, & Cotman, 1995; Van Praag et al., 1999, 2005). This research has demonstrated proliferative effects of exercise on molecular and cellular function in the hippocampus and parahippocampus, regions commonly associated with the DMN and which are particularly disrupted in mild to moderate AD (Greicius et al., 2004; Lustig et al., 2003; Sorg et al., 2007; Supekar et al., 2008; Zhou et al., 2008). Recent studies have also started to link the findings in the animal literature to humans. For example, increased aerobic fitness level was associated with increased volume in the right and left hippocampi in older adults, and this relationship, in part, mediated the relationship between aerobic fitness and spatial memory performance (Erickson et al., 2009). Also, a three-month aerobic exercise intervention increased cerebral blood volume, considered an in vivo correlate of neurogenesis, in the human adult hippocampus and was correlated with improved cognitive performance (Pereira et al., 2007).
Additionally, several other DMN regions are positively associated with individual differences in aerobic fitness in healthy elderly adults, including the frontal and parietal cortices (Colcombe et al., 2004); albeit these associations were found in the context of a selective attention task and not during passive rest. Thus it remains to be seen whether aerobic fitness affects the intrinsic functional integration of these regions, outside the context of a cognitive task set. In light of this, coupled with the parallels of cognitive and Alzheimer disease outcomes, the current study examines whether increased functional connectivity of the DMN as a function of aerobic fitness level reflects one mechanism by which increased fitness is associated with better cognitive performance on tests of executive function and memory.
To test this prediction, we performed seed-based functional connectivity analysis of the whole brain to find regions most characteristic of age-related dysfunctional connectivity with the PCC/rsp between groups of college-age and elderly participants (Gusnard & Raichle, 2001; Buckner et al., 2008). Next, we examined whether in a group of elderly adults aerobic fitness was associated with increased functional connectivity in the same areas that showed the largest age-related differences in connectivity. Finally, we examined the cognitive relevance of increased connectivity in regions associated with greater aerobic fitness levels by testing whether increased connectivity as a function of increased fitness, was associated with better cognitive performance on tests of executive function and spatial memory. We hypothesized a mediation model whereby the direct relationship between aerobic fitness and cognition is mediated by functional DMN connectivity.
2. Methods
2.1. Participants
Participants were recruited from the local community of Urbana-Champaign, Illinois. Eligible participants had to (1) demonstrate strong right handedness, with a 75% or above on the Edinburgh Handedness Questionnaire (Oldfield 1971), (2) be between the ages of 18 and 35 for young adults and between 55 and 80 years for elderly adults (3) score ≥ 51 on the modified Mini-Mental Status Exam (mMMSE, (Stern, Sano, Paulsen, & Mayeux, 1987)), a screening questionnaire to rule out potential neurological pathology, (4) score < 3 on the Geriatric Depression Scale (GDS) (Yesavage et al., 1983; Sheikh & Yesavage 1986), (5) have normal color vision (6) have a corrected visual acuity of at least 20/40 and (7) sign an informed consent. Participants completed a mock MRI session, wherein they were screened for their ability to complete an experiment in an MRI environment. Participants who passed the mock screening subsequently completed a series of structural and functional MRI scans, and for the elderly subjects, a graded maximal exercise test. Prior to MR scanning all participants were tested for visual acuity and (if need be) corrective lenses were provided within the viewing goggles to ensure a corrected vision of at least 20/40 while in the scanner. Participants were compensated for their participation.
The final sample consisted of N=32 young adult participants (mean age = 24.1, SD = 5.1, 85% female), and N=120 elderly participants (mean age = 66.5, SD = 5.7, 71% female). Elderly participants were not cognitively impaired, with an average mMMSE score of 54.8 out of 57 (SD = 1.9), and were well educated with average years of education of 15.8 (SD = 3). Neuroimaging measures were collected as part a larger task battery, and were originally developed to be passive viewing tasks for localizing stimulus-specific processing regions of the ventral visual cortex. Participants in this study represent a superset of a previously published investigation of age-related differences in stimulus processing specificity (Voss et al., 2008). Note it is not uncommon to use data with simple task-related epochs to examine intrinsic functional connectivity (e.g., Andrews-Hanna et al., 2007; Greicius & Menon, 2004)
2.2. Measures
2.2.1. Task Switching
Participants completed a task that required them to switch between judging whether a number (1, 2, 3, 4, 6, 7, 8, or 9) was odd or even and judging whether it was low or high (i.e., smaller or larger than 5). Numbers were presented individually for 1500 ms against a pink or blue background at the center of the screen, with the constraint that the same number did not appear twice in succession. If the background was blue, participants used one hand to report as quickly as possible whether the letter was high (“X” key) or low (“Z” key). If the background was pink, participants used their other hand to report as quickly as possible whether the number was odd (“N” key) or even (“M” key). Participants completed four single task blocks (2 blocks of odd/even and 2 blocks of high/low) of 24 trials each. Due to the difficulty of this task, participants were provided with a practice block in which they switched from one task to the other for 120 trials. This practice block allowed participants to become acquainted with the switching block and ensured compliance with task instructions. Finally, they completed a dual task (switching) block of 120 trials during which the task for each trial was chosen randomly. This task was similar to that of Kramer, Hahn, & Gopher (1999) and Pashler (2000); the version of the task reported in this study was programmed and administered using E-prime software (Psychology Software Tools, www.pstnet.com).
The primary executive function measure in this task is mean switch cost. The difference in performance for trials when the preceding trial involved the same task (non-switch trial) and those when the preceding trial was of the other task (switch trial) is a measure of local switch cost; local switch cost measures attentional set re-configuration and inhibition. The difference in performance for trials in the single task block and those in the dual task block when the preceding trial involved the same task (non-switch trial) is a measure of global switch cost; global switch cost measures the attentional overhead resulting from maintenance of two distinct mental task sets. Response time during the single task blocks represents a measure of processing speed, thus cost measures represent specific costs over and above of general slowing. All reaction time measures are based on mean reaction times.
2.2.2. Wisconsin Card Sorting Task (WCST)
In this task, participants completed a computerized version of the WCST, assessing working memory, inhibition, and switching processes (O’Sullivan et al., 2001). This task was chosen because of its reliance on executive functions and set switching, which should show similar patterns of association to the local switch cost measure described above. The participant’s task was to sort cards displayed on a computer screen. The cards contained geometric designs and could be sorted into categories by shape, color, or number of the design. Participants were asked to match each card that appeared in the lower portion of the computer screen with one of four cards displayed at the top of the screen. The participants were told that the computer would provide feedback about the accuracy of their decision, but that the examiner could not give them any additional instructions about the task. For the present study, we computed a metric of perseverative errors that measures the inability to flexibly adapt to a changing rule set. The primary dependent variable for this measure was computed by taking the average of the standardized number of perseverative responses and perseverative errors, and computing their residuals after regression onto the total number of errors, to control for overall level of performance (Raz, Rodrigue, & Acker, 2003). Due to data collection error, 11 elderly subjects’ data was lost, resulting in N=109 elderly adults with WCST data.
2.2.3. Spatial memory
Participants performed a spatial memory paradigm that has been previously associated with aerobic fitness and hippocampal volume in a sample of 165 older adults (Erickson et al., 2009). First, a fixation crosshair appeared for one second and participants were instructed to keep their eyes on the crosshair. Following the fixation, either one, two, or three black dots appeared at random locations on the screen for a duration of 500 ms. The dots were removed from the display and the fixation cross re-appeared on the screen for a period of three seconds. During this time, participants were instructed to try and remember the locations of the previously presented black dots. At the end of the three-second delay, a red dot appeared on the screen in either one of the same locations as the target dots (match condition) or at a different location (nonmatch condition). Participants had two seconds to respond to the red dot by pressing one of two keys on a standard keyboard – the ‘x’ key for a nonmatch trial, and the ‘m’ key for a match trial. Forty trials were presented for each set size (1, 2, or 3 locations), with 20 trials as match trials and 20 trials as nonmatch trials. Participants were instructed to respond as quickly and accurately as possible. Several practice trials were performed before the task began in order to acquaint the participants with the task instructions and responses. Since our goal was to characterize general spatial memory performance, the primary dependent variable was computed as the average mean reaction time across all three conditions, and average accuracy across all three conditions. Throughout the manuscript this is referred to generally as spatial memory response time and accuracy.
2.2.4. Aerobic fitness assessment
Elderly participants were required to obtain consent from their personal physician before cardiorespiratory fitness testing was conducted. Aerobic fitness (VO2 max) was assessed by graded maximal exercise testing on a motor-driven treadmill. The protocol involved the participant walking at a speed slightly faster than their normal walking pace (approximately 30 – 100m per minute) with increasing grade increments of 2% every 2 minutes. A cardiologist and nurse continuously monitored measurements of oxygen uptake, heart rate and blood pressure. Resting heart rate was measured while the participant lay in the supine position after ECG preparation, and before the treadmill test began. Oxygen uptake (VO2) was measured from expired air samples taken at 30-second intervals until a maximal VO2 was attained or to the point of test termination due to symptom limitation and/or volitional exhaustion. VO2 max was defined as the highest recorded VO2 value when two of three criteria were satisfied: (1) a plateau in VO2 peak between two or more workloads; 2) a respiratory exchange ratio >1.00; and (3) a heart rate equivalent to their age predicted maximum (i.e. 220 -age).
2.3. Imaging Methods
For all participants, high resolution T1-weighted brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 144 contiguous axial slices, collected in ascending fashion parallel to the anterior and posterior commissures, echo time (TE)=3.87 ms, repetition time (TR)=1800 ms, field of view (FOV)=256 mm, acquisition matrix 192 mm × 192 mm, slice thickness=1.3mm, and flip angle=8°. All images were collected on a 3T head-only Siemens Allegra MRI scanner.
Functional MRI (fMRI) scans were acquired during three passive viewing tasks: 1) a checkerboard task comprised of luminance-matched flashing black-and-white checkerboards and flashing color checkerboards at a rate of 8 Hz, each checkerboard condition was presented in two separate 30-second blocks that alternated with 20-second blocks of fixation baseline; 2) a word viewing task comprised of 30-second blocks of words, pseudo-words, and letter strings, presented separately in two 30-second blocks that alternated with 20-second blocks of fixation baseline, each block consisted of 20 unique stimuli that were each presented for one-second with a 500-ms fixation between each word presentation; and 3) a face/building viewing task comprised of three 20-second blocks of faces and buildings that alternated with 20-second blocks of luminance matched scrambled images (taken from the face and building stimulus set) as the baseline condition, each block consisted of 20 unique black-and-white images (controlled for luminance and dimension) that were each presented for one-second. In each task participants were instructed to keep their eyes open and to pay attention to the screen.
Visual stimuli were presented with MRI-safe fiber optic goggles (Resonance Technologies, Inc.). Participants completed the passive viewing tasks as part of a larger battery of cognitive paradigms within the scanner. For the fMRI tasks, T2* weighted images were acquired using a fast echo-planar imaging (EPI) sequence with Blood Oxygenation Level Dependent (BOLD) contrast (64 × 64 matrix, 4 mm slice thickness, TR = 1500 ms, TE = 26 ms, flip angle = 60). A total of 150 volumes were acquired per participant for the checkerboard task, 220 volumes for the word task, and 180 volumes for the face/building task.
2.4. Image Analysis
2.4.1. Structural MRI preprocessing
Each participant’s low-resolution EPI image was registered to his or her high-resolution T1 structural image, which was subsequently registered to stereotaxic space (study-specific template generated using 152 T1 MNI as the target volume, Montreal Neurological Institute) using FLIRT 12-parameter affine linear registration (Jenkinson et al. 2002). A study-specific template was made, comprised of 64 representative brains chosen randomly from the sample (32 young and 32 old). This was done to ensure that the reference image was not biased towards older adults. To make the study-specific template, high-resolution structural images were first skull-stripped using BET (Smith, 2002), and manually inspected and corrected for any skull-stripping errors. Next, the structural images were registered to the 152 T1 MNI volume using FLIRT 12-parameter affine linear registration (Jenkinson et al. 2002). Finally, registered volumes were averaged to form a representative reference volume. This was done to minimize the amount of warping during the registration process, and to protect against registration bias. Before group analyses, functional data were registered to stereotaxic space using transforms generated with the alignment of high-resolution T1 images.
2.4.2. fMRI Preprocessing
fMRI data preprocessing was carried out using FSL 4.1.2 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). The following pre-statistics processing was applied: rigid body motion correction using MCFLIRT (Jenkinson et al. 2002), removal of non-brain structures using BET (Smith, 2002), spatial smoothing using a Gaussian kernel of FWHM 6.0-mm, grand-mean intensity normalisation of the entire 4D dataset by a single multiplicative factor, and temporal filtering with a high pass frequency cut-off of 120 seconds.
2.4.3. General Linear Model Analysis for initial seed selection
Regression-based analysis of fMRI data was carried out using FEAT (FMRI Expert Analysis Tool, http://www.fmrib.ox.ac.uk/analysis/research/feat/) Version 5.98, part of FSL. At the individual level, a separate individual level analysis was done for each of the passive viewing scans. The hemodynamic response to each block was convolved with a double-gamma HRF function. We chose to use a double-gamma HRF function since preliminary analysis of the checker stimulation task showed that the convolution of the double-gamma function had a better fit to the data than the gamma function. Primarily this was due to the small dip in signal approximately ten seconds after the initial rise at the start of the block, followed by a steady signal for the duration of the block and fall at the end of the block epoch. The double-gamma models an initial overshoot, followed by a post-HRF-undershoot, and this showed to be the best fit to the signal for both young and elderly adults. Furthermore, the same high-pass temporal filtering that was applied to the data was applied to the GLM for the best possible match between the model and data. For each task there was a linear contrast that tested for the comparison of activation during rest greater than visual processing blocks (rest > task). The three statistical maps that resulted (rest > task for each of the three passive viewing tasks) were then combined into a higher level fixed-effects analysis, which generated a statistical map representing where activation was greatest for rest > visual processing across all three fMRI scans, within a subject. Statistical maps from the fixed-effects analysis were then forwarded to a higher-level mixed-effects analysis to find areas across participants that were more active during rest than visual processing. Higher-level mixed-effects analyses were carried out using FLAME (Beckmann, Jerome, & Smith, 2003). To ensure regional differences in gray matter volume did not confound our analyses, gray matter partial volume 3D images were added as voxel-wise covariates in the functional imaging analysis (see Oakes et al., 2006; implemented as part as FMRI Expert Analysis Tool Version 5.98, part of FSL 4.1.2, FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl).
Since the aim of this paper is to examine functional connectivity and not amplitude of activation, the primary purpose of the GLM analysis was to confirm that the DMN was activated in a comparison of rest > visual processing, so as to identify an unbiased seed of the PCC/rsp region for an initial seeding to examine functional connectivity. A PCC/rsp initial group seed ROI for functional connectivity analysis was chosen from this overall mean statistical map; the resulting PCC/rsp group ROI spanning 390 anatomical voxels (approx 40 functional voxels) centered on the MNI coordinates x = 8, y = −56, z = 30, see Figure 1. To account for potential individual differences in local anatomy, subject-specific peaks were found within this initial group ROI and new subject-specific ROIs were formed. A subject-specific ROI was made around subject-specific peaks for each subject, in each task, spanning 125 anatomical voxels (approximately 8 functional voxels). Thus, subject-specific peaks in the PCC/rsp were used for the functional connectivity analyses described next.
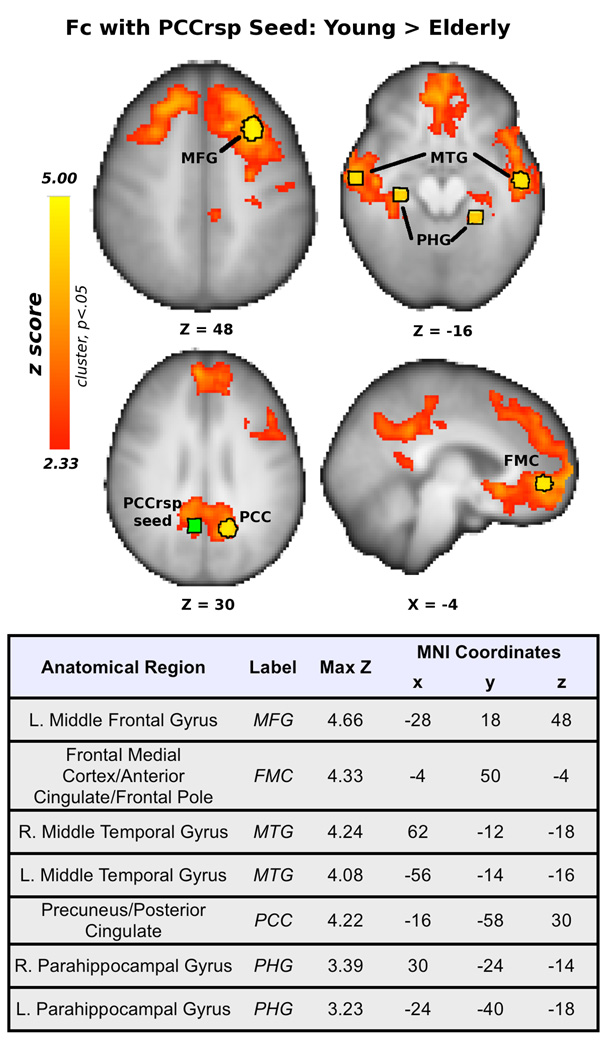
Figure 1. Regional age effects for the young > elderly contrast.
Statistical parametric maps shown above represent where young adults have significantly greater functional connectivity with the PCC/rsp seed compared to elderly adults. The table lists statistical peaks of the corresponding statistical maps. As shown in the table, FMC=Frontal medial cortex, MTG=middle temporal gyrus, PCC=posterior cingulate cortex, and PHG=parahippocampal gyrus. The initial PCC/rsp seed is shown in green, and the derived peaks based on between-group age effects are shown in yellow. All renderings are in radiological orientation (L=R, R=L).
2.4.4. Functional Connectivity seeding analysis
To examine age-related differences in functional connectivity, a seeding analysis was done based on the PCC/rsp seed described above, for each passive viewing task for each subject. Individual-level seeding procedures followed those described in Andrews-Hanna et al., 2007 and Kelly et al., 2009 (e.g., Kelly et al also used FSL framework). Data preprocessing specific to the functional connectivity analysis included the addition of a lowpass temporal filter, to ensure the bandwidth of the fMRI signal fell between .008 < f < .080 Hz. The DMN has been reliably shown to fluctuate within this frequency band, and so this was done to remove high-frequency fluctuations that could confound estimates of intrinsic functional connectivity. Next, the mean time-series from the PCC/rsp seed was extracted, and in addition, the time-series from three nuisance ROIs were extracted including a region in deep white matter in left temporal cortex, a region in the left lateral ventricle, and a whole brain mask to extract global signal changes. Note resting heart rate was not significantly correlated with aerobic fitness in our sample (p >.15); additionally, differences in heart rate variability on intrinsic fMRI BOLD changes have been associated with signal change in the CSF (de Munck et al., 2008), so the CSF nuisance regressor should add additional protection against spurious results as a function of heart rate. Also, since the tasks used here are passive viewing tasks, we have no reason to believe the task would induce individual variation in signal change related to differential task-related heart-rate modulation as a function of fitness level (van Buuren et al., 2009). Furthermore, global signal has been shown to correlate with cardiac and respiration-induced changes in the fMRI BOLD signal (Chang & Glover, 2009; Birn et al., 2006; Wise et al., 2004). Therefore, removal of global signal variance should help towards removal of non-neural sources of activation synchrony. However, it is possible that fitness variation would be correlated with respiration-induced variation in DMN signal and controlling for global signal would overly constrict our ability to study individual differences in fitness on DMN functional connectivity. We examined this concern with exploratory whole-brain analysis of the association between aerobic fitness and functional connectivity with the PCC/rsp seed, both with and without global signal regression. Results showed greater sensitivity in both spatial extent and magnitude of connectivity associations as a function of individual differences in fitness when global signal was regressed out compared to when it was not (see supplementary Figure 1). This suggests that global signal removal was not overly corrective but rather enhanced sensitivity to individual differences in fitness. Moreover, the conjunction analysis of these two analyses showed clusters consistently in the left and right parahippocampal cortex, the frontal medial cortex, and the posterior cingulate – suggesting that relevant DMN regions were revealed under both analyses - however, based on these results global signal correction was performed in all following analyses.
To further isolate our examination to intrinsic functional connectivity, we also extracted signal from a bilateral ROI in the primary visual cortex (125 anatomical spheres centered at ±18, −98, −4; defined with the same central coordinates as the left and right visual cortex ROI in Andrews-Hanna et al., 2007). This visual cortex regressor, along with the global signal regressor, served as cautionary measures to ensure our estimates of functional connectivity were not inflated do to the additive influence of synchronized signal change as a result of visual stimulation.
Finally, the PCC/rsp time-series was entered as a regressor of interest in an individual-level GLM analysis, while covarying out variance from six motion parameters computed by rigid body translation and rotation in preprocessing (Jenkinson et al. 2002) and four nuisance ROIs (including the bilateral visual cortex ROI). To obtain voxel-wise partial correlation coefficients from this regression, the time-series of ROI regressors (including motion and nuisance ROI regressors) and the functional data were normalized to a common variance. This procedure was done for each passive viewing task. Individual-level analyses of voxel-wise regression of the PCC/rsp seed onto the whole brain were then aggregated within subject (across the three functional runs) for greater statistical power, using a fixed-effects analysis as described above. Finally, the fixed-effects maps were converted to Z values using Fisher’s r-to-z transformation (Zar, 1996) and forwarded to a mixed-effects group analysis that considered between-subject variation (Beckmann, Jerome, & Smith, 2003). Aggregating data from three passive viewing tasks, in addition to providing greater statistical power, also ensures that the results are not dependent on one type of visual processing task.
In the mixed-effects between-subjects analysis, we compared young and elderly adults’ mean functional connectivity with the PCC/rsp seed, while (in a voxel-wise manner) controlling for the variance associated gray matter volume. The Young > Old contrast of interest resulted in a statistical map representing where aging is associated with decreased functional connectivity with the PCC/rsp. The resulting ROIs were determined from this analysis based on statistical peaks in separable anatomical brain areas known to comprise the default mode network and as demarcated in the Harvard-Oxford cortical atlas that is packaged with the FSL software package (FSL 4.1.2, FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Peaks are listed in table format and illustrated in Figure 1. Note, following mixed-effects higher-level analysis, the distribution of statistical parametric maps was determined as Gaussian and statistical thresholding was based on Z-statistic-scores of parameter (partial correlation) estimates; statistical map threshold set at voxel Z = 2.33, and cluster p<.05 (Worsley, Evans, Marrett, & Neelin, 1992). The cross-correlation of timeseries’ from the resulting age-sensitive ROIs was then computed for each participant and averaged across the three runs; before further analysis of individual differences, average correlation coefficients from each ROI pair from each participant were converted to Z values using Fisher’s r-to-z transformation (Zar, 1996).
The above analyses represent a focused examination of the effects of fitness on functional connectivity in regions showing the most age-related decline in DMN connectivity. This also ensured that ROIs were chosen independent of fitness, and permitted an unbiased analysis of the inter-relationships between functional connectivity, aerobic fitness, and cognition.
2.5. Statistical Analysis of brain-behavior relationships
For examination of brain-behavior relationships, we hypothesized that functional connectivity in the DMN would serve as a significant source of variance in the association between aerobic fitness and cognition. We justify the causal directions of the model such that it is unlikely functional connectivity would cause aerobic fitness; similarly it is unlikely in this cross-sectional analysis that cognition would cause functional connectivity. While causal predictions in correlational data deserve caution, we believe the proposed model provides the best theoretical framework for the associations between fitness, cognition, and functional connectivity. In the model the paths between the independent variable (IV) (fitness) and the mediator variable (M) (functional connectivity) and M to the dependent variable (DV) (cognition) are represented with the coefficients a and b respectively. Here a represents the coefficient of the relationship between the IV and M, and b represents the coefficient of the relationship between M and the DV; together a*b represents the indirect effect of functional connectivity on cognition. A third coefficient, c, then represents the coefficient of the relationship between the IV and DV, or the direct effect of fitness on cognition. In this paper, we will assess whether mediation is statistically significant using a bootstrapping procedure that repeatedly resamples from the data to build an empirical estimation of the indirect effect (a*b) and non-parametric confidence intervals for testing the statistical significance of the effect (Preacher & Hayes, 2008). This analysis technique is used in the multiple mediator model developed by Preacher & Hayes and relies on few assumptions about the normality of the indirect effect. In our analyses we ran 5000 resamples, and tested the indirect path with a 90% CI (one-tailed, p<.05), following the procedures outlined in Preacher & Hayes, 2008. Finally, we also report an effect size measure of the mediation effect using the method developed by Fairchild et al. (2009). For continuous outcomes this R2 mediation measure estimates the proportion of variance in the DV accounted for by the indirect effect of the IV (fitness) on the DV (cognition) through the mediator (functional connectivity). For further details on the mediation analyses employed here see Preacher & Hayes (2008) and Fairchild et al. (2009).
Before the mediator analysis, we first examined bivariate associations between aerobic fitness and functional connectivity, functional connectivity and cognition, and aerobic fitness and cognition. Subsequently, we tested the proposed mediator model, considering only statistically significant relationships between fitness (IV) and cognition (DV), and fitness (IV) and functional connectivity (M). That is, we entered into the mediator model a set of ROI pairs that showed increased functional connectivity in association with increased fitness, and examined the statistical significance of both specific (ROI-pair specific) and total (as a set of ROI-pairs) indirect (or, mediation) effects. A multiple mediator model uniquely allows for the test of multiple variables as potential mediators both as specific indirect effects and as total indirect effects of the mediator variable set as whole. In turn, we believe this approach keeps with the spirit of functional connectivity by not considering each connection in isolation in their association with cognition, and provides the most parsimonious model for examining whether and how much functional connectivity accounts for variation in the direct relationship between aerobic fitness and cognition.
3. Results
3.1. Age-related differences in functional connectivity with the PCC/rsp
Results of the comparison of elderly and young adults are illustrated in Figure 1 and show age differences in functional connectivity in five separable functional areas of the DMN, including the posterior cingulate, the posteromedial region of the middle frontal gyrus (bordering the posterolateral region of the superior frontal gyrus), the frontal medial cortex, bilateral middle temporal gyri and bilateral parahippocampal gyri. Subsequently, we identified the statistical peaks within each region of the DMN and these are listed in the Table in Figure 1. Bilateral anatomical peaks were combined into bilateral ROIs to gain statistical power, and because we had no pre-existing hypotheses about the laterality of functional connectivity differences as a function of aerobic fitness. This resulted in five ROIs of interest, derived from the age-group analysis, including ROIs representing localized age effects in the left middle frontal gyrus (MFG), frontal medial cortex (FMC), bilateral middle temporal gyri (MTG), posterior cingulate cortex (PCC), and bilateral parahippocampal gyri (PHG) (as labeled in Figure 1). Mean fisher’s Z transformed correlation coefficients for each age group and statistical comparison of their mean effects is shown below the diagonal in Table 1. Finally, Table 1 illustrates that young had greater variability in ROI time-series data; however, age-group comparisons remained unchanged when differential group variances were accounted for in independent-samples t-tests.
Table 1.
| PCCrsp | PCC | PHG | MTG | MFG | FMC | |||
|---|---|---|---|---|---|---|---|---|
| PCCrsp | yng | 1.00 | ||||||
| old | 1.00 | −0.03 | 0.08 | 0.09 | 0.13 | 0.08 | r with vo2 | |
| PCC | yng | 0.63 | 1.00 | |||||
| old | 0.43** | 1.00 | 0.07 | 0.11 | 0.23** | 0.17* | r with vo2 | |
| PHG | yng | 0.17 | 0.10 | 1.00 | ||||
| old | 0.08* | 0.04* | 1.00 | −0.04 | 0.20* | 0.08 | r with vo2 | |
| MTG | yng | 0.43 | 0.29 | 0.23 | 1.00 | |||
| old | 0.19** | 0.04** | 0.19 | 1.00 | 0.16* | 0.16* | r with vo2 | |
| MFG | yng | 0.23 | 0.17 | 0.05 | 0.26 | 1.00 | ||
| old | 0.03** | 0.00** | −0.04* | 0.06** | 1.00 | 0.18* | r with vo2 | |
| FMC | yng | 0.49 | 0.31 | 0.17 | 0.46 | 0.27 | 1.00 | |
| old | 0.27** | 0.13** | 0.07** | 0.23** | 0.09** | 1.00 | ||
| Variance | yng | 0.43 | 0.45 | 0.32 | 0.38 | 0.43 | 0.44 | |
| old | 0.35 | 0.36 | 0.28 | 0.29 | 0.33 | 0.37 | ||
| 2-tailed p | 0.001 | 0.001 | 0.058 | 0.000 | 0.000 | 0.006 | ||
Note: fisher transformed z(r) correlation coefficients between the initial PCCrsp seed and derived seeds (PCC, PHG, MTG. MFG. FMC) from age group contrast. PCC/rsp=initial posterior cingulate/retrosplenial seed; FMC=Frontal medial cortex; MTG=middle temporal gyrus; PCC=posterior cingulate cortex; PHG=parahippocampal gyrus. Age group comparisons are shown below the diagonal, and associations with fitness in the elderly are shown above the diagonal;
p<.05;
p<.003 (bonferroni corrected p-value for 15 correlations).
3.2. Association of aerobic fitness with cognition
Before examining indirect effects, this analysis focused on the direct effects of aerobic fitness on cognition in the elderly adults. Results showed that increased aerobic fitness was associated with fewer perseverative errors on the WCST, r = −.19, p<.05; this association was unaffected when the variance associated with age was controlled, pr = −.19, p<.05. For the dependent variables associated with task-switching, global switch cost was not associated with fitness (p>.05), however local switch cost did show a statistically significant relationship with aerobic fitness, such that higher fit elderly adults had decreased local switch cost, r = −.15, p<.05; after controlling for the variance associated with age, the correlation was reduced to marginal statistical significance, pr = −.13, p=.09. Finally, aerobic fitness was associated with better average accuracy and mean response time across all levels of the spatial memory task (Accuracy: r = .33, p<.05; RT: r = −.31, p<.05); after controlling for the variance associated with age, performance on the spatial memory task was still associated with higher aerobic fitness (Accuracy: pr =.17, p<.05; RT: pr = −.20, p<.05).
3.3. Association of aerobic fitness with functional connectivity in the DMN and cognition
The next series of analyses focused on demonstrating (a) the association between aerobic fitness in elderly adults and functional connectivity in age-sensitive regions of the DMN and (b) the association between functional connectivity in the DMN and cognition. Table 1 shows the results from the first of these analyses, indicating that six functional connections showed a statistically significant positive association with aerobic fitness at p<.05; note only the connection between the PCC and MFG, however, had a p-value surviving bonferonni correction of 15 correlations, p<.003. All statistically significant ROI-ROI correlations with aerobic fitness remained significant at p<.05 when the variance associated with age was controlled. Further, to confirm that ROI differences in variance didn’t contribute to the association with fitness, we did partial correlations for each reported significant bivariate relationship above, controlling for the variance in timecourses for the two ROIs in the functional connectivity pair. All correlations remained unchanged (all still p<.05 and PCC-MFG still p<.003). All connectivity associations showing statistically significant correlations with p<.05 were considered in the mediation model.
Next we examined associations between connectivity in the DMN and cognition. Results showed that connectivity of the PCC-FMC connection was associated with fewer perseverative errors, p = −.19, p<.05; this association was unchanged after controlling for variance associated with age, pr =−.19, p<.05. Decreased local switch cost was associated with increased functional connectivity for the pairs of MTG-MFG (p = −.28, p<.05) and MFG-FMC (p = −.17, p<.05); after controlling for the variance associated with age, MTG-MFG (pr = −.27, p<.05; MFG-FMC (pr = −.16, p<.05). Increased spatial memory accuracy was associated with increased connectivity of the PCC-MFG pair: r = .19, p<.05; this was reduced after controlling for age, pr = .12, p=.09. Finally faster response speed in the spatial memory task was associated with greater connectivity in the following ROI pairs: MTG-MFG: r = −.16, p<.05; MTG-FMC: r = −.17, p<.05; MFG-FMC: r = −.20, p<.05; after controlling for the variance associated with age the correlations were reduced but remained marginally significant, MTG-MFG: pr = −.14, p=.07; MTG-FMC: pr = −.14, p=.07; MFG-FMC remained significant: pr = −.18, p<.05.
3.4. Multiple Mediator Model
Here we examined the extent to which increased connectivity, as positively associated with aerobic fitness, was associated with individual differences in cognitive function, based on performance in three relevant measures of cognition: task-switching, WCST, and spatial memory. Also, in the elderly adult group age was not associated with decreased functional connectivity (all p >.05), thus whatever variance functional connectivity shares in the relationship between aerobic fitness and cognition should not be confounded with age. In turn, mediation models were carried out with variables uncorrected for variance associated with age.
A full listing of the multiple mediation results is shown in Table 2. Shown in the table are the product of coefficients (a*b) for each specific indirect effect and the total indirect effect (sum of specific indirect effects) through functional connectivity for the association between aerobic fitness and cognition. This represents the strength of the indirect effect, or the mediation effect of functional connectivity, on the direct effect of fitness on cognition. Variables showing mediation effects in the expected direction are illustrated in paired scatter plots in Figure 2. Results showed that there were unique functional contributions to each cognitive outcome, except spatial memory accuracy.
Table 2.
| Bootstrapped CI Bias Corrected and Accelerated |
||||
|---|---|---|---|---|
| Product of Coefficients | 90% CI | |||
| Point Estimate | SE | Lower | Upper | |
| Indirect Effects for DV Perseverative Errors | ||||
| PCCMFG | 0.0038 | 0.0041 | −0.0005 | 0.0140 |
| PCCFMC* | −0.0077 | 0.0055 | −0.0210 | −0.0012 |
| PHGMFG | 0.0014 | 0.0046 | −0.0046 | 0.0114 |
| MTGMFG | 0.0010 | 0.0037 | −0.0035 | 0.0101 |
| MTGFMC | −0.0024 | 0.0040 | −0.0114 | 0.0016 |
| MFGFMC | 0.0009 | 0.0037 | −0.0038 | 0.0090 |
| TOTAL | −0.0031 | 0.0084 | −0.0176 | 0.0103 |
| Total Adjusted R-squared: .041, p=.13 | ||||
| *Partial Model Adjusted R-squared: .067. p<.05; R-Squared mediation: .0126 | ||||
| Indirect Effects for DV Local Switch Cost | ||||
| PCCMFG | −0.4941 | 0.8050 | −2.2216 | 0.4418 |
| PCCFMC | 0.2489 | 0.6387 | −0.5007 | 1.6385 |
| PHGMFG | 0.1312 | 0.8120 | −0.8491 | 1.8437 |
| MTGMFG* | −1.5007 | 1.2157 | −4.4232 | −0.0930 |
| MTGFMC | −0.4762 | 0.9248 | −2.6787 | 0.5138 |
| MFGFMC | −0.3222 | 0.7583 | −2.0932 | 0.4090 |
| TOTAL* | −2.4131 | 1.8192 | −6.4331 | −0.2454 |
| Total Adjusted R-squared: .040. p=.12 | ||||
| *Partial Model Adjusted R-squared: .070. p<.05; R-Squared mediation: .0103 | ||||
| Indirect Effects for DV Spatial Working Memory RT | ||||
| PCCMFG | −0.0321 | 0.9211 | −1.4483 | 1.5810 |
| PCCFMC | −0.1526 | 0.7106 | −1.4759 | 0.7902 |
| PHGMFG* | 1.6144 | 1.1170 | 0.3232 | 4.2277 |
| MTGMFG | −0.7589 | 0.7827 | −2.7238 | 0.0184 |
| MTGFMC | −0.5135 | 0.7135 | −2.3974 | 0.1509 |
| MFGFMC* | −0.8505 | 0.8079 | −2.9762 | −0.0190 |
| TOTAL | −0.6932 | 1.6517 | −3.4739 | 1.9148 |
| Total Adjusted R-squared: .114. p<.05 | ||||
|
*Partial Model Adjusted R-squared: .121, p<.05; R-Squared mediation for MFGFMC: .0185: R-Squared mediation for PHGMFG: −.0138 | ||||
Note: significant indirect path, p<.05 * one-tailed; Partial Model includes only ROI-ROI connectivity of significant unique effects in multiple mediator model. Bootstrap estimates based on 5.000 bootstrap samples. PCC=Posterior Cingulate Cortex: MFG=Middle Frontal Gyrus. FMC=Frontal Medial Cortex; MTG=Middle Temporal Gyrus; PHG=Parahippocampal Gyrus; ROI conjunctions represent the functional connectivity between that ROI pair (e.g., PCCFMC represents the functional connectivity z(r) between the PCC and the FMC).
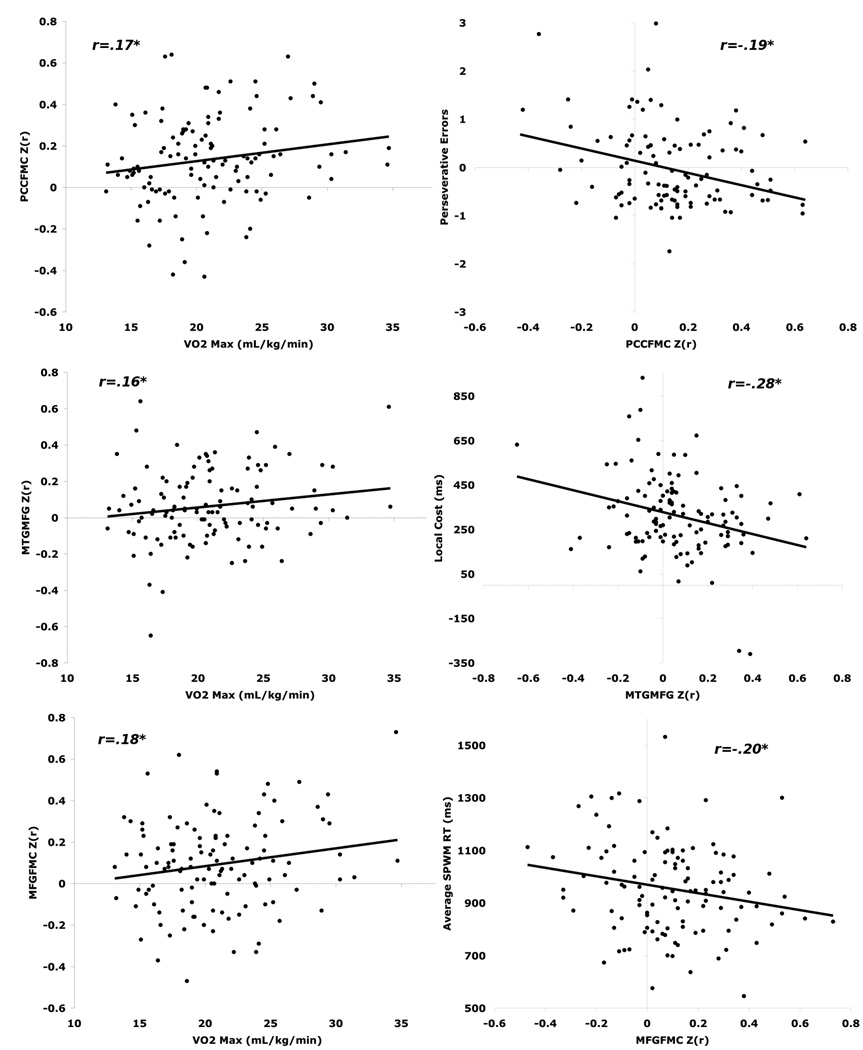
Figure 2. Scatter plots for indirect path associations that showed significant mediation of the direct path between fitness and cognition.
Scatter plots of associations that formed significant indirect pathways between aerobic fitness and cognition. PCCFMC = connectivity z(r) of the posterior cingulate cortex with the frontal medial cortex; MTGMFG = connectivity z(r) of the middle temporal gyrus with the left middle frontal gyrus; MFGFMC = z(r) of the left middle frontal gyrus with the frontal medial cortex. VO2 Max = maximal oxygen uptake (mL/kg/min).
Perseverative errors were mediated through functional connectivity between the PCC and FMC, as this is the only connection showing a significant indirect effect in the mediator model. Further, R2 mediation when just PCC-FMC was considered with fitness (partial model) was .0126, or 1.26% of the variance associated with perseverative errors is attributable to the indirect effect of functional connectivity between the PCC and FMC. Given that the total R2 for the partial model was .067, this suggests that 18.8% of the total effect in this model (.0126/.067) is attributable to PCC-FMC connectivity.
Results for local switch cost, however, showed there were both specific and total indirect effects of functional connectivity on the direct relationship between fitness and local switch cost. Functional connectivity between the MTG and MFG was statistically significant in the mediator model. Further, in the partial model R2 mediation was .0103, or approximately 1% of the variance associated with local switch cost is attributable to MTG-MFG connectivity. Moreover, the total R2 for the partial model was .070, suggesting that 14.7% of the total effect in this model is attributable to MTG-MFG connectivity. Additionally functional connectivity of all the ROI pairs – taken as a set - mediated the relationship between fitness and local switch cost (see Table 2).
Results for spatial memory ability were less straightforward. Here we found that the connectivity between MFG and FMC mediated the direct relationship between fitness and spatial memory reaction time (RT), such that greater connectivity was associated with faster spatial memory response speed. However, we also found a specific indirect effect in the opposite direction, where the connection between PHG and MFG mediated spatial memory speed in the positive direction. In the partial model R2 mediation for MFG-FMC was .0185, which accounted for 15.2% of the total effect. In contrast, the R2 mediation effect was -.0138 for PHG-MFG, suggesting that controlling for the variance associated with the MFG-FMC connection creates a positive association between the PHG-MFG and response speed. This dissociation highlights the need to consider multiple ROIs in one model when examining the impact of functional connectivity on cognition, rather than isolating specific ROI-ROI correlations.
4. Discussion
This is the first study to examine functional connectivity differences in the brain, as a function of individual differences in aerobic fitness, a lifestyle factor that has important implications for the mental and physical health of elderly adults. Furthermore, the present study demonstrates not only an important link between the DMN, aerobic fitness, and cognitive aging, it also presents a framework for examining exercise effects on the integration of large-scale brain systems.
While the functional purpose of the DMN remains somewhat elusive, we replicated previous findings that have shown that functional connectivity of the DMN supports improved executive function in elderly adults (Andrews-Hanna et al., 2007, Damoiseaux et al., 2008). Specifically, one study showed that long-range connectivity between the PCC/rsp and the frontal medial cortex was associated with better cognitive performance in elderly adults (Andrews-Hanna et al., 2007). We extend on these results and show that functional connectivity along the same dimension (anterior-posterior) mediates the association between aerobic fitness and performance on a classic test of executive function, the Wisconsin Card Sorting Test. While Andrews-Hanna et al. (2007) found the PCC-FMC connection was associated with increased processing speed, perseverative errors is not an executive function measure that is wholly accounted for by general slowing (Head et al., 2009). It should also be noted that the FMC ROI used in this analysis is ventral to the ROI in Andrews-Hanna et al. (2007).
In addition, MTG-MFG connectivity showed significant unique effects on the association between fitness and local switch cost, as well as a significant total indirect effect of the set of six ROI-ROI pairs. This suggests that as a set, the functional connectivity between the ROIs that showed a positive association with fitness, significantly mediates the association between fitness and decreased local switch cost, and that the effects are particularly strong for the MTG-MFG pair. While fitness has been shown to be preferentially beneficial to the structural and functional integrity of the frontal cortex, such a relationship has not been shown with fMRI outside the context of a cognitive task. The MFG aspect of the DMN may be particularly important as a transition zone, as it borders or may even overlap with prefrontal regions associated with task positive networks. It is also interesting that functional connectivity was preferentially associated with local switch performance, suggesting the effects are not global, in agreement with previous theories of the effects of exercise on the brain and cognition (Kramer & Erickson, 2007). These results, coupled with the results showing a positive association between PCC-FMC connectivity and performance on the WCST task, suggests that aerobic fitness training may lead to increased DMN connectivity and in turn provide a pathway for improved executive function. Of course this is speculative based on cross-sectional data, yet the current study provides supporting evidence for investigating this question further.
We also found that local MFG-FMC frontal connectivity mediated the association between fitness and spatial memory response speed; however, while controlling for this effect, there was an association in the opposite direction for the PHG-MFG pair (associated with slower response speed). This result is hard to interpret as task adaptive connectivity for the MFG in one connection relative to another, since the fMRI data were collected during passive viewing tasks. Further, when considered on its own the PHG-MFG connection was uncorrelated with spatial memory task performance, while the MFG-FMC connection was statistically significant on its own. Yet at least two different studies have shown increased frontal connectivity in association with AD populations (Wang et al., 2006; Supekar et al., 2008). Our results may therefore suggest the relative strength of correlations among regions in the DMN are as important as the mere strength of connections, in determining the association between functional connectivity and cognition. Perhaps when the connectivity between distal regions is stronger than particular proximal regions (violating small world properties), there is functional disruption that has negative consequences for specific cognitive outcomes. Our evidence for this interpretation is admittedly weak, and the cognitive relevance of multivariate patterns of disruption remains an important topic of future study.
Another important future direction is a greater understanding of the interaction between intrinsic resting functional connectivity and network coherence during action-oriented (i.e., exogenously directed) cognitive operations. For example, future research should address the relative predictive power of functional connectivity measured during rest or low-level cognitive states compared to during high-level exogenous demands, for understanding individual differences in cognitive performance. What remains attractive about resting state data is the simplicity in experimental design and the parsimony of tapping into fundamental networks with broad predictive power. This also facilitates comparison across a broad spectrum of populations (e.g., with respect to age or pathology) without confounds such as task difficulty or strategy that could otherwise mask (or be driven by) individual differences in intrinsic functional organization.
In considering how our results fit in with what is known about the neurobiological effects of exercise on the brain, some important issues deserve discussion. First, given that aerobic exercise has been linked to improved aspects of cortical vasculature (Black et al. 1999; Clark et al., 2009; Swain et al. 2003; Ding et al. 2006), one may ask whether our effects are driven exclusively by increased vascularization in higher fit individuals compared with lower fit individuals. However, increased neurogenesis and synaptogenesis as a function of aerobic exercise training in rodents has shown concomitant increase in vascularization (angiogenesis); this is consistent with the idea that greater tissue volume couples with increased vasculature to provide metabolic support for neuronal activity and regulation (Cotman et al., 2007). Furthermore, since our results are based on differences in functional connectivity the vasculature argument fails to fully explain these results, since functional connectivity requires the dynamic modulation of temporally coordinated activation in distinct brain regions. Additionally, at least two modes of evidence support the notion that the DMN is based on neural connectivity. First is the evidence that functional DMN maps are mirrored by patterns of structural connectivity, with particularly strong anatomical connections between the posterior cingulate, medial temporal, and the frontal medial cortex (Greicius et al., 2008; Kondo et al., 2005; Kobayashi et al., 2007). Second is evidence from a recent direct electrophysiological mapping of the DMN (Miller et al., 2009). Concerning fitness effects, it is also possible that compromised neurovascular coupling is a mechanism for age-related disruption of large-scale brain networks, ameliorated by increased aerobic fitness level. To test this idea, resting state functional imaging before and after aerobic fitness training of aged macaque monkeys would provide an exciting avenue of future research.
Our results may be viewed in the context of some potential limitations. First, the nature of the tasks (passive viewing of various stimuli, interspersed with rest blocks) were not pure resting state paradigms (resting quietly with eyes closed in the scanner). We can only speculate whether the same results would be observed using a pure resting state paradigm. However, given that previous research on the DMN and cognitive aging has utilized both resting state (Damoiseaux et al. 2008) and task block or event-related designs (Persson et al. 2007; Andrews-Hanna, et al. 2008), and come to similar conclusions, we expect similar results would be found. It is also possible that intermittent external processing demands act as a probe for network disruption, eliciting increased individual variability in network coherence. For example, Andrews-Hanna et al. (2007) observed a significant negative correlation between age and DMN connectivity both between old and young and when just older adults were considered, whereas none of our ROI pairs showed a significant negative correlation with age in just the older adults. However, the cognitive demand during the functional runs differed between their study and ours (semantic decision task vs. passive viewing tasks). Future research should examine the extent to which pure resting state, rest interspersed with task, and event-related designs, along with variations in task difficulty, elicit meaningful individual differences in functional connectivity.
Another important factor to consider when comparing results across studies is the metric of functional connectivity. For example, Damoiseaux et al. (2008) measured connectivity during pure rest, but their metric for functional connectivity was different than either the present study or Andrews-Hanna et al. (2007). Damoiseaux et al. (2008) used multivariate blind source separation to produce a metric representing a complex aspect of network connectivity (coherence of whole-head spatial patterns of activation over time and across subjects). As a result, their metric may produce a more stringent requirement for participants to score high on network connectivity, which could result in greater sensitivity to age-related dysfunction. However, future research should test this by using multiple methods and examine their convergence (or lack of) in association with age, cognitive performance, and disease status. A noted advantage of the multivariate approach is a parsimonious metric of network coherence compared to multiple ROI pairs derived from a seeding analysis. In our ROI analysis we tried to control for multiple comparisons by entering all ROI pairs into a single mediation model, instead of running multiple separate mediation tests. We acknowledge this doesn’t completely correct for multiple comparisons; thus a caveat of our brain-behavior analysis is the potential for spurious effects due to an increased number of ROI pairs. Yet, given this is the first study to explore the relationships between DMN functional connectivity, fitness, and cognition, the multiple mediator model provided a viable framework for characterizing regional interactions while still preserving some protection against multiple comparisons.
Another potential limitation may be the influence of undetected pre-clinical pathology. That is, since we did not measure biomarkers of brain pathology such as PIB-PET amyloid imaging (e.g., Andrews-Hanna et al., 2007; Hedden et al., 2009), we cannot be certain that a proportion of our participants weren't in pre-clinical states of pathology that were undetectable by our screening methods. However, such contamination is less likely to have significantly affected our results given the lack of association between age and network disruption in older adults.
Related to ROI generation, our ROIs were determined by using an initial seed based on amplitude of activation maxima for rest > task in the same group of participants then examined for functional connectivity. We don’t believe this biased the results, as Table 1 shows that all of the significant fitness associations were actually with an adjacent posterior cingulate region that was not the initial seed. Another limitation of the current study is that it is cross-sectional. For example, it is possible that a third variable associated with older adults who have both increased DMN function and higher baseline levels of aerobic fitness, such as genetic or social-behavioral factors, could be involved in the effects of the current study. Examining functional connectivity of the DMN after an aerobic exercise training intervention could test this limitation, and presents an important area of future research. Furthermore, our study was restricted to self-reported relatively sedentary individuals; the current results may therefore represent a conservative estimate of the relation between fitness level, DMN, and cognition.
Finally, a potential limitation of the current study is our exclusive examination of the DMN. Yet, the DMN provides a starting point for understanding perhaps the most fundamental network from which other networks are engaged for exogenous processing; we therefore see this study as an important first step for characterizing the role of functional connectivity as a source of variance in the association between cardiorespiratory fitness and cognition. For example, animals with a PCC/rsp lesion have difficulty integrating multiple streams of information, particularly when information from external and internal sources must be integrated for guidance of a future decision such as spatial navigation (for review, see Aggleton, 2009). This is consistent with a model of the DMN in cognition as an “integrator” or “translation center” that in turn represents a convergence zone for interacting brain systems (Burgess et al., 2001; Byrne et al., 2007; Treserras et al., 2009; Vann et al., 2009). Thus, the PCC/rsp may provide a vantage point for switching between an internal representation of the world and past experience (allocentric) and action-oriented representations (egocentric). This would also be consistent with the abilities supported by the DMN such as autobiographical memory and planning (e.g., Buckner et al., 2008 reviews support for these).
This model also provides support for the preferential role of the DMN in executive function (Hampson et al., 2006; Damoiseaux et al., 2007). Specifically a translation model emphasizes the role of the PCC/rsp in monitoring and linking endogenous mentation with initiation of goal-directed action, supporting the overall role of the DMN in dynamically linking interacting neurocognitive networks. In turn, the network's efficacy would be most vulnerable when multiple networks must effectively interact to maintain a high level of cognitive performance, such as during executive functions. From here it follows that increased DMN connectivity as a function of aerobic fitness may help explain some of the preferential benefit of aerobic fitness on executive functions (Colcombe & Kramer, 2003; Kramer & Erickson, 2007). It remains imperative, however, for future research to test whether connectivity of additional networks (e.g, frontoparietal or motor) is associated with individual differences in aerobic fitness and whether there is specificity in exercise training-induced plasticity of brain networks that supports specific cognitive gains. These research questions comprise a few of the critical next steps for understanding the role of aerobic exercise in promoting healthy cognitive aging.
Supplementary Material
Both analyses included regression of nuisance variables including a deep white matter ROI and lateral ventricle. The figure illustrates comparison of regional whole-brain analyses to assess the effect of preprocessing on the association of fitness with regional functional connectivity. Illustrated are results from: 1) regression of motion correction parameters only and 2) regression of motion correction + global signal. Results suggest that analysis while controlling for global signal variance is more sensitive to individual variation in fitness. Results were unchanged with respect to fitness when visual cortex activation was also corrected for. All renderings are in radiological orientation (L=R, R=L).
Acknowledgements
We would like to thank the National Institute on Aging [RO1 AG25667, RO1 AG25032] for supporting this research. We would also like to thank Nancy Dodge, Holly Tracy, and the EPL laboratory for their help in data collection. Finally, we would like to thank the reviewers for their very helpful comments on a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: None of the authors have actual or potential conflicts of interest related to this work.
References
- Aggleton JP. Understanding retrosplenial amnesia: Insights from animal studies. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2009.09.030. (in press). [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jerome GJ, Smith SM. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. NeuroImage. 2005;25:294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nature Reviews Neuroscience. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S. Annals of the New York Academy of Sciences. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychological Review. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The State of Aging and Health in America 2007. U.S. Department of Health and Human Services; 2007. [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. Journal of Neuroscience. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Medicine. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Weronika JB, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology: MEDICAL SCIENCES. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C, Berchtold NC, Christie L. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cerebral Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Mathews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- de Munck JC, Goncalves SI, Faes ThJC, Kuijer JPA, Pouwels PJW, Heethaar RM, Lopes da Silva FH. A study of the brain’s resting state based on alpha band power, heart rate and fMRI. NeuroImage. 2008;42:112–121. doi: 10.1016/j.neuroimage.2008.04.244. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neuroscience and Biobehavioral Reviews. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild AJ, MacKinnon DP, Taborga MP, Taylor AB. R2 effect-size measures for mediation analysis. Behavior Research Methods. 2009;41:486–498. doi: 10.3758/BRM.41.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, VanPraag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. The American journal of psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, U.S.A. 2002;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-Mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. Journal of Cognitive Neuroscience. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. Journal of Neuroscience. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Yucel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophrophrenia Research. 2007;91:82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Head D, Kennedy K, Rodrique K, Raz N. Age differences in preservation: cognitive and neuroanatomical mediators on the Wisconsin Card Sorting task. Neuropsychologia. 2009;47:1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. Journal of Neuroscience. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Marguilies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. The Journal of Comparative Neurology. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. The Journal of Comparative Neurology. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends in Cognitive Sciences. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen N, Banich M, McAuley E, Harrison C, Chason J, Vakil E, Bardell L, Boileau L, Boileau RA, Colcombe A. Ageing, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychologica. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation Analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly C, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (macaca mulatta) European Journal of Neuroscience. 1999;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels J, Taylor SF, Marshuetz C. Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues in Clinical Neuroscience. 2001;3:151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Task switching and multi-task performance. In: Monsell S, Driver J, editors. Attention and performance XVIII: Control of cognitive processes. Cambridge: MIT Press; 2000. pp. 277–309. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Broeckhoven CV, Adolfsson R, Nilsson LG, Nyberg L. Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia. 2008;46:1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: A link to cognitive control? Journal of Cognitive Neuroscience. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediatior models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press; 1986. Geriatric depression scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences, USA. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulsen J, Mayeux R. Modified mini-mental state examination: Validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- Supekar K, Menon M, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s Disease. PLOS Computational Biology. 2008;4:1–11. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treserras S, Boulanouar K, Conchou F, Simonetta-Moreau M, Berry I, Celsis P, Chollet F, Loubinoux I. Transition from rest to movement: Brain correlates revealed by functional connectivity. NeuroImage. 2009;48:207–216. doi: 10.1016/j.neuroimage.2009.06.016. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, Heuvel M, Ramsey NF, Kahn RS, Vink M. Cardiorespiratory effects on default-mode network activity as measured with fMRI. Human Brain Mapping. 2009;30:3031–3042. doi: 10.1002/hbm.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences, U.S.A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Neuroscience Reviews. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Doerksen S, Hu L, McAuley E, Kramer AF. Dedifferentiation in the visual cortex: An fMRI investigation of individual differences in older adults. Brain Research. 2008;1244:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. NeuroImage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three dimensional statistical analysis for cbf activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Upper Saddle River: Prentice-Hall; 1996. [Google Scholar]
- Zhang Y, Brady M, Smith SM. Segmentation of brain MR images through a hidden markov random field model and the expectation maximization algorithm. IEEE Transactions in Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Dougherty JH, Jr, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimer’s & Dementia. 2008;4:265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Both analyses included regression of nuisance variables including a deep white matter ROI and lateral ventricle. The figure illustrates comparison of regional whole-brain analyses to assess the effect of preprocessing on the association of fitness with regional functional connectivity. Illustrated are results from: 1) regression of motion correction parameters only and 2) regression of motion correction + global signal. Results suggest that analysis while controlling for global signal variance is more sensitive to individual variation in fitness. Results were unchanged with respect to fitness when visual cortex activation was also corrected for. All renderings are in radiological orientation (L=R, R=L).