Abstract
Purpose
To report the short-term efficacy and safety of intravitreal bevacizumab (Avastin) injection with panretinal laser photocoagulation (PRP) in patients with high-risk proliferative diabetic retinopathy (PDR) according to the Early Treatment Diabetic Retinopathy Study criteria.
Methods
A prospective, interventional case series study was conducted in 17 patients (20 eyes) with high-risk PDR, who were treated with intravitreal bevacizumab (2.5 mg) followed by PRP when the peripheral vitreous became clear or 2 weeks after injection. Patients underwent complete ophthalmic evaluation, including Snellen visual acuity and fluorescein angiography at baseline, 1, 3, and 6 months after bevacizumab injection. Main outcome measures included the serial changes in visual acuity, vitreous clear-up time, and neovascularization on the disc (NVD) regression time.
Results
All patients had obvious reduction in angiographic leakage and involution of retinal neovascularization (NV) at the 1- and 3-month follow-up. The mean follow-up time was 7.5 months. The vitreous hemorrhage (VH) showed a partial resolution as early as 1 week, and complete regression at 3 months. The mean vitreous clear-up time after intravitreal Avastin was 8.5±2.2 weeks. The mean time interval from intravitreal Avastin to NVD regression was 10.8±3.4 weeks. Mean logarithm of the minimum angle resolution visual acuity improved from 1.03 at baseline to 0.36 at 1-month, 0.38 at 3-month, and 0.48 at the 6-month follow-up (P<0.01). Three eyes (18%) required vitrectomy surgery during follow-up. The indication for vitrectomy was dense, persistent VH in 2 eyes, and focal tractional retinal detachment (TRD) in 1 eye. Recurrent retinal NV with minor preretinal hemorrhage was observed in 6 eyes (30%) 3 months after the first injection, and resolved after repeated bevacizumab injections. Patients received an average of 1.4 injections (range: 1–2). Seven eyes (35%) underwent 2 injections. One eye (5%) had ocular complication of PDR progression to TRD. No systemic adverse events were observed following injections.
Conclusions
Short-term results suggest combined intravitreal bevacizumab and PRP achieved rapid clearance of VH, regression of retinal NV, and visual improvement in the treatment of high-risk PDR. Long-term study is warranted to assess the long-term efficacy and safety.
Introduction
Diabetic retinopathy remains the most common cause of visual loss in working aged adults in developed countries.1 Without timely treatment, 50% of the patients with proliferative diabetic retinopathy (PDR) will lose their sight within 5 years.2 The occurrence and growth of retinal new vessels [neovascularization (NV)], either on the disc or elsewhere, is an important risk factor for severe loss of vision in patients with diabetic mellitus.3 The retinal NV is generally thought to occur as a response to retinal hypoxia and ischemia, and secondary release of the vascular endothelial growth factor (VEGF) into the vitreous cavity.4–6
VEGF is a potent stimulus for vascular leakage and endothelial proliferation and migration.7 The vitreous levels of VEGF were elevated by about 3-fold in patients with severe PDR.8 Since PDR is associated with an increased level of VEGF in the vitreous,4,6,8 and VEGF has been shown to play a key role in the development of NV,5 blocking VEGF theoretically should reverse many complications resulting from PDR. Thus, anti-VEGF pharmacologic therapy could be a promising alternative therapy for PDR with retinal NV.
Bevacizumab (Avastin; Genetech, San Francisco, CA) is a recombinant monoclonal antibody that binds all isoforms of VEGF. The systemic administration of bevacizumab is approved for metastatic colon cancer.9 Recently, regression of retinal NV and resolution of vitreous hemorrhage (VH) were reported after a single injection of bevacizumab.10,11 The author suggested the mechanism that bevacizumab can regress the NV and stop the multiple rebleeding episodes during the VH clearing process.10 However, this effect seemed not to be durable because NV tended to recur after 12 weeks.12 Herein, we conducted a prospective study to investigate the efficacy and safety of intravitreal bevacizumab (Avastin) injection in combination with subsequent panretinal laser photocoagulation (PRP) to treat patients with high-risk PDR.
Patients and Methods
In this prospective, uncontrolled interventional case series study, patients diagnosed with high-risk PDR, complicated by VH and/or retinal neovascularization on the disc (NVD), were enrolled between January 2010 and August 2011 at Taipei Veterans General Hospital (Taipei, Taiwan). The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Study design and baseline evaluation
Demographics and baseline clinical findings, including age, sex, duration, and category of diabetic mellitus, metabolic control (hemoglobin A1c), associated systemic diseases (hypertension), and previous laser treatment, were recorded. The ocular and systemic safety of intravitreal injection of bevacizumab were also evaluated and analyzed.
Patients were included if they had high-risk PDR, which was defined according to the guidelines by the Early Treatment Diabetic Retinopathy Study,3,13 as any one of the following: (1) presence of moderate to severe NVs at the disc (NVDs) with 1/4–1/3 disc area in size or larger; (2) presence of any NVDs associated with vitreous or preretinal hemorrhage, or (3) NVs elsewhere with more than a half disc area associated with vitreous or preretinal hemorrhage. Exclusion criteria included (1) history of prior vitreoretinal surgery (vitrectomy, intravitreal triamcinolone injection) in the study eye; (2) history of any thromboembolic event (including myocardial infarction or cerebral vascular accident); (3) major surgery within the prior 6 months or planned within the next 28 days; (4) uncontrolled hypertension; (5) known coagulation abnormalities or current use of anticoagulative medication other than aspirin; or (6) evidence of external ocular infection (conjunctivitis, keratitis, or significant blepharitis).
Each patient received detailed complete ophthalmologic examinations, including best-corrected visual acuity (BCVA) measurements converted to a logarithm of the minimum angle resolution (logMAR), intraocular pressure (IOP), slit lamp biomicroscopy, indirect ophthalmoscopy, fundus photography, and fluorescein angiography (FA) at the first visit (baseline evaluation), 1, 3, and 6 months after injection.
All patients signed a written informed consent form. The nature of off-label use of this drug and its potential side effects of endophthalmitis, retinal detachment, and the possibility of thromboembolic events were discussed with patients before obtaining informed consent.
Ophthalmic follow-up examinations and main outcome measures
Postinjection follow-up was scheduled at 1 day and 1 week, and then every 4 weeks thereafter. At each visit, complete ophthalmologic examinations were performed. Echographic evidence of vitreoretinal traction (VRT) was also noted during follow-up. Systemic or ocular adverse events were monitored. In eyes with VH, attempt of some scatter PRP was performed when the peripheral vitreous became clear, and supplemental PRP was performed during monthly return visits. In eyes without VH, full scatter PRP was performed 2 weeks after injection.
Two outcome measures were used to evaluate the short-term effects of bevacizumab. The primary study outcome measure was defined as the serial change in mean logMAR BCVA. The secondary outcome measure was the vitreous clear-up time and NVD regression time. The vitreous clear-up time was defined as clearly visible main retinal vessels and optic disc in the posterior pole with the peripheral retina clear enough for a successful PRP in at least 3 quadrants. Panretinal photocoagulation was performed by independent ophthalmologists unaware of this study. The NVD regression time was defined as the total absence of previous fluorescein leakage from the active NVD before Avastin injections. The following results were also recorded to evaluate the effects of bevacizumab: number of patients who need second injection, rate of persistent or recurrent VH, and frequency of eyes underwent vitrectomy during follow-up.
Injection technique
Injection was performed via pars plana under aseptic conditions in the operation room. Before injection, topical anesthetic eye drops were applied at least 3 times. The conjunctival sac and eyelid margin were rinsed with the povidone-iodine solution. After application of a sterile drape, a lid speculum was inserted. The dosage for intravitreal bevacizumab injections was 2.5 mg (0.1 mL), injected using a syringe with a 30G needle at a distance of 4.0 mm from the limbus at phakic eyes, and 3.5 mm in pseudophakic eyes. Before injection, anterior chamber paracentesis was performed to maintain adequate postoperative IOP. The needle was removed carefully using a sterile cotton applicator to prevent reflux. After injection, antibiotic eye drops and topical corticosteroid eye drops were applied 4 times a day for 1 week.
Statistical analysis
Statistical analysis of the data was performed using SPSS software (version 18.0 SPSS, Inc., Chicago, IL). The serial changes in mean BCVA were presented. BCVA was reported as logMAR visual acuity and the Snellen equivalent. Continuous outcomes were compared using the Wilcoxon t test. A P-value<0.05 was considered statistically significant.
Results
A total of 17 consecutive patients (20 eyes) with high-risk PDR, complicated by VH or NVD, were enrolled and investigated in this study. The baseline characteristics of patients are summarized and shown in Table 1. Of the 17 patients, 10 were female and 7 were male. Thirteen eyes presented with VH, and 7 eyes presented with moderate to severe NVD. The mean age was 54.9±9.1 years (age range: 44–81). The mean follow-up period was 7.5 months (range: 6.5–12 months). Eight patients had a history of hypertension (47%). PRP has been previously performed on 5 (29%) patients.
Table 1.
Baseline Characteristics of Patients with Intravitreal Injection of Bevacizumab
| Characteristics | Study patients (n=17) |
|---|---|
| Number of patients | 17 |
| Gender | |
| Male | 7 |
| Female | 10 |
| Number of eyes | 20 |
| Right eye | 9 |
| Left eye | 11 |
| Age of onset (mean±SD) | 54.9±9.1 |
| Range | 44–81 |
| Type of DM (n) | |
| Type 1 insulin-dependent diabetes mellitus (IDDM) | 3 |
| Type 2 non-insulin-dependent diabetes mellitus (NIDDM) | 14 |
| Duration of DM (years) (mean±SD) | 15.1±8.6 |
| Hemoglobin A1c (mean±SD) | 9.5±2.1 |
| Presence of hypertension [n (%)] | 8 (47) |
| Prior PRP [n (%)] | 5 (29) |
SD, standard deviation; DM, diabetes mellitus; PRP, panretinal laser photocoagulation.
After intravitreal injection of bevacizumab, all patients had obvious reduction in angiographic leakage and involution of NV at the 1- and 3-month follow-up. The VH showed a partial resolution as early as 1 week, and complete regression at 3 months. In the 12 patients (71%) without previous laser PRP treatment (naive eyes), bevacizumab also resulted in rapid clearance of VH and avoided vitrectomy surgery in these high-risk patients. Patients received an average of 1.4 injections during follow-up (range: 1–2). Thirty-five percent of eyes (7 eyes) underwent repeated injections. The detailed data and clinical outcomes of patients after intravitreal injection of bevacizumab are presented in Table 2. Seventeen eyes (85%) underwent laser PRP after the intravitreal injection of bevacizumab. The time interval from the first intravitreal bevacizumab to further PRP was 4.5±3.5 weeks during follow-up.
Table 2.
Clinical Outcomes for Study Eyes After Intravitreal Injection of Bevacizumab
| Clinical outcome | Study eyes (n=17) |
|---|---|
| Number of IVIA (mean±SD) | 1.4±0.5 |
| Time of NVD regression (weeks) (mean±SD) | 10.8±3.4 |
| VCUT without vitrectomy (weeks) (mean±SD) | 8.5±2.2 |
| Eyes underwent vitrectomy during follow-up [n (%)] | 3 (18) |
| Indication for vitrectomy [n (%)] | |
| Dense VH | 2 (66.7) |
| Focal TRD | 1 (33.3) |
| Baseline BCVA (logMAR) | 1.03±0.64 |
| 6 months BCVA (logMAR) | 0.48±0.43 |
| Difference between baseline and 6 months BCVAa (logMAR) | 0.56±0.55 |
Statistical analysis of the difference between baseline and 6 months BCVA was performed using the Wilcoxon t test: P<0.01 (P<0.05 was considered statistically significant).
IVIA, intravitreal injection of Avastin; NVD, neovascularization on the disc; VCUT, vitreous clear-up time; TRD, tractional retinal detachment; VH, vitreous hemorrhage; BCVA, best-corrected visual acuity; logMAR, logarithm of the minimum angle resolution.
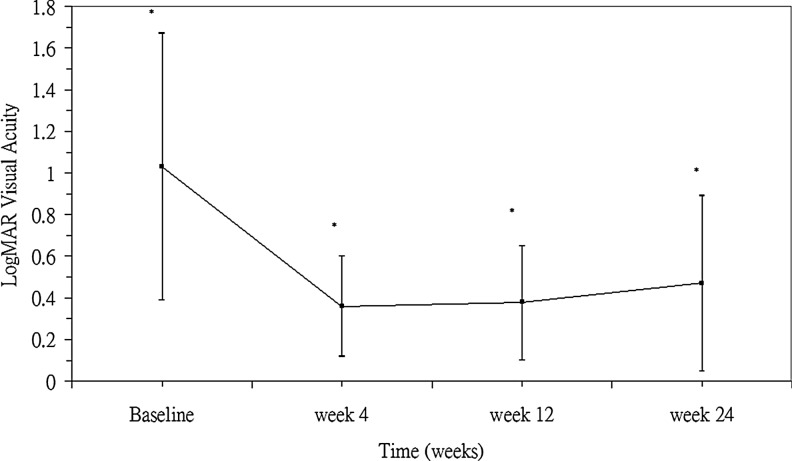
The mean Snellen visual acuity improved from 20/214 at baseline to 20/46 at 1-month, 20/48 at 3-month, and 20/59 at the 6-month follow-up. Nine of the 17 eyes (53%) showed an improvement in VA of more than 3 lines on the Snellen chart. Baseline mean BCVA with the logMAR scale was 1.03±0.64. LogMAR BCVA improved to 0.36±0.23, 0.38±0.27, and 0.48±0.43 at 1, 3, and 6 months, respectively, following the injection of bevacizumab (P<0.01 at each time points) (Fig. 1). No patient had loss of vision greater than one line at the last visit. One eye (5%) had ocular complication of PDR progression to tractional retinal detachment (TRD). No systemic adverse events were observed following injections.
FIG. 1.
Mean best-corrected visual acuity [logarithm of the minimum angle resolution (logMAR)] changes in eyes with proliferative diabetic retinopathy (n=17) at baseline and different time points after intravitreal bevacizumab injections (*P<0.01 at each time points).
In 13 eyes of high-risk PDR complicated with VH, the mean vitreous clear-up time after intravitreal Avastin was 8.5±2.2 weeks. The increased fibrovascular retinal traction was noted in 6 eyes (30%) during follow-up. A patient who presented with VH and was treated with intravitreal injections of bevacizumab is shown in Fig. 2. Three eyes required vitrectomy surgery during follow-up. The mean time interval from intravitreal Avastin to vitrectomy was 7.3 weeks. Among those 3 eyes, the indication for vitrectomy was dense, persistent VH in 2 eyes (66.7%), and development and progression of focal TRD in 1 eye (33.3%). The risk factors of making an eye most likely to fail combined intravitreal bevacizumab and PRP therapy include severe PDR with preexisting fibrovascular proliferation and focal TRD.
FIG. 2.
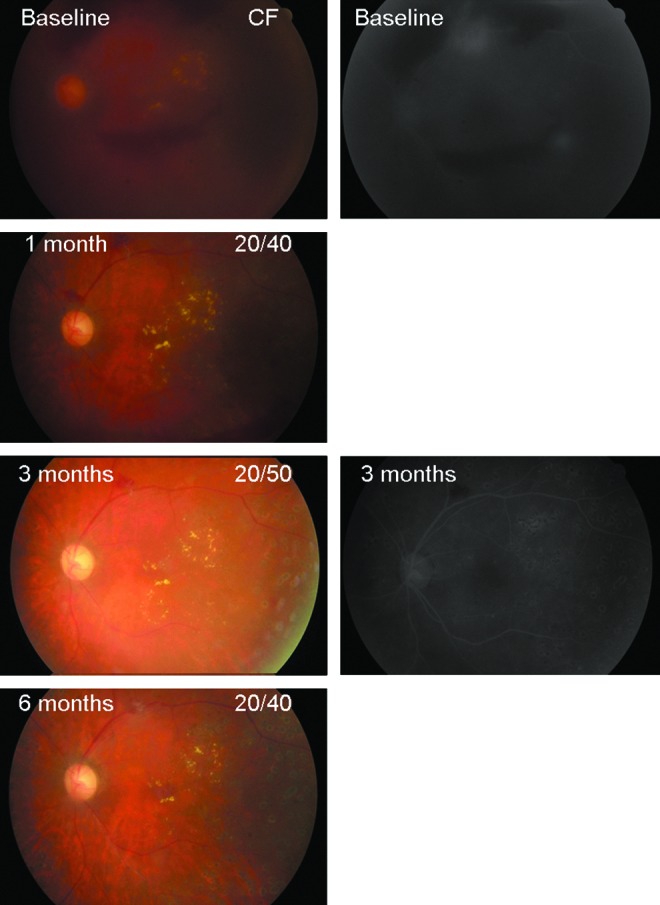
A 51-year-old female presented with moderate (1/2 disc area) neovascularization elsewhere (NVE) and vitreous hemorrhage (VH) in the left eye, and fluorescein angiography (FA) showed large regions of NVE leakage compatible with the diagnosis of high-risk proliferative diabetic retinopathy. She received the first intravitreal injection of bevacizumab at baseline. After 1 month, VH remarkably cleared, and visual acuity improved from counting fingers (CF) at baseline to 20/40. Three months after bevacizumab injection, VH completely cleared up and FA disclosed no more fluorescein leakage from NVE. After 6 months, NVE completely regressed with fibrous tissue left. Color images available online at www.liebertpub.com/jop
In 7 eyes of high-risk PDR complicated with moderate to severe NVD, the mean time interval from intravitreal Avastin to complete NVD regression on FA examination was 10.8±3.4 weeks. A patient who presented with high-risk PDR and severe NVD and was treated with repeated injections of bevacizumab is shown in Fig. 3. Partially reperfused retinal NV with minor preretinal hemorrhage was observed in 6 eyes (30%), 3 months after the first injection, and resolved after repeated bevacizumab injections. There was no new NV or enlarged area of prior retinal NV noted in any patients during the follow-up period.
FIG. 3.
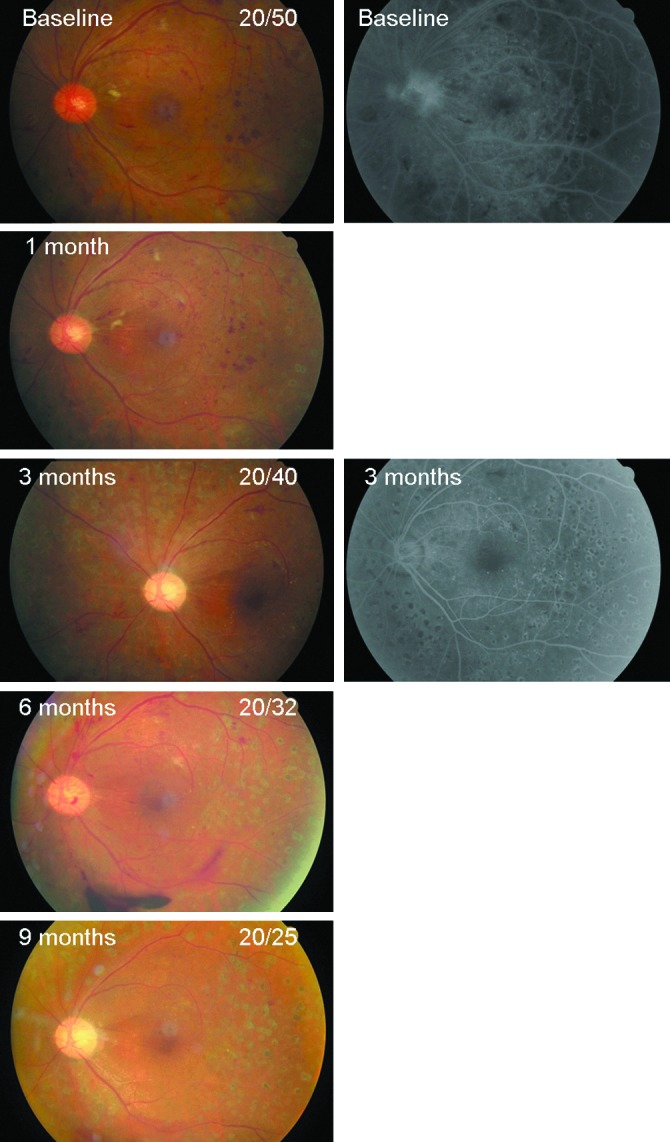
A 50-year-old male presented with severe neovascularization on the disc (NVD), compatible with the diagnosis of high-risk proliferative diabetic retinopathy. FA showed profuse NVD leakage and a large area of capillary dropout. He received the first intravitreal injection of bevacizumab at baseline. After 3 months, NVD completely regressed without any fluorescein leakage. However, reperfused NVD with preretinal hemorrhage occurred in the 6-month follow-up, thus, he received repeated intravitreal bevacizumab injection. NVD regressed again at the 9-month follow-up, and visual acuity improved from 20/50 at baseline to 20/25. Color images available online at www.liebertpub.com/jop
Discussion
Our study demonstrated that intravitreal bevacizumab in combination with PRP showed promising short-term functional and anatomic effects in the treatment of the complication associated with high-risk PDR. Intravitreal bevacizumab achieved marked regression of retinal NV, rapid resolution of VH, and concomitant visual improvement. Mean VA had a statistically significant improvement from 20/214 to 20/59 during a mean 7.5-month follow-up (P<0.01). Twelve patients (71%) without previous laser PRP treatment (naive eyes), bevacizumab also resulted in rapid clearance of VH, and avoided vitrectomy surgery in these high-risk patients. Since the currently used treatment modality of laser PRP has its limitations and drawbacks, combined anti-VEGF pharmacologic therapy and PRP appear as an alternative or adjunctive therapeutic option for PDR.
The healthy human retina contains little VEGF; however, patients with active PDR were associated with elevated vitreous levels of VEGF.5 Increased VEGF, triggered by hypoxia, is a key mediator of retinal NV and macular edema.4–6 Previous study has also demonstrated that the VEGF concentration declined after successful laser PRP.5 Furthermore, injection of VEGF in primates can produce an ischemic retinopathy similar to diabetic retinopathy and even produce iris NV.14 Therefore, inhibition of VEGF by intravitreal bevacizumab could theoretically provide a potential therapeutic advantage for retinal NV in PDR.
PRP currently is the mainstay and gold standard therapy for PDR since the Diabetic Retinopathy Study was published.15 It is estimated that about 60% PDR patients respond to laser PRP with retinal NV regression within 3 months.16 However, it is a destructive procedure, often painful, and may be associated with a decreased peripheral visual field and an increased risk of macular edema.15 Many diabetic patients need additional laser therapy and 4.5% of them eventually require vitrectomy surgery despite laser PRP.17 Moreover, NV regression may take several weeks after completion of PRP, and NV continues to grow despite the first session of PRP in one-third of patients.18 Therefore, VH may lead to visual loss and preclude complete laser PRP in these patients. The current study demonstrated the advantage of intravitreal bevacizumab on the management of patients with PDR and VH. It may play as a new therapeutic option or an adjuvant agent to PRP in some patients of PDR, such as when VH precludes the visualization of fundus and prevents adequate laser PRP. However, the possibility of worsening TRD is a major concern. The main shortcoming of bevacizumab is the short duration of its effect. Conversely, laser PRP has better durability. In the current study, combined bevacizumab and supplemental PRP accelerated the VH clear-up and NV regression. In the present study, intravitreal bevacizumab may also have synergistic effects, when used in combination with PRP for the treatment of high-risk PDR with severe NVD.
Although PRP was performed, partially recurrent retinal NV with minor preretinal hemorrhage was observed in 6 eyes (30%), 3 months after the first injection, and resolved after repeated bevacizumab injections. Similar observations have been reported in the literature.10–12 Reperfused NV after first injection may be attributed to an insufficient amount of bevacizumab after the loading injection. Jorge et al. reported that the maximal bevacizumab effect on retinal NV regression was evident at week 6, with recurrent NV leakage observed in 93% of eyes at the 12th week.12 However, the area of recurrent NV leakage at week 12 was significantly decreased compared to the baseline area.12 Matsumoto et al. also reported that rebound macular edema was observed following intravitreal bevacizumab in patients with retinal vein occlusion of a chronic nature.19 The authors hypothesized that bevacizumab-induced upregulation of VEGF receptors may be more sensitive to the VEGF in the underlying ischemic state.19 The onset of recurrent retinal NV varied among our patients. In the current study, we chose to defer the reinjection only when recurrent NV occurred. We hypothesized that the factors influencing the recurrence of retinal NV after the first injection may include the larger area of ischemic retina, absence of prior laser PRP, staging of preexisting retinal new vessels, and the inadequacy of subsequent laser PRP. Therefore, a regular follow-up with repeated bevacizumab injections and subsequent PRP may be necessary to sustain a steady outcome in patients with high and prolonged VEGF levels.
The optimal dosing of intravitreal bevacizumab in treating PDR is still undetermined. Doses of bevacizumab used in the literature have ranged from 1.25 to 2.5 mg.10,11,20,21 We chose the higher dose of 2.5 mg in this study in the hopes of prolonging the therapeutic effect and reducing the number of repeated injections. The average number of injections in this study was 1.4 in a 6-month follow-up. Furthermore, Arevalo et al. reported a dose-dependent response that 2.5 mg dosage seemed to be more effective than the 1.25 mg to have complete NV regression in naïve eyes.20 Nevertheless, in a recent biologic study, a possible therapeutic effect in the fellow eye raises concern that systemic side effects are possible in patients undergoing intravitreal bevacizumab (6.2 μg–1.25 mg) treatment, and a lower dose regimen may achieve a therapeutic result with less risk of systemic side effects.11 Further study of the optimal dosing of intravitreal bevacizumab in treating PDR is indicated.
Three eyes required vitrectomy surgery despite intravitreal bevacizumab during follow-up. Among those 3 eyes, the indication for vitrectomy was dense, persistent VH without any sign of hemorrhage absorption in 2 eyes, and PDR progression to focal TRD in 1 eye. Theoretically, the anti-VEGF pharmacologic agent has fibrosis-inducing ability and increases VRT. In literature, the progression to TRD from a preexisting fibrovascular tissue contraction is a risk in few cases after intravitreal bevacizumab.10,20,22 Furthermore, Arevalo et al. reported the development or progression of TRD in 5.2% patients with severe PDR following intravitreal bevacizumab.23 Cautious echographic examinations should be done before intravitreal bevacizumab to exclude the patients with broad vitreoretinal adhesion. Thereafter, careful ophthalmologic examinations and echographic evidence of VRT should be carefully monitored at the follow-up visit after intravitreal bevacizumab therapy.
The limitations of this current study included the fact that it had relatively small number of patients (sufficient for statistical purposes) and short-term follow-up period. There was no control group of PDR without intravitreal bevacizumab treatment. A long-term prospective study is needed to confirm the maintenance of therapeutic benefit suggested in this study. Evaluation of possible long-term ocular and systemic adverse effects is also essential.
In conclusion, the observed anatomic (by ophthalmic examination and FA) and visual acuity improvements after combined intravitreal bevacizumab injection and PRP demonstrated that it was a useful alternative or adjunctive treatment for high-risk PDR. However, multiple reinjections are necessary to maintain the visual improvement in some patients. Long-term study is mandatory to determine the optimal dosing regimen.
Acknowledgments
This study was supported by grants from the National Science Council (NSC98-2314-B075-003-MY2) and Taipei Veterans General Hospital (V100C-043, V99C1-009), Taiwan.
Author Disclosure Statement
No proprietary interest is held by any of the authors in this manuscript.
References
- 1.Klein R. Retinopathy in a population-based study. Trans. Am. Ophthalmol. Soc. 1992;90:561–594. [PMC free article] [PubMed] [Google Scholar]
- 2.Chew E.Y. Major clinical trials of vitreoretinal diseases. In: Regillo C.D., editor; Brown G.C., editor; Flyn H.W., editor. Vitreoretinal Disease, the Essentials. New York: Theme Medical Publishers, Inc.; 1999. pp. 667–677. [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98:823–833. ETDRS Report No. 12. [PubMed] [Google Scholar]
- 4.Adamis A.P. Miller J.W. Bernal M.T., et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 5.Aiello L.P. Avery R.L. Arrigg P.G, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 6.Pe'er J. Shweiki D. Itin A., et al. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab. Invest. 1995;72:638–645. [PubMed] [Google Scholar]
- 7.Noma H. Funatsu H. Yamasaki M., et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am. J. Ophthalmol. 2005;140:256–261. doi: 10.1016/j.ajo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Malik R.A. Li C. Aziz W., et al. Elevated plasma CD105 and vitreous VEGF levels in diabetic retinopathy. J. Cell. Mol. Med. 2005;9:692–697. doi: 10.1111/j.1582-4934.2005.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manzano R.P.A. Peyman G.A. Khan P., et al. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Spaide R.F. Fisher Y.L. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Avery R.L. Pearlsman J. Pieramici D.J., et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–1705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 12.Jorge R. Costa R.A. Calucci D., et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE Study) Retina. 2006;26:1006–1013. doi: 10.1097/01.iae.0000246884.76018.63. [DOI] [PubMed] [Google Scholar]
- 13.Diabetic Retinopathy Study Research Group. Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. Arch. Ophthalmol. 1979;97:654–655. doi: 10.1001/archopht.1979.01020010310003. [DOI] [PubMed] [Google Scholar]
- 14.Tolentino M.J. Miller J.W. Gragoudas E.S., et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 15.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. Ophthalmology. 1991;98:766–785. ETDRS report number 9. [PubMed] [Google Scholar]
- 16.Vander J.F. Duker J.S. Benson W.E., et al. Longterm stability and visual out-come after favourable initial response of proliferative diabetic retinopathy to panretinal photocoagulation. Ophthalmology. 1991;98:1575–1579. doi: 10.1016/s0161-6420(91)32085-2. [DOI] [PubMed] [Google Scholar]
- 17.Flynn H.W., Jr Chew E.Y. Simons B.D., et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. Ophthalmology. 1992;99:1351–1357. doi: 10.1016/s0161-6420(92)31779-8. ETDRS Report No. 17. [DOI] [PubMed] [Google Scholar]
- 18.Doft B.H. Blankenship G.W. Single vs multiple treatment sessions of argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1982;89:772–779. doi: 10.1016/s0161-6420(82)34734-x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto Y. Freund K.B. Peiretti E., et al. Rebound macular edema following bevacizumab (Avastin) therapy for retinal venous occlusive disease. Retina. 2007;27:426–431. doi: 10.1097/IAE.0b013e31804a7af2. [DOI] [PubMed] [Google Scholar]
- 20.Arevalo J.F. Wu L. Sanchez J.G., et al. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye. 2009;23:117–123. doi: 10.1038/sj.eye.6702980. [DOI] [PubMed] [Google Scholar]
- 21.Wu L. Arevalo J.F. Roca J.A., et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008;28:212–219. doi: 10.1097/IAE.0b013e3181619bee. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.H. Yeh P.T. Chen M.S. Yang C.H. Yang C.M. Intravitreal bevacizumab and panretinal photocoagulation for proliferative diabetic retinopathy associated with vitreous hemorrhage. Retina. 2009;29:1134–1140. doi: 10.1097/IAE.0b013e3181b094b7. [DOI] [PubMed] [Google Scholar]
- 23.Arevalo J.F. Maia M. Flynn H.W., Jr., et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br. J. Ophthalmol. 2008;92:213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]