Abstract
Purpose
Vascular endothelial growth factor (VEGF) may contribute to the scarring process resulting from glaucoma filtration surgery, since this cytokine may stimulate fibroblast proliferation. The aim of this study was to describe a new bevacizumab-loaded polyurethane implant (BPUI) and to evaluate its effectiveness as a new drug delivery system of anti-VEGF antibody in a rabbit model of glaucoma filtration surgery.
Methods
An aqueous dispersion of polyurethane was obtained via the conventional process. Bevacizumab (1.5 mg) was then incorporated into the dispersion and was subsequently dried to form the polymeric films. Films with dimensions of 3×3×1 mm that either did (group BPUI, n=10) or did not contain bevacizumab (group PUI, n=10) were implanted in the subconjunctival space, at the surgical site in 1 eye of each rabbit. The in vitro bevacizumab release was evaluated using size-exclusion high-performance liquid chromatography (HPLC), and the in vivo effects of the drug were investigated in a rabbit experimental trabeculectomy model by examining the bleb characteristics and collagen accumulation, and by performing immunohistological analyses of VEGF expression.
Results
HPLC showed that only 10% of the bevacizumab in the implants had been released by postoperative day 5. In vivo studies demonstrated that the drug had no adverse effects; however, no significant differences in either the bleb area score or the collagen deposit intensity between the group PUI and the group that BPUI were observed. Moreover, the group BPUI presented a significantly lower proportion of VEGF-expressing fibroblasts than group PUI (0.17±0.03 vs. 0.35±0.05 cells/field, P=0.005).
Conclusions
This study demonstrated that bevacizumab release from the BPUIs only occurred for a short time probably from the surface of the films. Nevertheless, they were well tolerated in rabbit eyes and reduced the number of VEGF-expressing fibroblasts.
Introduction
Intraocular pressure (IOP) reduction is considered the main objective of many glaucoma treatment modalities to maintain vision.1,2 Surgical procedures that involve draining the aqueous humor to the sub-Tenon's space are specifically associated with decreases in the rates of visual-field progression.3
Trabeculectomy is the most commonly performed glaucoma-filtering surgery procedure. Its outcome depends on the amount of resistance that develops due to scarring during wound healing. After a trabeculectomy, the development of conjunctival and episcleral fibrosis occurs as a result of progressive fibroblast migration, fibroblast proliferation, collagen accumulation, and angiogenesis at the site of filtration.4,5
The primary auxiliary treatment after a trabeculectomy to curb excessive scarring is to use antifibrotic agents, including 5-fluorouracil (5-FU) and mitomycin C (MMC). However, these agents may have drawbacks that possibly lead to thin-walled avascular blebs, which are susceptible to further complications.6–8
These side effects have prompted that increasing interest in the use of angiogenesis-inhibiting compounds [especially those that act against vascular endothelial growth factor (VEGF), termed anti-VEGF compounds] that are aimed at modulating the effects of VEGF on both the proliferation and migration of human fibroblasts in Tenon's capsule has arisen.5 Bevacizumab, which is an anti-VEGF monoclonal antibody that contains human interaction regions, has been successfully used to aid in the wound-healing process that is associated with glaucoma surgery.9–11
There remains uncertainty regarding how to optimally deliver bevacizumab. One possibility is subconjunctival delivery, but it is expected to have a short duration of action and would therefore require repetitive injections.12
Polyurethane implants (PUIs) have been investigated extensively for use in biomedical applications as scaffolds for tissue regeneration and as controlled-/sustained-release drug delivery systems because of their excellent biocompatibility, chemical versatility, and mechanical properties.13–15 No previous study has evaluated the use of anti-VEGF antibodies associated with PUIs in the eye, so the aim of the present study was to demonstrate the release of active bevacizumab from PUIs in an experimental model of glaucoma-filtering surgery in rabbits.
Methods
Preparation of polyurethane films
The PUIs were prepared from an aqueous dispersion of polyurethane according to conventional methods. In a nitrogenous environment, poly-Å-caprolactone and polyethylene glycol dimethyl propionic acid (DMPA) were transferred to a reactor, stirred for ∼30 min, and were then added to a solution that contained the reactor isophorone diisocyanate until the NCO/OH ratio was equal to 2.3. This mixture was then heated to 70°C–75°C for 2 h, after which a catalyst, dibutyltin dilaurate, was added at a temperature of 70°C–75°C for 1 h. Triethylamide prepolymers were added to the mixture, and the mixture was vigorously shaken at a temperature 40°C for 40 min to neutralize the COOH groups of the DMPA. The prepolymer was then dispersed in water, and hydrazine was immediately added as a chain extender to complete the synthesis (40°C/1 h).
Bevacizumab incorporation to the PUIs
To incorporate bevacizumab into the PUIs, 300 mg/12 mL of bevacizumab (Avastin®—25 mg/1 mL; Genentech) was added to 4.0 mL of an aqueous polyurethane dispersion, and the mixture was mildly agitated. This method resulted in a film weight of 2,492.44 mg, reaching a final bevacizumab concentration of 12.04% (w/w). The aqueous polyurethane dispersion that contained the dissolved bevacizumab was then transferred to plastic molds and was dried at room temperature for 7 days. PUIs that did not contain the drug were prepared via the same process. After the drying process was complete, both of the implants that contained bevacizumab (BPUIs) and those that did not contain the drug (PUIs) had a final appearance that was similar to that of transparent plastic. The films were then cut and shaped in the form of flat cobbles that had dimensions of 3×3×1 mm; each BPUI contained a final dose of 1.0 mg bevacizumab (Fig. 1).
FIG. 1.
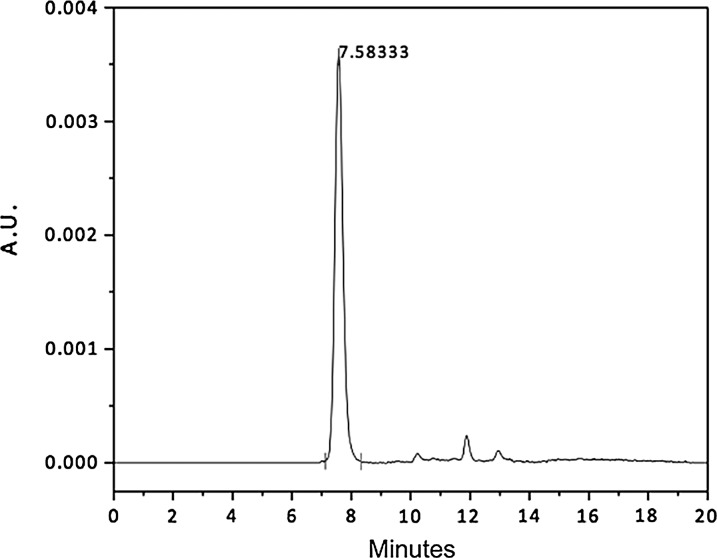
Size-exclusion high-performance liquid chromatogram of a 24-h sample of a bevacizumab-loaded polyurethane implant (BPUI) that was incubated in phosphate-buffered saline (PBS) for the detection of bevacizumab at a flow rate of 1.0 mL/min (Waters BioSuite 250; 5 μm, HR SEC–7.8×300 mm). AU, absorbance unit (279 nm)×104.
In vitro degradation study
The following procedure was performed in an incubator at 30 rpm and 37°C. BPUIs and PUIs were placed in glass vials with 2.0 mL of phosphate-buffered saline (PBS; pH 7.4). At a series of pre-established intervals, the incubation medium was removed from the implants, and the quantity of liquid in the buffer vials was renewed by adding 2.0 mL of freshly prepared PBS to each flask. The amount of drug that had been released into the incubation medium was quantified with a size-exclusion high-performance liquid chromatography (HPLC) using a Waters® apparatus that was equipped with an autosampler (model 717plus; Waters) and according to the method that was described by Gomes et al.16 Chemical separation was carried out in an isocratic manner using a pump (model 515; Waters) with a constant flow rate of 1.0 mL/min A BioSuite 250® HR SEC, 300×7.8 mm×5 μm (Waters). An HPLC column was used in which the mobile phase consisted of PBS at a pH of 7.4 was used. An ultraviolet detector (model 2487; Waters) at a wavelength of 279 nm was used, and the area under peak was used to determine the quantity of the drug in the test solution.
Scanning electron microscopy of the films
The films were directly examined with a scanning electron microscope (JEOL; model JSM-6360LV—operated at 5 kV) both before and after 5 days of incubation. All of the films underwent a cryogenic fracture procedure, so that both the surface and the region of fracture could be analyzed. No metallization of the samples was necessary.
Fourier-transform infrared spectroscopy
To evaluate the potential bevacizumab–polyurethane interactions, the infrared spectra of the PUI and BPUI samples were collected in a Fourier-transform infrared spectrophotometer (FTIR; Perkin Elmer, model Spectrum 1000). Each sample was pressed against a Zinc selenide crystal, and measurements were carried out using the attenuated total reflectance technique. Each spectrum was a result of 32 scans with a resolution of 4 cm−1, in the range 4,000–650 cm−1, with posterior interpretation of the bands plotted on graphs.
Surgical procedures
Twenty female New Zealand rabbits (Oryctolagus cuniculus), each weighing between 2 and 2.5 kg, were used in this study. All of the animals were acquired from the Central Bioterium of the University of São Paulo (Ribeirão Preto Campus). The BPUIs and PUIs were sterilized with ethylene oxide before being soaked in a sterile physiological saline solution before surgery. Intramuscular injection of ketamine hydrochloride (35–50 mg/kg, Ketalar; Pfizer) and Xylazin (5–10 mg/kg; König do Brasil Ltda) was used to induce general anesthesia for the surgeries. A subconjunctival pocket was created via a blunt dissection at the limbus and a 5–6-mm incision in the superior-temporal quadrant of the right eye of each rabbit. One of the 2 films (10 animals received PUIs, and 10 animals received BPUIs) was then inserted into the subconjunctival pocket 3 mm from the limbus using a conjunctival forceps, and the film was affixed with a central single nylon 10.0 suture (Ethicon®; Johnson & Johnson). After the film insertion, a previously described surgical technique that results in a filtering bleb was performed on each animal.17 Topical applications of a combination of ciprofloxacin 3.5 mg/mL and dexamethasone 1 mg/mL (Ophthalmic Suspension; Alcon Laboratories) were administered 3 times a day for 5 days after surgery.
All of the animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. We also obtained approval from the Ethics Committee on Animal Experiments of the School of Medicine of Ribeirão Preto–University of São Paulo (protocol no. 124/2009).
Clinical monitoring
The eyes of the rabbits were observed for any changes at the insertion sites, to evaluate the gross appearances of the implants, and to determine whether there was any evidence of surgical complications using slit-lamp examinations. The IOPs of both eyes of each rabbit were measured using a tonometer (Tonopen Avia; Reichert) under topical anesthesia (proximetacaine 1%; Alcon Laboratories) before surgery and on the fifth postoperative day. Bleb characteristics were graded based on the daily photographic evaluations of the surgical sites in conjunction with comparisons that were tailored to the Moorfields Bleb Grading System by 2 of the authors of this manuscript (V.R.C.R. and R.C.).18
Histological analysis
The rabbits were euthanized using intravenous pentobarbital (60–150 mg/kg), after which the eyes were enucleated. Immediately after enucleation, the eyes were immersed in 10% neutral buffered formalin for 24 h. The globes were dehydrated and embedded in paraffin. They were then sectioned with a microtome and stained with the appropriate stain (both hematoxylin & eosin and Sirius Red stain). Fibrosis was evaluated by measuring the intensity of the collagen capsule that had formed around each implant. Photographs of the sections that had been stained with Sirius Red and that were taken at a magnification of 200×were evaluated using the ImageJ 1.44 (National Institute of Health) software program. Analysis of the extraocular muscles in the same photographs was used as controls.
Immunohistochemistry
Immunohistochemical stains were used to quantify the amount of VEGF-expressing fibroblasts that were located near the implants. The sectioned, paraffin-embedded slides were heated to 60°C for 1 h, deparaffinized, and rehydrated. The endogenous peroxidase activity was quenched by means of a 30-min incubation in a 3% H2O2/PBS solution, washed, and blocked with a solution of 20% Aquablock (East Coast Biologics, Inc.) in PBS/0.2% Tween-20 for 30 min. The sections were then incubated with a human anti-VEGF monoclonal antibody at a dilution of 1:50 (VEGF C-1; SC 7269; BD Biosciences Pharmingen). Primary antibodies were detected using biotinylated IgG secondary antibodies (2 μg/mL), after which the sections were incubated with 3,3′-diaminobenzidine (DAB substrate kit; Vector Laboratories) and counterstained with hematoxylin.
Statistical analysis
Statistical analyses consisted of descriptive statistics, including means, medians, and standard errors, and the frequency distributions and percentages were used for categorical variables. The Mann–Whitney U test was used to compare the results between groups. P values of<0.05 were defined as statistically significant. The Prism 5.0 software program (GraphPad Software, Inc.) was used for all analyses.
Results
In vitro bevacizumab release from films
The HPLC size-exclusion method was developed to determine bevacizumab release from the BPUI films. This method was validated by showing that neither compounds of the incubation medium nor those of the polymer interfered with drug retention time. These findings decrease the risks of overestimation. The results revealed that this quantification method was specific, precise, accurate, robust, and linear (r2=0.998) over a range of concentrations from 5 to 75 μg/mL, so this method can be used in drug-release studies. Drug stability was tested by HPLC and electrophoresis, which demonstrated that 96% or more of the initial concentration of bevacizumab (dissolved in PBS, pH 7.4; 37°C) was recovered after the 7-day period described for preparation of the implants.
The BPUIs failed to provide a sustained release of bevacizumab, because no drug release peak was detected by the analytical method over a period ranging up to 40 days, with the exception of a 10% of free drug only on the first day. Its presence was noted based on displaying a symmetrical peak at a mean retention time of 7.58 min (Fig. 1). Six BPUIs, identical to those used for the in vitro release tests, were also dissolved in methanol to verify the amount of drug that was present in the implants. The HPLC analysis was unable to provide any evidence of the drug after 1 day.
Fourier-transform infrared spectroscopy
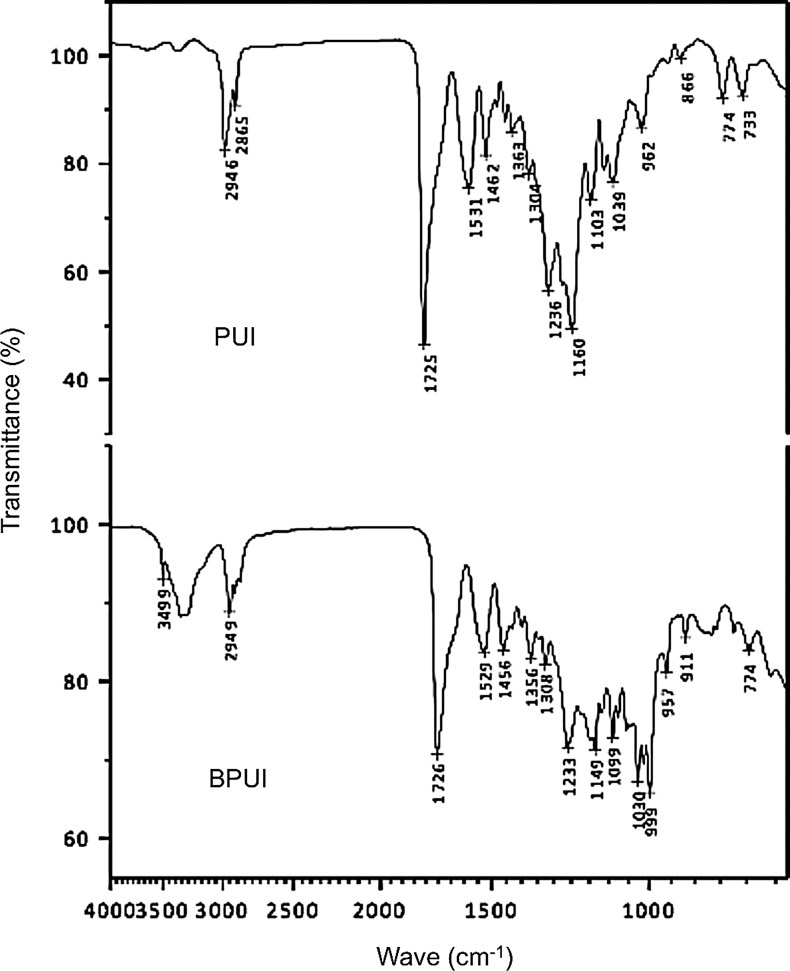
The spectrum related to the sample containing PUI displayed typical polyurethane absorption regions. Regions of 2,946 and 2,865 cm−1 represent the characteristic bands of asymmetric and symmetric stretching of –CH3, respectively. Another characteristic band was observed at 1,725 cm−1, and can be attributed to stretching of the carbonyl groups. These groups are related to urethane, urea, and ester, and may be involved in hydrogen bonds with –NH groups of the urethane linkage (Fig. 2). In the BPUI spectrum, a band in the region of 3,499 cm−1 was evidenced and was attributed to free –NH groups present in the proteic structure of the drug. However, the band near 3,300 cm−1 appeared more intense than in the spectrum of PUI, indicating that most of the –NH groups of the proteic structure of the drug participated in hydrogen bonds with polar groups of the polymer (Fig. 2).
FIG. 2.
Fourier-transform infrared spectra of the polyurethane implant (PUI) and the BPUI for evaluation of differences in the polymer structure. The graphs show transmittance (%) at each wave number (cm−1).
Scanning electron microscopy
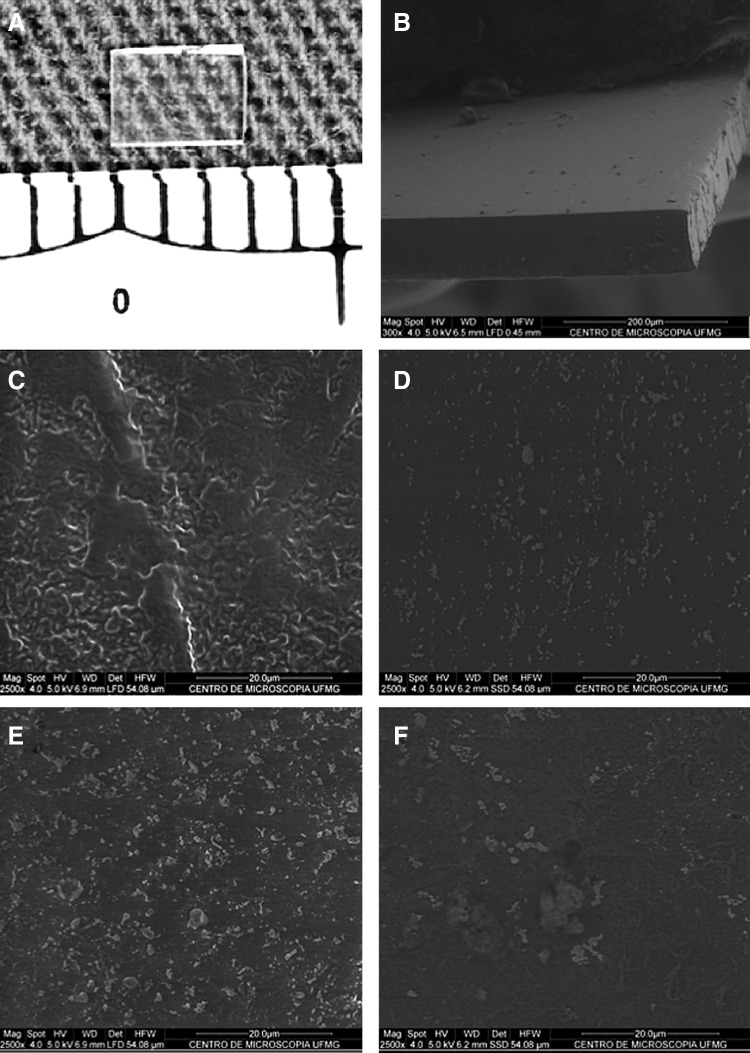
The BPUI displayed a compact and homogeneous appearance both on the surface and in the region of the fracture, which is characteristic of a successful polymer synthesis (Fig. 3). Images of both a PUI and a BPUI from before and after 5 days of incubation in PBS are shown in Fig. 3. Compared with the BPUI, the PUI presented a rougher surface texture; this finding is indicative of a greater degree of degradation after 5 days. The BPUI showed a smooth surface both before and after incubation; this finding is most likely related to an interaction between polyurethane and bevacizumab.
FIG. 3.
Photographs showing the appearance of the polyurethane films. Macroscopic aspect and dimensions (3×3 mm) of a BPUI (A) and a scanning electron microscope image showing the details of the fracture region of the same implant (B). Note the compact and homogeneous appearance of both the surface of the implant and the region of the fracture (original magnification–300X). Images of the surfaces of a PUI and a BPUI showing that the PUI has a rougher surface than the BPUI both before (C vs. D, respectively) and after 5 days of incubation in PBS (E vs. F, respectively) (original magnification 2500X).
Effects of BPUI in the experimental glaucoma-filtering surgery
The clinical characteristics of the animals from the BPUI group, including the mean IOP differences and the median of all of the bleb scores that were determined based on the Moorfields Bleb Grading System, were not significantly different from the clinical characteristics of the PUI group (Table 1). Histological sections showed that there was adequate placement of the silastic tubes over the PUIs; these sections also revealed the presence of neutrophilic infiltration and surrounding fibrosis accumulation (Fig. 4). An evaluation of the intensity of the collagen that had been stained with Sirius Red showed that there were no statistically significant differences in the intensity of the collagen staining between the BPUI and PUI groups (P=0.1905; Mann–Whitney U test) (Fig. 4).
Table 1.
Outcome Measures From the Fifth Postoperative Day Comparing the Results Between the Animals That Underwent Experimental Glaucoma-Filtering Surgery with the Insertion of Implants That Contain Bevacizumab or Did Not Contain Bevacizumab
| Groups | IOP difference (mmHg)a | Bleb area (central)b | Bleb area (maximum)b | Bleb highb | Vascularizationb |
|---|---|---|---|---|---|
| PUI | 1.1±1.0 | 2.0±0.1 | 3.0±0.1 | 2.0±0.1 | 3.0±0.1 |
| BPUI | 2.9±0.8 | 2.0±0.4 | 3.0±0.3 | 3.0±0.4 | 3.0±0.1 |
| P value | 0.413 | 0.72 | 0.72 | 0.057 | 0.72 |
IOP difference=postoperative IOP (on day 5) −preoperative IOP, expressed as mean±SE.
Scores were based on the Moorfields Bleb Grading System, were expressed as medians±SE, and were analyzed with a Wilcoxon signed-rank test.
PUI, polyurethane implant; BPUI, bevacizumab-loaded polyurethane implant; IOP, intraocular pressure; SE, standard errors.
FIG. 4.
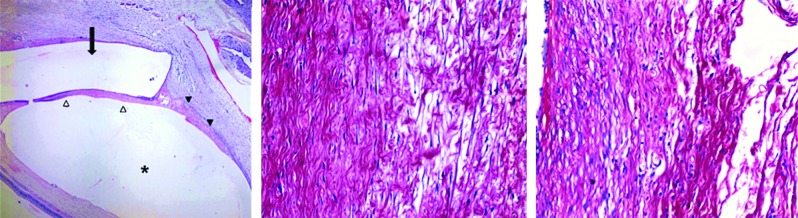
Photomicrograph of the surgical site of an animal from the group that received BPUIs (left) showing a silastic tube in the subconjunctival space (arrow) above the implant (asterisk). The implant was attached to the juxtascleral space and surrounded by a tissue reaction. Note the formation of a fibrous capsule between the implant and the silastic tube (white arrowhead) and around the edge of the implant (black arrowhead) (hematoxylin & eosin, original magnification 25X). Photomicrographs showing Sirius Red staining of areas that are adjacent to the implants. Note the collagen deposits, which are stained in red, and the numerous fibroblasts in both the BPUI and the PUI animals (center and right, respectively) (Sirius Red, original magnification 200X). Color images available online at www.liebertpub.com/jop
Expression of VEGF in Tenon fibroblasts
The immunohistochemical analysis of slides of the surgical site showed VEGF staining of several different cell types, including endothelial cells, fibroblasts, and macrophages (Fig. 5). Compared to the animals in the PUI group, animals from the BPUI group had a significantly lower proportion of VEGF-expressing fibroblasts (0.17±0.03 vs. 0.35±0.05 cells/field, P=0.005; Mann–Whitney U test).
FIG. 5.
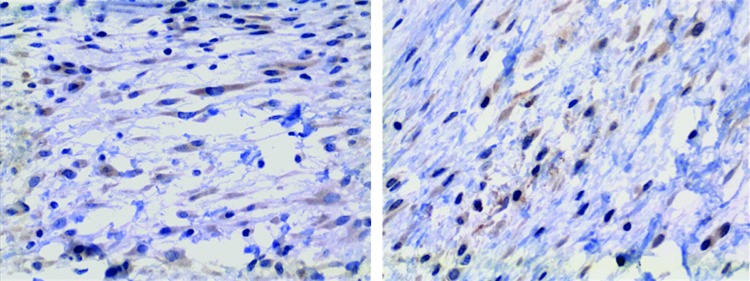
Immunohistochemical staining for vascular endothelial growth factor in the areas adjacent to the implants in the surgical sites. Observe the staining of fibroblasts, which are present in a noticeably smaller number in the group that received BPUIs (right) than in the group that received PUIs (left) (original magnification 400X). Color images available online at www.liebertpub.com/jop
Discussion
Current wound modulators that are utilized as adjuvant therapies after trabeculectomies, such as MMC and 5-FU, interfere with wound healing by decreasing the proliferation of fibroblasts. Unfortunately, these medications are commonly associated with thin cystic blebs that predispose to several complications.19 A growing number of studies have been published that focus on angiogenesis as a potential target for increasing the surgical success associated with routine trabeculectomies; these studies mainly focus on the effects of bevacizumab and ranibizumab.9–11,20
The dose, route of administration, and new drug release mechanisms should be studied to improve the effectiveness of a bevacizumab-augmented trabeculectomy to reduce fibrosis. Several issues need to be addressed regarding the off-label use of bevacizumab, such as the duration of action of the drug and its toxicity profile in ocular tissues.
In general, drugs that are injected into the subconjunctival space, such as bevacizumab, are cleared via trans-scleral or conjunctival blood and lymphatic flow. However, a longer half-life in ocular tissues after subconjunctival injection bevacizumab compared with intravitreal injection or even topical application via eye drops suggests that bevacizumab accumulates in the scleral matrix.12
Attempting to control excessive scarring during the short-to-medium term after surgery is associated with higher success rates, and instances in which failing surgeries were rescued by using subsequent procedures have been described over the last 20 years.21,22
After considering some pharmacokinetic issues and the necessity of the prolonged release of antiscarring drugs during the trabeculectomy postoperative period, we proposed the use of subconjunctival implants as a new bevacizumab delivery system. A drug containing polyurethane film was developed with a size that was acceptable for subconjunctival insertion. These implants (BPUI) can be produced by incorporating bevacizumab in an aqueous polyurethane dispersion in the absence of additional complex chemical reactions due to its hydrophilicity.
Our results showed no adverse effects of administering the implants, and that free bevacizumab could be detected with HPLC. Unfortunately, only a small fraction of the incorporated drug could be detected during a very short period of time. Nevertheless, the assumption of the final amount of bevacizumab in the implants described above was based on chemical calculations. Other limitations are that an in vitro potency assay was not undertaken, and no evaluation of binding between bevacizumab released to tissue and its local receptors.
It is possible that a chemical bond formed between the polyurethane and the bevacizumab, and that the quantification of 10% of the drug observed on the first day could be related to the release of the small proportion of the drug that was present on the surface of the films. To investigate this possibility, we probed for a chemical interaction drug and a polymer using FTIR spectroscopy. This analysis displayed waves that were related to hydrogen bonds between the polar groups of the PUI and NH groups of proteic structure of the drug.
This interaction may produce a higher hydrolytic stability of the polymer, which is most likely due to an increase in the number of crosslinks from hydrogen interactions and then hinders the entry of water into the polymeric matrix.23,24 FTIR also pointed to the occurrence of interactions mainly in the hard segments of PUI than in the soft ones, and further tests should aim modifications in the hard segments during synthesis of the films.
Despite uncertainties regarding the amount of bevacizumab released from BPUIs, there is an indication that whatever amount was released, it was sufficient to at least lower in vivo the proportion of VEGF-expressing fibroblasts. Although the local inhibition of VEGF could be associated with a decrease in the number of VEGF-producing cells, free bevacizumab available locally was insufficient to inhibit fibrosis. Either the small amount of bevacizumab released or the predominant VEGF isoform that was already present in the surgical site and involved in the subconjunctival healing process25 could explain these disappointing results. Indeed, both VEGF-121 and VEGF-165 (which are mainly involved in the angiogenesis process), but not VEGF-189 (which is associated with the healing process), are produced by Tenon fibroblasts in culture. Moreover, these cells also express these cognate VEGF receptors.11,25
Other systems that permit the sustained release of bevacizumab have been described by Abrishami et al.26 and Andrew et al.,27 but these systems utilized a liposomal delivery system (nanoliposomes) and an inorganic polymer (mesoporous silicon oxide), respectively. They were developed to treat diseases of the posterior segment of the eye, so bevacizumab was encapsulated and administered directly into the vitreous body. The current study is the first one to describe the application of a sustained drug delivery system that contains bevacizumab in the anterior segment of the eye or in conjunction with glaucoma-filtering surgery.
In conclusion, our results indicate that bevacizumab was probably only released from the surface of polyurethane films (BPUI). The low proportion of the drug released may stem from chemical interactions that may have developed between the drug and the polymeric matrix. BPUIs can be produced easily and inexpensively, but further pharmacokinetic studies using BPUIs are necessary to optimize the bioavailability of the drug while maintaining minimal side effects.
Acknowledgment
Financial support: CAPES and CNPq.
Author Disclosure Statement
All the authors have no financial conflict of interest with this study.
References
- 1.Heijl A. Leske M.C. Bengtsson B. Hyman L. Bengtsson B. Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 2.Maier P.C. Funk J. Schwarzer G. Antes G. Falck-Ytter Y.T. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ. 2005;331:134. doi: 10.1136/bmj.38506.594977.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folgar F.A. de Moraes C.G.V. Prata T.S. Teng C.C. Tello C. Ritch R., et al. Glaucoma surgery decreases the rates of localized and global visual field progression. Am. J. Ophthalmol. 2010;149:258–264. doi: 10.1016/j.ajo.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Skuta G.L. Parrish R.K., 2nd Wound healing in glaucoma filtering surgery. Surv. Ophthalmol. 1987;32:149–170. doi: 10.1016/0039-6257(87)90091-9. [DOI] [PubMed] [Google Scholar]
- 5.Wong J. Wang N. Miller J.W. Schuman J.S. Modulation of human fibroblast activity by selected angiogenesis inhibitors. Exp. Eye Res. 1994;58:439–451. doi: 10.1006/exer.1994.1037. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.W. Huang H.T. Bair J.S. Lee C.C. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J. Ocul. Pharmacol. 1990;6:175–182. doi: 10.1089/jop.1990.6.175. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield D.S. Liebmann J.M. Jee J. Ritch R. Late-onset bleb leaks after glaucoma filtering surgery. Arch. Ophthalmol. 1998;116:443–447. doi: 10.1001/archopht.116.4.443. [DOI] [PubMed] [Google Scholar]
- 8.Kitazawa Y. Taniguchi T. Nakano Y. Shirato S. Yamamoto T. 5-Fluorouracil for trabeculectomy in glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1987;225:403–405. doi: 10.1007/BF02334165. [DOI] [PubMed] [Google Scholar]
- 9.Grewal D.S. Jain R. Kumar H. Grewal S.P.S. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008;115:2141–2145. doi: 10.1016/j.ophtha.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Kahook M.Y. Schuman J.S. Noecker R.J. Needle bleb revision of encapsulated filtering bleb with bevacizumab. Ophthalmic Surg. Lasers Imaging. 2006;37:148–150. [PubMed] [Google Scholar]
- 11.Li Z. Van Bergen T. Van de Veire S. Van de Vel I. Moreau H. Dewerchin M. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest. Ophthalmol. Vis. Sci. 2009;50:5217–5225. doi: 10.1167/iovs.08-2662. [DOI] [PubMed] [Google Scholar]
- 12.Nomoto H. Shiraga F. Kuno N. Kimura E. Fujii S. Shinomiya K., et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest. Ophthalmol. Vis. Sci. 2009;50:4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 13.Da Silva G.R. Silva Cunha A. Behar-Cohen F. Ayres E. Oréfice R.L. Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. Polym. Degrad. Stab. 2010;95:491–499. [Google Scholar]
- 14.Da Silva G.R. Silva Cunha A. Saliba J.B. Berdugo M. Goldenberg B.T. Naud M., et al. Polyurethanes as supports for human retinal pigment epithelium cell growth. Int. J. Artif. Organs. 2011;34:198–209. doi: 10.5301/ijao.2011.6398. [DOI] [PubMed] [Google Scholar]
- 15.Moura S.A.L. Lima L.D.C. Andrade S.P. Silva Cunha A. Órefice R.L. Ayres E., et al. Local drug delivery system: Inhibition of inflammatory angiogenesis in a murine sponge model by dexamethasone-loaded polyurethane implants. J. Pharm. Sci. 2011;100:2886–2895. doi: 10.1002/jps.22497. [DOI] [PubMed] [Google Scholar]
- 16.Gomes E.C.L. Cunha Junior A.S. Yoshida M.I. Jorge R. [Desenvolvimento e validação de método analítico para quantificação do fármaco Bevacizumabe por cromatografia liquid de alta eficácia] Quim Nova. 2012;35:608–611. (In Portuguese.) [Google Scholar]
- 17.Cordeiro M.F. Constable P.H. Alexander R.A. Bhattacharya S.S. Khaw P.T. Effect of varying the mitomycin-C treatment area in glaucoma filtration surgery in the rabbit. Invest. Ophthalmol. Vis. Sci. 1997;38:1639–1646. [PubMed] [Google Scholar]
- 18.Wells A.P. Crowston J.G. Marks J. Kirwan J.F. Smith G. Clarke J.C.K., et al. A pilot study of a system for grading of drainage blebs after glaucoma surgery. J. Glaucoma. 2004;13:454–460. doi: 10.1097/00061198-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Khaw P.T. Chang L. Wong T.T. Mead A. Daniels J.T. Cordeiro M.F. Modulation of wound healing after glaucoma surgery. Curr. Opin. Ophthalmol. 2001;12:143–148. doi: 10.1097/00055735-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kahook M.Y. Bleb morphology and vascularity after trabeculectomy with intravitreal ranibizumab: a pilot study. Am. J. Ophthalmol. 2010;150:399–403. doi: 10.1016/j.ajo.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Greenfield D.S. Miller M.P. Suner I.J. Palmberg P.F. Needle elevation of the scleral flap for failing filtration blebs after trabeculectomy with mitomycin C. Am. J. Ophthalmol. 1996;122:195–204. doi: 10.1016/s0002-9394(14)72010-0. [DOI] [PubMed] [Google Scholar]
- 22.Hodge W. Saheb N. Balazsi G. Kasner O. Treatment of encapsulated blebs with 30-gauge needling and injection of low-dose 5-fluorouracil. Can. J. Ophthalmol. 1992;27:233–236. [PubMed] [Google Scholar]
- 23.Apekis L. Pissis P. Christodoulides C. Spathis G. Niaounakis M. Kontou E., et al. Physical and chemical network effects in polyurethane elastomers. Progr. Colloid Polym. Sci. 1992;90:144–150. [Google Scholar]
- 24.Król P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Progr. Mat. Sci. 2007;52:915–1015. [Google Scholar]
- 25.Van Bergen T. Vandewalle E. Van de Veire S. Dewerchin M. Stassen J.M. Moons L., et al. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery. Exp. Eye Res. 2011;93:689–699. doi: 10.1016/j.exer.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Abrishami M. Zarei-Ghanavati S. Soroush D. Rouhbakhsh M. Jaafari M.R. Malaekeh-Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29:699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- 27.Andrew J.S. Anglin E.J. Wu E.C. Chen M.Y. Cheng L. Freeman W.R., et al. Sustained release of a monoclonal antibody from electrochemically prepared mesoporous silicon oxide. Adv. Funct. Mater. 2010;20:4168–4174. doi: 10.1002/adfm.201000907. [DOI] [PMC free article] [PubMed] [Google Scholar]