Abstract
To measure the levels of interleukin-34 (IL-34) in serum and synovial fluid (SF) of patients with rheumatoid arthritis (RA) and to evaluate the effect of recombination human (rh) IL-34 on IL-17 production by peripheral blood mononuclear cells (PBMC) in RA patients, the serum and SF levels of IL-34, and the production of IL-17 by rhIL-34-treated PBMC of RA patients were measured by enzyme-linked immunosorbent assay. We also tested the change of IL-34 level after tumor necrosis factor (TNF)-α blockade therapy in 30 RA patients. In contrast to almost no detectable IL-34 in osteoarthritis (OA) and healthy serum, IL-34 could be detected in 93 out of the 125 RA cases (74.4%). Sera IL-34 levels were significantly higher in RA patients compared with the controls and correlated with disease activity. IL-34 levels were higher in SF samples than in sera in 11 RA patients. The level of serum IL-34 decreased after anti-TNF treatment. In the presence of rhIL-34, stimulation of PBMC from RA patients resulted in increased production of IL-17. These findings suggest that IL-34 may play a role in the pathogenesis of RA.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease and characterized by inflammatory infiltration of the synovium, leading to cartilage and bone destruction. In recent years, interleukin-17 (IL-17)–producing cells (Th17 cells) have been described and play an important role in pathogenesis of RA (Yang and others 2008; Shen and others 2009). One important question arises, how is Th17 regulated in RA? Some reports showed that other cytokines, such as IL-27 and IL-35 are able to suppress the production of IL-17 and modulate arthritic inflammation (Niedbala and others 2007, 2008). Moreover, inhibition of IL-33 can attenuate the severity of experimental arthritis by inhibition the Th17 cells (Palmer and others 2009).
IL-34 is a newly discovered cytokine without significant amino acid sequence homology to other cytokines (Lin and others 2008). Currently, very limited knowledge is known about this new cytokine. This cytokine shares common receptor with macrophage-colony stimulating factor (M-CSF) (Droin and Solary 2010). M-CSF plays an important role in the pathogenesis of RA (Hamilton 2008). How about IL-34? Recently, study showed that IL-34 could promote osteoclastogenesis in vitro (Chen and others 2011). IL-34 can also increase IL-6 and chemokine levels in human whole blood (Eda and others 2010). Little is known about the function of IL-34 in the pathogenesis of RA. IL-34 expression has been identified in the synovial tissue by immunohistochemistry and a significant association was found between IL-34 expression and synovitis severity (Chemel and others 2012). However, whether IL-34 is released into circulation in RA remains unclear. In our study, we investigated the serum and synovial fluid (SF) levels of IL-34. Moreover, we showed for the first time that IL-34 can induce the production of IL-17 by activated peripheral blood mononuclear cells (PBMC) from patients with RA.
Patients and Methods
Patients
Sera were obtained from 125 RA patients, who fulfilled the American College of Rheumatology criteria for RA (Arnett and others 1988). In addition, 40 osteoarthritis (OA) patients and 55 age- and sex-matched healthy persons served as controls. SF samples were obtained from 11 RA patients at the same time. The present study was approved by the ethics committee in our institution according to the Declaration of Helsinki and written informed consent was given by all patients. Clinical data, including swollen joint count (SJC) and tender joint count (TJC) were obtained from medical records on admission. Blood tests, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), and anti-cyclic citrullinated peptide (anti-CCP) antibody were measured by standard methods. Interstitial lung disease (ILD) was identified on high-resolution computed tomography. Disease activity in RA was scored with the Disease Activity Score 28 based on CRP levels (DAS28-CRP).
Tumor necrosis factor-α blockade therapy
Sera from other 30 patients with RA who received either infliximab therapy (3 mg/kg, infused 4 times, at weeks 0, 2, 6, 14) or etanercept therapy (25 mg/time, twice a week, infused 16 weeks) were collected. Sera samples were collected before and after treatment (Weeks 0 and 16).
Measurement of IL-34 and matrix metalloproteinase-3 levels in serum and SF samples
IL-34 and matrix metalloproteinase (MMP)-3 were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's directions (R&D systems). Each sample was measured in triplicate.
Preparation, stimulation of PBMCs and detection of IL-17 levels in supernatants
PBMCs were purified from peripheral blood of 15 RA patients by centrifugation, using a Ficoll-Hypaque gradient (Amersham Pharmacia Biotech). For measurement of cytokine secretion, PBMCs were seeded into the wells of 96-well culture plates (Nunc) at 105/200 μL/well in triplicate and stimulated with beads coated with antibodies to CD3/CD28 (105 beads/well; Miltenyi Biotech). Recombinant human (rh) IL-34(0, 10, 20, 50,100 ng/mL) (R & D Systems) was added to the media. After incubation for 4 days, cell-free supernatants were collected and the concentrations of IL-17 were assessed using an ELISA kit, according to the manufacturer's instructions (eBioscience).
Statistical analysis
All data are presented as the mean±SD. Unpaired Student's t-test or paired t-test was used to compare variables between groups. Pearson's correlation coefficient was used to test the correlations between two variables. All analyses were performed using SPSS 17.0 and GraphPad 5 software.
Results
Clinical features of patients with RA
125 RA patients were recruited. Clinical features are indicated in Table 1.
Table 1.
Clinical Data of Rheumatoid Arthritis Patients and Correlation Analysis with Serum Interleukin-34
| |
|
Correlation with IL-34 |
|
|---|---|---|---|
| Characteristics | Values | Correlation coefficient (r) | P |
| Age (years) | 56.5±17.1 | −0.003 | 0.971 |
| Sex (F/M) | 111/14 | 0.101 | 0.343 |
| Disease duration (years) | 6.1±3.3 | −0.214 | 0.017a |
| ESR (mm/h) | 59.7±35.2 | 0.254 | 0.004a |
| CRP (mg/L) | 37.8±41.3 | 0.314 | <0.001a |
| RF (IU/mL) | 52.8±317.9 | 0.390 | <0.001a |
| Anti-CCP | 70 (55) | 0.401 | <0.001a |
| ILD | 56 (69) | 0.090 | 0.831 |
| TJC (0–46 joints) | 8.0±7.5 | 0.205 | 0.030a |
| SJC (0–48 joints) | 5.9±3.4 | 0.175 | 0.052 |
| DAS28-CRP | 4.9±1.5 | 0.246 | 0.006a |
| MMP3 (ng/mL) | 36.8±8.3 | 0.301 | <0.001a |
P<0.05.
ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; Anti-CCP, anticitrullinated peptide antibody; ILD, interstitial lung disease; TJC, tender joint count; SJC, swollen joint count; DAS28, 28-joint count disease activity score.
Increased serum and SF levels of IL-34 in patients with RA
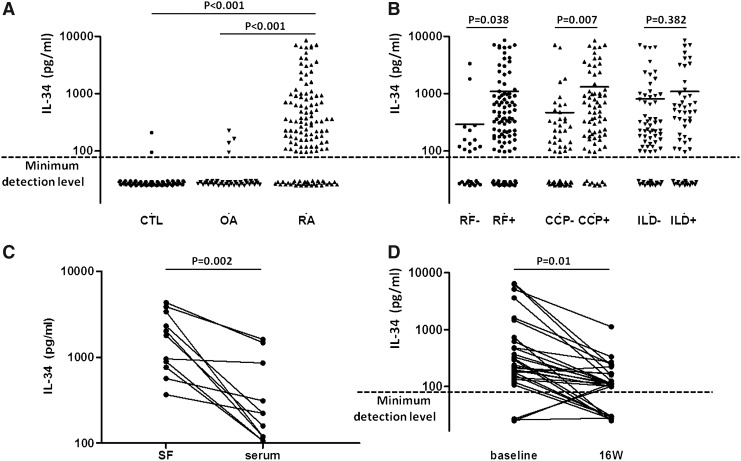
In contrast to almost no detectable IL-34 in OA and healthy serum, IL-34 could be detected in 93 out of the 125 RA cases (74.4%). Serum IL-34 levels were significantly higher in patients with RA than in those with OA and healthy controls (P<0.001, P<0.001, respectively) (Fig. 1A). IL-34 was significantly higher in the RF-positive group compared with the RF-negative group (1098.0±188.9 pg/mL versus 290.2±145.1 pg/mL; P=0.038) (Fig. 1B). Similarly, the IL-34 was significantly higher in the CCP-positive RA patients compared with the CCP-negative RA patients (1306.0±236.3 pg/mL versus 468.1±171.8 pg/mL; P=0.007) (Fig. 1B). There was no difference in IL-34 levels between RA patients with and without ILD (1090.0±258.4 pg/mL versus 813.3±190.9 pg/mL; P=0.382) (Fig. 1B).
FIG. 1.
(A) Serum interleukin (IL)-34 in patients with rheumatoid arthritis (RA), osteoarthritis (OA) and healthy controls. (B) Compare serum IL-34 in RA patients with positive rheumatoid factor (RF), positive anticitrullinated peptide antibody (anti-CCP) and with interstitial lung disease (ILD) to RA patients with negative RF, negative anti-CCP and without ILD. (C) IL-34 levels in paired serum and SF samples in RA patients (n=11). (D) Serum IL-34 before and after antitumor necrosis factor-α treatment in RA patients.
The levels of IL-34 were also measured in 11 matched samples of both RA SF and serum. IL-34 levels were all detectable in SF all of these patients. The SF levels of IL-34 were higher than the serum levels in these 11 patients measured simultaneously (1930.0±420.7 pg/mL versus 482.7±171.0 pg/mL, P=0.002) (Fig. 1C).
Serum IL-34 concentrations in RA patients decreased after antitumor necrosis factor (TNF)-α treatment (1021.0±334.4 pg/mL versus 152.6±366.6 pg/mL; P=0.01) (Fig. 1D).
Serum IL-34 was correlated with disease activity in RA patients
The correlations between the clinical features and IL-34 levels are presented in Table 1. Most interestingly, serum IL-34 was negative correlated with disease duration in RA patients (Table 1).
Significant positive correlations were found between IL-34 levels and inflammation parameters in the RA patients, including ESR and CRP (Table 1). Serum IL-34 was also correlated with the antibodies production in RA patients, including RF and anti-CCP (Table 1). Additionally, there were statistically significant positive correlations between the levels of IL-34 and disease activity indexes, including TJC and DAS28 (Table 1). As MMP-3 is an important index for bone erosion in RA patients, we found that there was also a strong correlation between serum levels of IL-34 and MMP-3 (Table 1).
In contrast, there were no correlation between the levels of IL-34 and other clinical parameters, including age, SJC, and ILD.
rhIL-34 increases IL-17 production of PBMC from RA patients
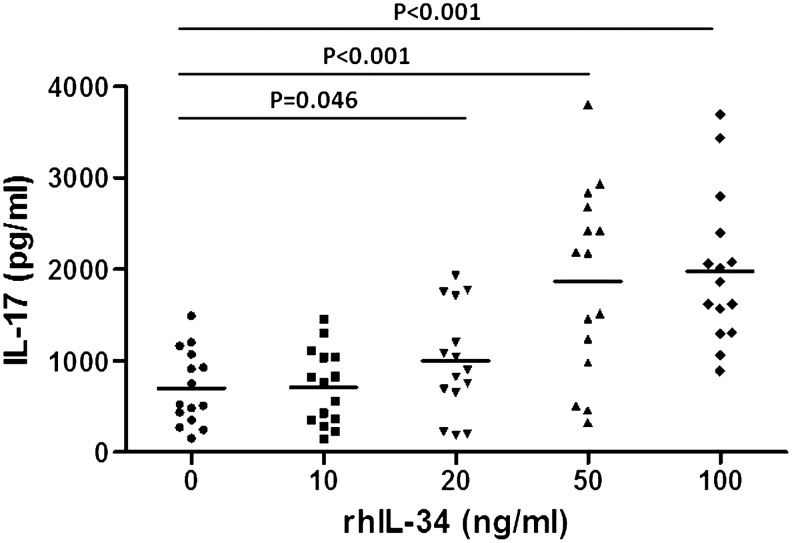
To investigate the effect of IL-34 on IL-17 production, we measured IL-17 in the culture supernatants from the PBMCs of patients with RA in the presence of anti-CD3/CD28 with or without rhIL-34. The levels of IL-17 increased dose-dependently after rhIL-34 treatment (995.60±585.51 pg/mL at 20 ng/mL, P=0.046; 1863.89±1034.02 pg/mL at 50 ng/mL, P<0.001; 1979.83±815.79 pg/mL at 100 ng/mL, P<0.001) (Fig. 2). We also investigated the effect of rhIL-34 on PBMCs from healthy donors. In contrast, no significant production of IL-17 occurred.
FIG. 2.
IL-17 levels in the culture supernatants of peripheral blood mononuclear cells (PBMC) from RA patients. PBMC were stimulated with anti-CD3/CD28 antibodies and rhIL-34.
Discussion
To our knowledge, this study is the first to show that IL-34 levels were elevated in RA serum and correlated with disease activity. SF samples from patients with OA are rarely available, so we compared the results in SF with those results in sera from patients with RA simultaneously. We demonstrated that levels of IL-34 in SF were much higher than that in serum. Previous study showed IL-34 expression in synovial tissue of RA patients and associated with synovitis severity (Chemel and others 2012). Collectively, these results may suggest that IL-34 can be produced by fibroblast-like synovial and released to circulation in RA patients.
IL-34 levels in serum correlated strongly with inflammation and disease activity as assessed by the TJC, ESR, CRP and DAS28-CRP. In accordance with this, elevated serum concentrations of IL-34, as well as the disease activity of RA patients were significantly decreased after TNF-α blockade therapy. Such a correlation was also seen in MMP-3. It has been reported that serum MMP-3 was a good indicator in the inflammatory and cartilage damage of CIA (Seeuws and others 2010). Meanwhile, the other study showed that IL-34 can promote osteoclast differentiation from human PBMC (Chen and others 2011). This indicated that IL-34 may contribute to the bone erosion in RA patients. Interestingly, IL-34 was also strongly associated with antibody levels in RA patients, including RF and anti-CCP antibody, suggesting that IL-34 may contribute to autoantibody production in RA. Our previous study showed that IL-27 and IL-33 were associated with ILD in RA (Shen and others 2011; Xiangyang and others 2012). In contrast, there was no correlation between the levels of IL-34 and ILD in this study.
Th17 cells and IL-17 are closely associated with the pathogenesis of RA (Shen and others 2009). Because upregulation of IL-34 in RA patients was observed in our study, whether IL-34 can affect the production of IL-17 is still unclear. We next investigated the IL-17 secretion of activated PBMC after stimulation with rhIL-34 from RA patients. To our knowledge, this has not been reported before. Interestingly, increased concentrations of IL-17 were seen in the supernatants after stimulation with rhIL-34. These results suggested that upregulated IL-34 in RA patients may exert its role by promoting IL-17 production.
In conclusion, this study has defined the increased serum and SF samples levels of IL-34 in patients with RA. We have shown that IL-34 was significantly associated with disease activity and can promote IL-17 production. This supports the hypothesis that IL-34 contribute to the pathogenesis of RA. This cytokine is potential future therapeutic target for RA.
Acknowledgments
Fund for Scientific Research of The First Hospital of China Medical University: fsfh1007 and Higher Education Department of Liaoning Province Research Grant: L2010604, L2010613.
Author Disclosure Statement
There is no conflict of interest in this study.
References
- Arnett FC. Edworthy SM. Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Chemel M. Le Goff B. Brion R, et al. Interleukin 34 expression is associated with synovitis severity in rheumatoid arthritis patients. Ann Rheum Dis. 2012;71:150–154. doi: 10.1136/annrheumdis-2011-200096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Buki K. Vaaraniemi J, et al. The critical role of IL-34 in osteoclastogenesis. PLoS One. 2011;6:e18689. doi: 10.1371/journal.pone.0018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droin N. Solary E. Editorial: CSF1R, CSF-1, and IL-34, a “menage a trois” conserved across vertebrates. J Leukoc Biol. 2010;87:745–747. doi: 10.1189/jlb.1209780. [DOI] [PubMed] [Google Scholar]
- Eda H. Zhang J. Keith RH, et al. Macrophage-colony stimulating factor and interleukin-34 induce chemokines in human whole blood. Cytokine. 2010;52:215–220. doi: 10.1016/j.cyto.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immuno. 2008;l8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Lin H. Lee E. Hestir K, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Niedbala W. Cai B. Wei X, et al. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- Niedbala W. Wei XQ. Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- Palmer G. Talabot-Ayer D. Lamacchia C, et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- Seeuws S. Jacques P. Van Praet J, et al. A multiparameter approach to monitor disease activity in collagen-induced arthritis. Arthritis Res Ther. 2010;12:R160. doi: 10.1186/ar3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. Goodall JC. Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- Shen H. Xia L. Xiao W, et al. Increased levels of interleukin-27 in patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:860–861. doi: 10.1002/art.30180. [DOI] [PubMed] [Google Scholar]
- Xiangyang Z. Lutian Y. Lin Z, et al. Increased levels of interleukin-33 associated with bone erosion and interstitial lung diseases in patients with rheumatoid arthritis. Cytokine. 2012;58:6–9. doi: 10.1016/j.cyto.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Yang L. Anderson DE. Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]