Abstract
Viral-mediated oncolysis is a promising cancer therapeutic approach offering an increased efficacy with less toxicity than the current therapies. The complexity of solid tumor microenvironments includes regions of hypoxia. In these regions, the transcription factor, hypoxia inducible factor (HIF), is active and regulates expression of many genes that contribute to aggressive malignancy, radio-, and chemo-resistance. To investigate the oncolytic efficacy of a highly virulent (velogenic) Newcastle disease virus (NDV) in the presence or absence of HIF-2α, renal cell carcinoma (RCC) cell lines with defective or reconstituted wild-type (wt) von Hippel-Lindau (VHL) activity were used. We show that these RCC cells responded to NDV by producing only interferon (IFN)-β, but not IFN-α, and are associated with increased STAT1 phosphorylation. Restoration of wt VHL expression enhanced NDV-induced IFN-β production, leading to prolonged STAT1 phosphorylation and increased cell death. Hypoxia augmented NDV oncolytic activity regardless of the cells' HIF-2α levels. These results highlight the potential of oncolytic NDV as a potent therapeutic agent in the killing of hypoxic cancer cells.
Introduction
Most cancer cells are resistant to the antiproliferative effects of interferons (IFNs) due to defects in their IFN signal transduction pathway (Toth and Thomas 1992; Reu and others 2006). This resistance makes these cells more susceptible to infection with a variety of oncolytic viruses, including Newcastle disease virus (NDV; Wong and others 2010; Mansour and others 2011). Due to their preferential killing of tumor cells, these viruses are widely studied as candidate agents in cancer virotherapy (Cassel and Garrett 1965; Reichard and others 1992; Elankumaran and others 2010; Alabsi and others 2011; Ali and others 2011; Mansour and others 2011; Jamal and others 2012). NDV was first identified and reported in Newcastle-upon-Tyne (United Kingdom) during an outbreak in poultry (Alexander 1988). It causes severe disease with high mortality in avian hosts (Maclachlan and Edward 2011), but is nonpathogenic for humans (Fiola and others 2006).
NDV stimulates the production of various cytokines, such as IFNs and tumor necrosis factor (Sinkovics and Horvath 2000). IFNs, as well as other immunomodulatory proteins, are known to activate the Janus kinase (JAK) and signal transducer and phosphorylation of the activator of transcription (STAT; Aaronson and Horvath 2002) pathways. The activated JAK/STAT signaling induces IFN-stimulated gene expression, which leads to the establishment of antiviral responses in infected cells (Rawlings and others 2004). One of the outcomes of these activation events is triggering of cell death pathways (Dranoff 2004). In NDV infection, induction of cytokines leads to enhanced NDV oncolytic activity (Zorn and others 1994). Recently, a local isolate of a viscerotropic-velogenic strain of NDV (reviewed in Yusoff and Tan 2001), designated AF2240, was shown to be oncolytic in several cancer cell lines (Alabsi and others 2011; Ali and others 2011). This highly oncolytic NDV strain may represent an unexplored avenue for developing a more potent cancer virotherapy agent.
Renal cell carcinoma (RCC) accounts for ∼3% of adult epithelial cancers and its worldwide incidence is on the rise (Koul and others 2011). Due to the lack of characteristic early warning signs, up to 30% of these cases are diagnosed at advanced stages. RCC is a chemoresistant tumor and late-stage cases are generally resistant to radiotherapy and chemotherapy. Prior to 2006, the standard treatment for RCC consisted of the use of cytokines (reviewed in Cowey and Hutson 2010). However, due to its limited beneficial properties, new treatment modalities, involving novel molecularly targeted agents, were approved by the U.S. Food and Drug Administration. Since then, agents that target angiogenesis (sunitinib, bevacizumab, and pazopanib) and a mammalian target of rapamycin (mTOR) inhibitor (temsirolimus) have been used as front-line treatments in place of cytokine therapy (Koul and others 2011). However, responses to these agents are relatively short-lived and relapses inevitably occur.
Clear cell RCC is the most common form of RCC, and is associated with loss of function mutations or silencing of the von Hippel-Lindau (VHL) tumor suppressor gene. The VHL protein is part of an E3 ligase complex that is critical for targeting the α subunit of the heterodimeric hypoxia-inducible factor (HIF) in normoxic conditions (Kaelin 2008). Under hypoxic conditions, the HIF-α subunit is not degraded and the heterodimeric HIF transcription factor transactivates many genes, including those regulating cell proliferation, angiogenesis, erythropoiesis, cellular pH, and glycolysis (Semenza 2012). Recently, a number of oncolytic viruses (Connor and others 2004) (Roos and others 2010) were shown to have a higher capacity for infecting and killing HIF-expressing cells. In contrast, another study (Hwang and others 2006) showed that elevated HIF conferred enhanced resistance to vesicular stomatitis virus (VSV)-mediated oncolysis. These studies offered conflicting implications on the potential use of oncolytic viruses in VHL disease such as clear cell RCC. In the present study, we explored the oncolytic activities of NDV in clear cell renal cancer cell lines and examined the mechanisms involved. Even though NDV has been proposed as an agent in anticancer virotherapy, information on its oncolytic activity, especially by a highly virulent strain, and IFN induction in HIF-stabilized hypoxic cancer cells is still lacking. To address this issue, we explored the oncolytic properties of a velogenic NDV strain, AF2240, on VHL-deficient, hence constitutively stabilized HIF-α and active HIF, human renal carcinoma cells (RCCs), and their variant wild-type (wt) VHL-reconstituted cells.
Materials and Methods
Cell lines and cell culture
The human renal clear cell carcinoma cell line 786-O, which is defective for the tumor suppressor gene VHL, was used in this study (Iliopoulos and others 1995). wt VHL cDNA used in the development of 786-O cells stably expressing the wt VHL (786-VHL) was a kind gift from Dr. Michael Ohh, University of Toronto (Hwang and others 2006). Both of the cell lines were maintained in Dulbecco's modified Eagle's medium (PAA, Pasching, Austria) supplemented with 10% fetal bovine serum (PAA) and 1% antibiotic–antimycotic (PAA) at 37°C with 5% CO2. For normoxic cultures, cells were incubated in a humidified CO2 incubator (Thermo Forma, Marietta, OH), while for hypoxic cultures cells were incubated in a 0.3% O2 environment in a ProOx in vitro chamber (BioSpherix, Redfield, NY), controlled by ProOx model 110 (BioSpherix).
Newcastle disease virus AF2240
A Malaysian-isolated velogenic strain of NDV, designated as AF2240 (reviewed in Yusoff and Tan 2001), was obtained from the Veterinary Research Laboratory, Ipoh, Malaysia. Virus propagation and purification were performed as previously described (Yusoff and others 1996). Time zero, or 0 h post-infection (hpi) began after a 1-h virus absorption period.
Measurement of IFN levels
786-O or 786-VHL cells were cultured in 6-well plates at a density of 5×104 cells per cm2 overnight and then treated with 0.1 or 1.0 multiplicity of infection (MOI) NDV under normoxic and hypoxic conditions. After 24 h, cell culture supernatants were collected. Levels of IFN-α and -β in the supernatants were determined using the VeriKine Human Interferon-Alpha and Interferon-Beta enzyme-linked immunosorbent assay (ELISA) kits (PBL Interferon Source, Piscataway, NJ), according to the manufacturer's suggested protocols. The concentrations of the relevant IFN in the test samples were calculated from a standard curve that was generated using a four-parameter fit.
Detection of late-stage apoptotic cells
Cells were seeded at a density of 5×104 cells per cm2, incubated overnight, and subsequently treated with NDV at 0.1 and 1 MOI for 24 h. To confirm that NDV-induced cell death in RCC cells was also via apoptosis, we monitored for the presence of DNA laddering, which is a hallmark of late-stage apoptosis (Collins and others 1997). At the end of the treatment, the culture media containing any floating cells were collected and subjected to centrifugation at 1,780 g, for 5 min. Floating cells were harvested since DNA laddering is readily detectable in floating (dead) compared to attached (viable) cells (Cooray and others 2003). The pelleted cells were then subjected to the DeadEnd™ Fluorometric terminal deoxynucleotidyl transferase duTP nick end labeling (TUNEL) System (Promega, Madison, WI) according to the manufacturer's suggested protocols. DNA was harvested from the cell pellets using a previously described method (Strauss 1998). The purified DNA was analyzed on a 2% agarose gel, stained with ethidium bromide, and visualized using the Gel Doc imaging System (BioRAD, Philadelphia, PA).
Cell viability assay
786-O or 786-VHL cells were seeded at 5×104 cells per cm2 in 6-well plates incubated overnight at 37°C and subsequently infected with NDV at MOI of 0.1 or 1.0 for 24 h under normoxic and hypoxic conditions. At the time of harvest, floating cells in the culture media were collected and then combined with the attached cells that were trypsinized. The cell mixtures were pelleted down at 1,780 g for 5 min. The cell pellets were resuspended in 2 mL of BD FACSFlow Sheath Fluid (BD Biosciences, Franklin Lakes, NJ) containing 1% fetal bovine serum (FBS), and stained with thiazole orange (TO) and propidium iodide (PI) from BD Cell Viability kit (BD Biosciences, San Jose, CA) for 5 min at room temperature. TO stains all cells, while PI stains only dead cells. The stained cells were then analyzed using a BD Accuri C6 flow cytometer (BD Biosciences, Ann Arbor, MI). Percent of viable cells was determined by the BD Accuri C6 software to represent the population of TO-positive/PI-negative cells in a sample. For DNA content measurement, cells were harvested and subjected to fluorescence-activated cell sorting (FACS) analysis using the BD Cycletest Plus DNA Reagent Kit (BD Biosciences, San Jose, CA). Data were collected from a minimum 50,000 gated events per sample to obtain the percentages of sub-G1 populations.
Immunodetection
Cells were lysed with 200 μL of radioimmunoprecipitation assay (RIPA) buffer (Thermo Scientific, Rockford, IL) containing ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor cocktail (Roche, Mannheim, Germany) for 1 h at 4°C. Fifty micrograms of each sample was then resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), electrotransferred, and probed with the required antibodies. Polyclonal anti-NDV was obtained from the Universiti Putra Malaysia. Other antibodies used were anti-hemagglutinin (anti-HA) (Sigma Aldrich, St. Louis, MO), anti-VHL (BD Pharmingen, San Diego, CA), and anti-β-actin (Sigma Aldrich). The antibodies for STAT-1, p-STAT-1 (S727), and p-STAT-1 (Y701) were purchased from Cell Signaling Technology (Danvers, MA). The blots were then probed with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (IgG). Protein bands were visualized using the SuperSignal West Dura Extended Duration Substrate kit or SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology, Rockford, IL). Band intensities were quantitated and normalized to the β-actin control using the ImageJ software (Wayne Rasband, NIH, Bethesda, MD). Values shown underneath each protein band represent the fold difference of the band intensity compared to the uninfected control for each cell line.
Statistical analysis
Student's t-test was used to analyze the experimental data throughout the study. The results were expressed as mean±standard error of the mean of at least two independent experiments with three technical replicates in each experiment. Statistical significance was defined as p-value<0.05. All the tests were performed using Windows Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA) or GraphPad Prism 5 (GraphPad Software, Inc., LaJolla, CA).
Results
NDV infection is affected by the VHL status of RCC cells
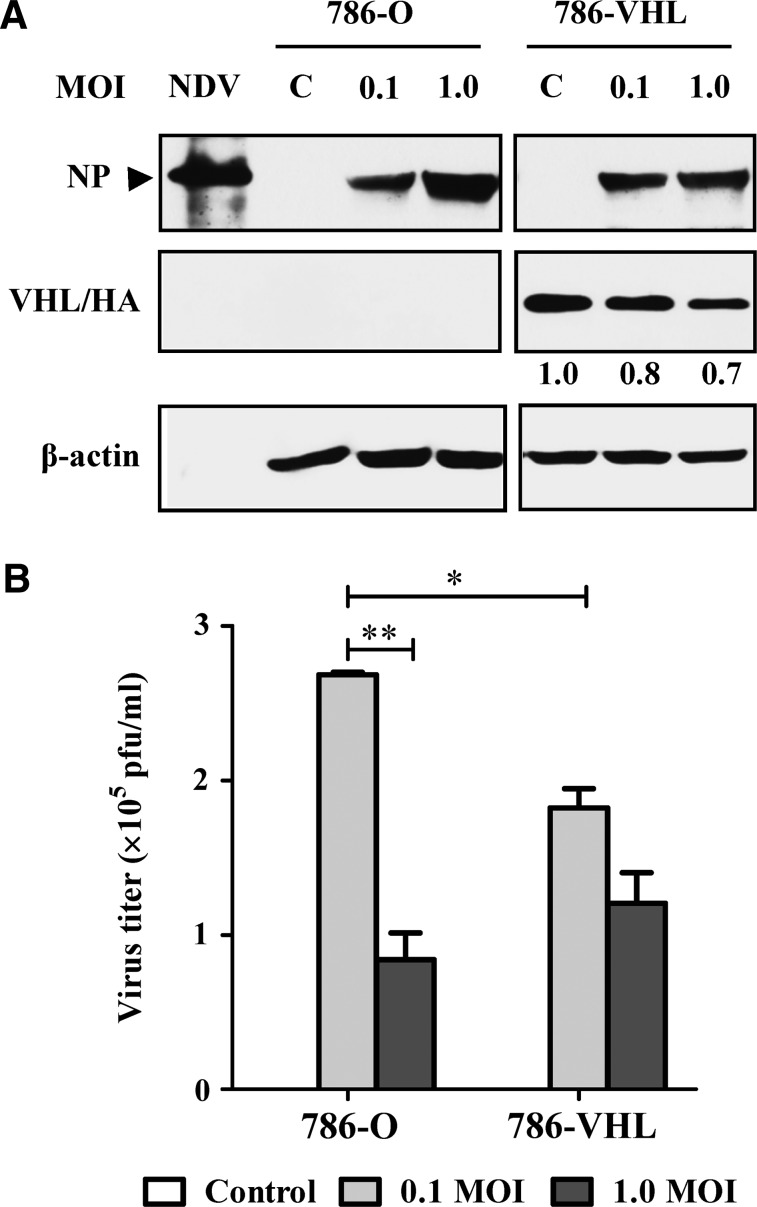
The expression status of the exogenous wt VHL in the transfected 786-VHL cells was confirmed via detection of the HA tag (Fig. 1A). Both 786-O and 786-VHL cells were susceptible to NDV infection, as evidenced by the detection of the viral nucleocapsid protein (NP) after 24 hpi (Fig. 1A). The pattern and intensities of the NP bands did not differ between the two cell lines at 0.1 MOI. However, at 1.0 MOI, 786-O has more NP protein than 786-VHL has at the similar MOI. To test whether the difference in the intracellular viral protein levels correlated with yield of released infectious virions, a plaque assay was performed using the infected cell culture media. The increase of intracellular viral proteins at 1.0 MOI did not correlate with the level of released infectious viral progenies in 786-O cultures (Fig. 1B). An inverse correlation was observed, where the increased levels of intracellular NP (Fig. 1A) were associated with lower virus titers (Fig. 1B). For 786-VHL, there was no difference in the level of NP protein for either 0.1 or 1.0 MOI infection (Fig. 1A). A slight but not statistically significant difference (p>0.05) in the corresponding virus titers was noted (Fig. 1B).
FIG. 1.
Effects of Newcastle disease virus (NDV) infection in 786-O and 786 VHL clear cell renal cell carcinoma cells. (A) Detection of the NDV nucleocapsid protein (NP) confirmed the presence of NDV infection in the cells. Anti-hemagglutinin (anti-HA) antibody was used to detect the presence of VHL protein containing an HA tag. (B) The plaque assay method was used to calculate the virus titers in the infected cell culture media. The titers are representative of experiments performed in triplicate. *p<0.05, **p<0.01.
NDV induced higher cytotoxicity in 786-VHL than in 786-O cells
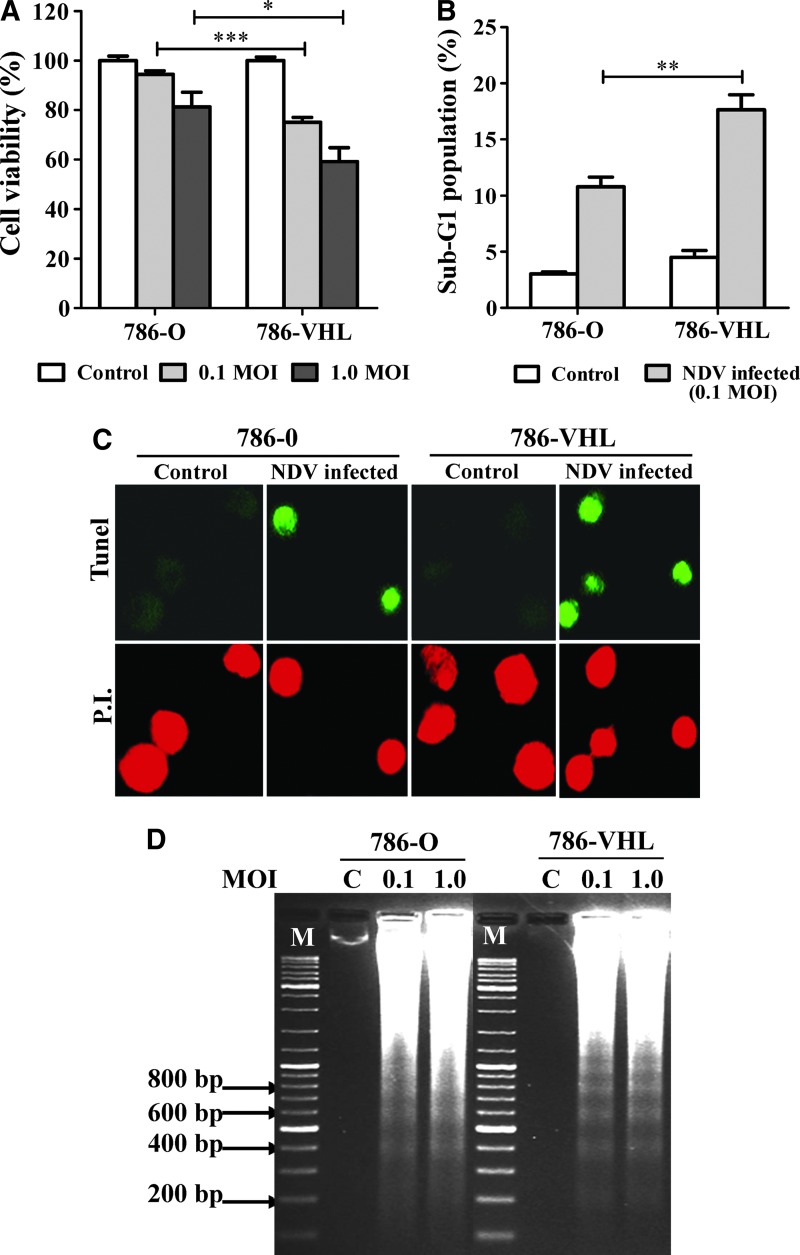
To investigate the level of cell death in the infected cultures, we measured their viability and determined whether the mechanism of cell death involved apoptosis. Cell viability assays showed that NDV infection of 786-VHL resulted in higher cell death compared to equivalent infection of 786-O cells (Fig. 2A). FACS analyses to gate for the sub-G1 population (Fig. 2B) showed fractions that correlated with the cell viability data in Fig. 2A. The dead cells were found to be apoptotic, as measured by the TUNEL staining method (Fig. 2C), and their DNA samples showed a laddering pattern of degradation (Fig. 2D) that is typical of apoptosis. A similar pattern of laddering was observed regardless of the MOI used in the infection. Overall, our data indicate that NDV induced apoptosis in both the 786-O and 786-VHL cells. This induction, however, was higher in 786-VHL than in 786-O. Since the specificity of NDV-mediated killing of cancer cells has been proposed to be due to defects in the type I IFN response of the cells (Stojdl and others 2000; Fiola and others 2006), we tested whether the difference in NDV-induced cytotoxicity in 786-O and 786-VHL was due to any difference in their IFN responses.
FIG. 2.
NDV induced higher cell death through apoptosis in 786-VHL than in 786-O cells. (A) Cell viability assay following NDV infection at 0.1 and 1.0 MOI. The results represent experiments performed in triplicate. (B) Percentage of sub-G1 populations in cells infected at 0.1 MOI correlate with the degree of cell death observed in (A). (C) terminal deoxynucleotidyl transferase duTP nick end labeling (TUNEL)-stained cell culture showed intense fluorescent green color in the nuclei of NDV-infected cells but not in the control cells. All the cells were counterstained with propidium iodide. Images shown are representative areas of each sample on glass slides. Magnification: 200×. (D) DNA laddering pattern of DNA samples purified from the floating cells in the infected culture media of both cell lines. C, Mock-infected control. M, DNA marker. *p<0.05, **p<0.01, ***p<0.001.
Restoration of VHL enhances NDV-induced IFN-β secretion and STAT1 phosphorylation
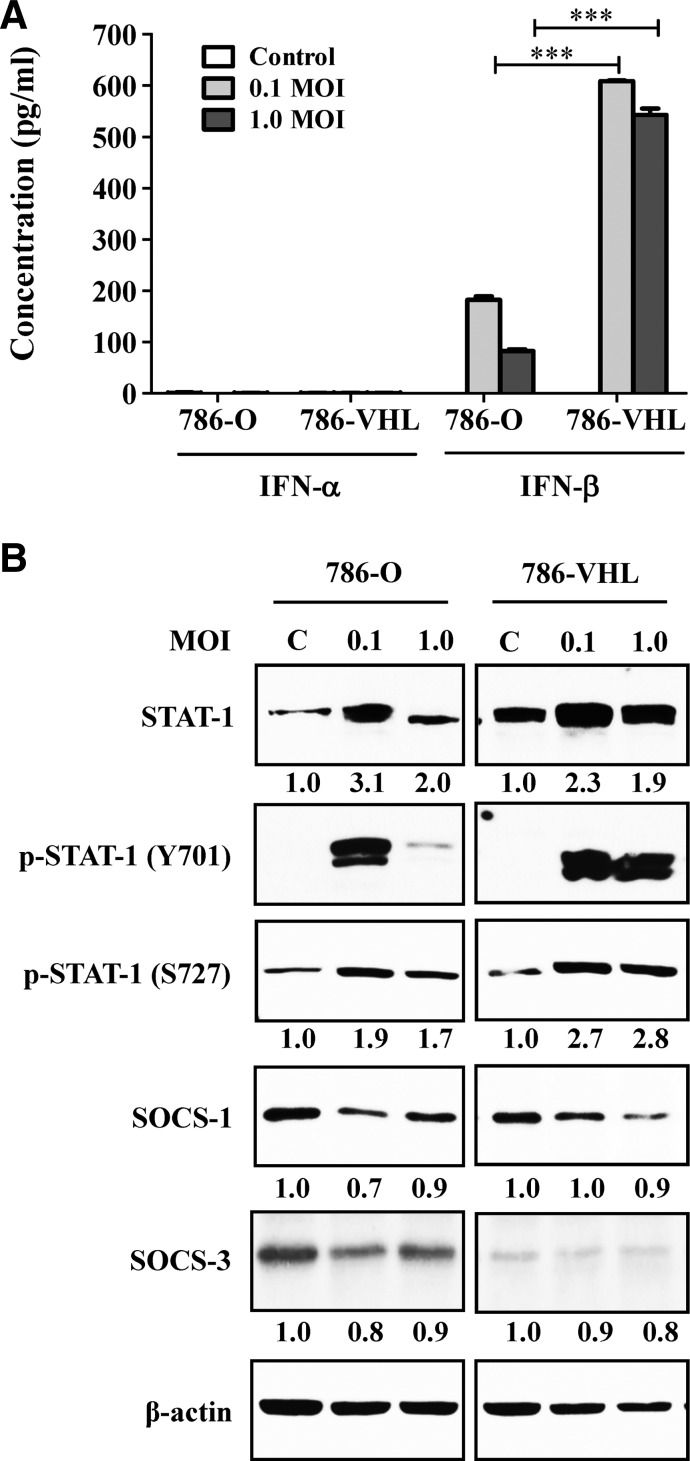
Type I IFNs (IFN-α/β) are commonly produced by cells in response to the formation of viral double-stranded RNA in the cytoplasm. To investigate the types of IFNs produced by the infected 786-O and 786-VHL cells, ELISA for IFN-α/β was performed using the infected culture media. Results showed that only IFN-β was detected upon NDV infection (Fig. 3A). The levels of IFN-β detected in the infected 786-VHL cell culture media were significantly higher than in the corresponding 786-O cultures. This higher IFN-β level may contribute toward an increased antiviral response in 786-VHL cells, leading to more cell death (Fig. 2A) and lower virus production (Fig. 1B).
FIG. 3.
Effects of VHL reconstitution on interferon (IFN) secretion, STAT1, and suppressor of cytokine signaling (SOCS) protein levels in the NDV-infected and mock-infected 786-O cells. (A) Enzyme-linked immunosorbent assay (ELISA) analysis, using the commercially available VeriKine Human Interferon kits (PBL Interferon Source), revealed the secretion of IFN-β but not IFN-α, which was dramatically higher in the VHL-containing cells upon NDV infection. The results represent experiments performed in triplicate. (B) NDV infection caused increased STAT1 phosphorylation, which was enhanced by VHL reconstitution. Values indicate fold difference of band intensities (that were initially normalized to their respective β-actin loading controls), compared to the uninfected control for each cell line. SOCS1 protein level was reduced following NDV infection, while SOCS3 protein was barely detectable in 786-VHL. C, Mock-infected control. ***p<0.001.
Since IFN-β was previously shown to affect mainly the phosphorylation of STAT1 in the JAK/STAT antiviral response pathway (Toshchakov and others 2002; Colonne and others 2011), we then tested the level of STAT1 phosphorylation in the cells following NDV infection. In the uninfected cell lines, there is an increased level of total STAT1 protein in 786-VHL compared to 786-O (Fig. 3B). NDV infection increases the level of this STAT1 in both cell lines. At 0.1 MOI, we see a more dramatic increase than the MOI of 1.0. Phosphorylation of STAT1 at tyrosine 701 (Y701) and serine 727 (S727) are required for homodimer formation and maximum induction of STAT1-mediated gene activation, respectively. In the case of S727 phosphorylation, an increase is seen at all MOIs in both cell lines, with a moderately higher level in 786-VHL (Fig. 3B). In the case of Y701 phosphorylation (required for dimer formation), there is a dramatic increase in 786-O cultures at the MOI of 0.1. Interestingly, that level of phosphorylation disappears at the MOI of 1.0. In 786-VHL cells, the dramatic increase in Y701 phosphorylation is seen at both MOIs. The increase in STAT1 phosphorylation is observed to coincide with reduction in the levels of the suppressor of cytokine signaling (SOCS) proteins that are inhibitors of the JAK/STAT signaling pathway (Kile and Alexander 2001). Nevertheless, our data demonstrate for the first time that NDV-induced IFN-β production is influenced by the VHL status of the RCC cells.
Since the difference between the two isogenic cell lines, 786-O and 786-VHL, is their wt VHL status, we wished to determine whether activity of the transcription factor HIF-2, which is constitutively active in 786-O cells (Maxwell and others 1999) and active only under hypoxic conditions in 786-VHL (Hwang and others 2006) cells, is also involved.
Hypoxia enhanced NDV-induced oncolysis of RCC cells
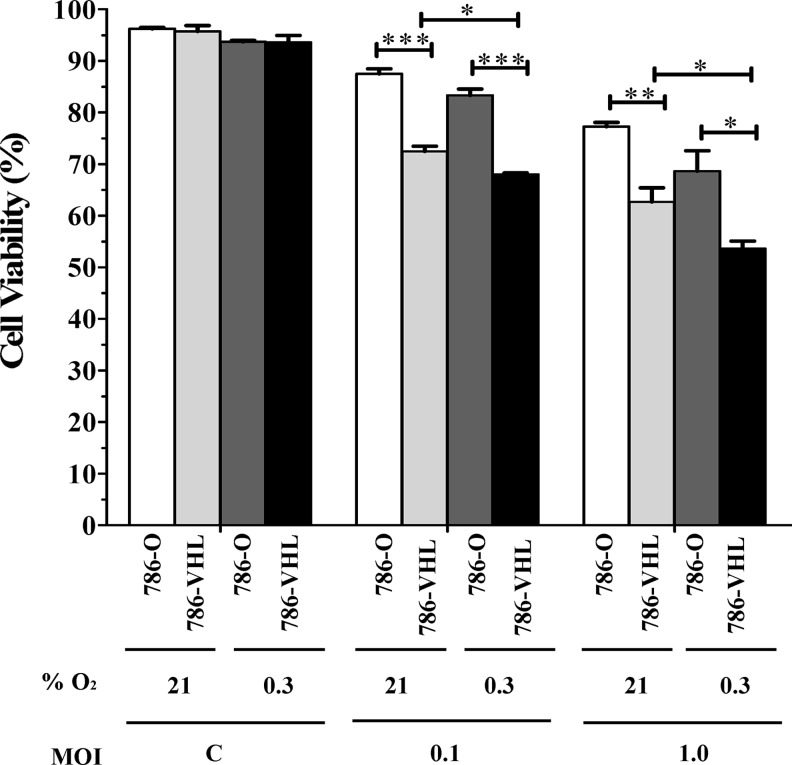
Having established that wt VHL status correlates with the degree of NDV-mediated oncolysis in RCC cells, we asked whether accumulation of HIF-2α in the 786-VHL cells under hypoxic conditions recapitulates the phenomenon observed in the 786-O cells where HIF-2α is stable under all conditions. To achieve this, we repeated the infection experiment in the presence of 0.3% O2 and compared the results to the normoxic condition. For all control cells, viability was the same under normoxic and hypoxic conditions (Fig. 4). For infected 786-O cells (stable HIF-2α under all conditions), there was a moderate increase in cell death under the hypoxic condition. For 786-VHL cells (stable HIF-2α under hypoxic condition only), there was a more significant (p<0.05) increase in cell death under hypoxic conditions. Thus, the increase in cell death under hypoxic conditions for both 786-O and 786-VHL is unlikely to be dependent on HIF-2 activity. To examine this in more detail, we measured the levels of both VHL and HIF-2α in the infected 786-O and 786-VHL cells.
FIG. 4.
Hypoxia enhanced NDV-induced oncolysis of clear cell renal cell carcinoma cells. Fluorescence-activated cell sorting (FACS) analysis, using the BD Cell Viability kit, was performed on the mock and infected cells after 24 h post-infection. A moderate increase in cell death under the hypoxic condition was observed for all the samples tested. The results represent experiments performed in triplicate. C, Mock-infected control. *p<0.05, **p<0.01, ***p<0.001.
NDV infection leads to a downregulation of VHL in 786-VHL cells
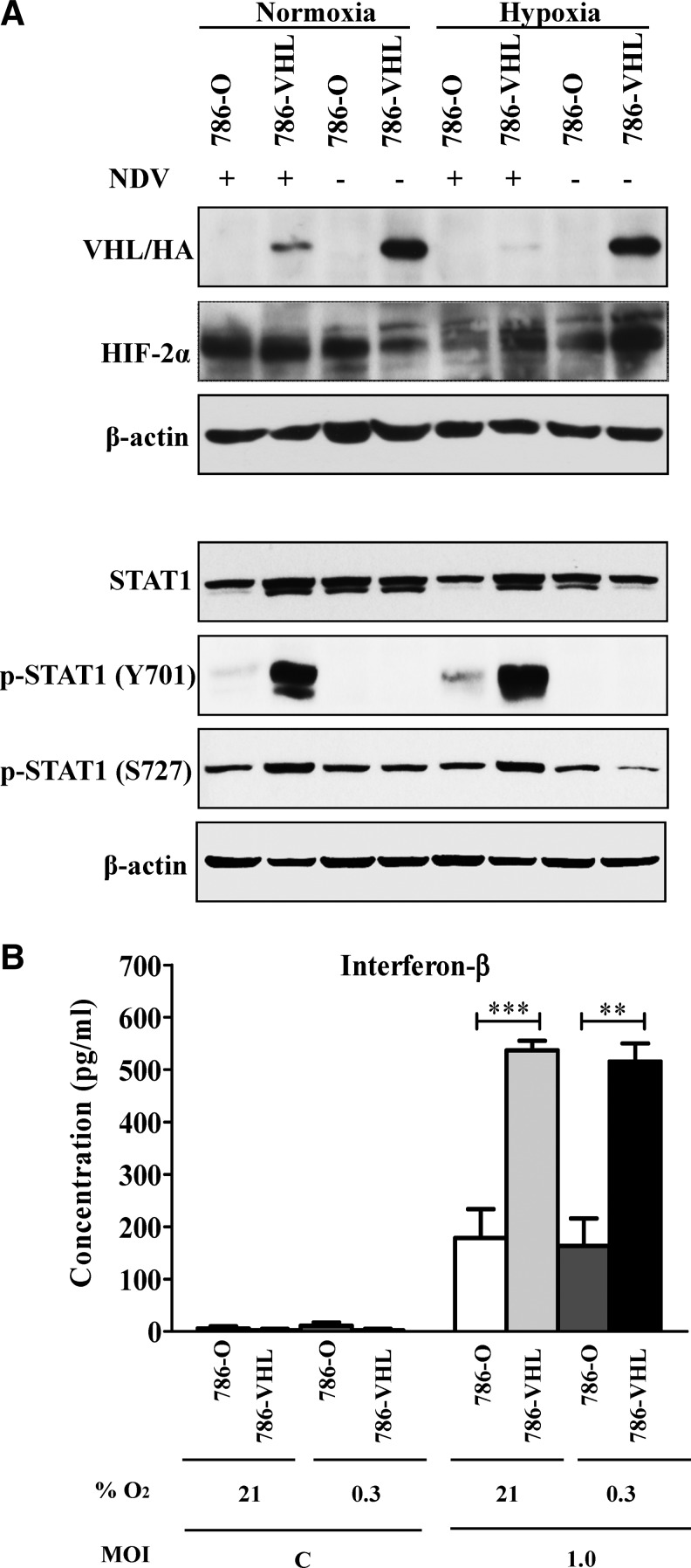
In the normoxic condition, a basal level of HIF-2α expression was noted in uninfected 786-VHL cells (Fig. 5A) despite the fact that subconfluent cultures were used for the experiments. Previous studies have shown that HIF-2α is less efficiently degraded via the prolyl hydroxylase domain (PHD)-mediated proteasomal degradation pathway compared to HIF-1α in physiological O2 conditions (Lofstedt and others 2007). It was also present at a higher basal level compared to HIF-1α in normoxic endothelial cells (Wiesener and others 1998; Ahmad and others 2009). Interestingly, NDV infection of 786-VHL cells induced downregulation of HA-VHL and this led to a further accumulation of HIF-2α (Fig. 5A). An even greater suppression of VHL was observed when the NDV-infected cells were cultured in a hypoxic condition. In this condition, the suppression of VHL by NDV did not alter the hypoxia-induced stabilization of HIF-2α. This is because, in limited O2 tension (hypoxia), prolyl-4-hydroxylases that require O2 for their activities will be unable to hydroxylate P405 and P531 within the HIF-2α O2-dependent degradation domain (Keith and others 2012). The absence of these hydroxylations results in the inability of VHL to recognize and ubiquitinate HIF-2α for targeted proteasomal degradation. This suggests that the increase in NDV-induced cytotoxic activity in 786-VHL cells is dependent on the expression of VHL during the early phases of NDV infection, thereby increasing the levels of IFN-β but is independent of HIF-2α levels and the corresponding HIF-2 activity. Further support for this notion is given with the observation that hypoxia did not significantly alter the pattern of STAT1 phosphorylation or IFN-β secretion in both cell lines (Fig. 5A, B) despite the increased level of cell death (Fig. 4).
FIG. 5.
Effects of hypoxia on the levels of VHL, HIF-2α, STAT proteins, and IFN production in NDV-infected clear cell renal cell carcinoma (RCC) cells. (A) NDV infection in hypoxic condition did not drastically change the patterns of VHL, HIF-2α, and STAT proteins compared to normoxic condition. (B) Hypoxia also failed to exert any effect on the level of NDV-induced IFN-β production by the infected clear cell RCC cells. The results represent experiments performed in triplicate. C, Mock-infected control. **p<0.01, ***p<0.001.
Discussion
Oncolytic virotherapy offers great promise as an alternative method for cancer treatment. Recently, several studies have shown that a number of oncolytic viruses have higher capacity to infect and kill HIF-expressing cancer cells (Connor and others 2004; Roos and others 2010), while others concluded that increased activity of HIF reduced the susceptibility of cancer cells to oncolysis (Hwang and others 2006). A recent addition to the armamentarium of the field of oncolytic viruses is the viscerotropic-velogenic strain of NDV, designated as AF2240 (Yusoff and Tan 2001), which induces high levels of apoptosis in the infected cancer cells (Alabsi and others 2011; Ali and others 2011).
The most common form of RCC is clear cell RCC (Lopez-Beltran and others 2009). Clear cell RCC is associated with loss of function mutations in the VHL gene or epigenetic silencing, leading to constitutive HIF-α stabilization and HIF activity, and increased angiogenesis. VHL is a tumor suppressor protein that is responsible for rapid proteasomal degradation of the HIF-α subunit under normoxic conditions (Lu and Kang 2010). We were interested to determine whether the oncolytic NDV was capable of killing these cell types under all conditions of O2 tension, that is, under the physiological conditions of heterogeneity of O2 tension encountered in most solid tumors that are either wt or deficient for VHL. To accomplish this we used a model of clear cell RCC cells where VHL is defective (786-O) and the isogenic 786-VHL cells, where the wt VHL function has been restored.
We found that NDV induced apoptosis in both 786-O and 786-VHL cells under both normoxic and hypoxic conditions, irrespective of the MOI used. However, some quantitative differences were seen. Infection using 1.0 MOI of NDV resulted in higher NP viral protein production in 786-O than in 786-VHL. This increase correlated with less virus progeny production and increased cell death. This pattern of virus-induced cytotoxicity might be due to the finite ability of the infected cells to produce viral proteins and release viral progeny. Increasing the MOI can lead to higher levels of cell killing, hence reducing virus yield (Flint and others 2008). Regardless of the amount of viral proteins produced and MOI used, NDV infection resulted in increased cell death in 786-VHL cells, particularly in a hypoxic environment. Importantly, this indicates that NDV will be an effective oncolytic agent for cancer cells in the hypoxic regions of tumors. NDV has been shown to be a strong type-I IFN inducer in susceptible cells (Fiola and others 2006; Elankumaran and others 2010). Since tumor cells have defects in their IFN responses, NDV preferentially replicates and induces cell death in these cells as compared to normal cells (Reichard and others 1992). In the present study, both 786-O and 786-VHL cells responded to NDV infection by producing only IFN-β but not IFN-α. This is consistent with a previous finding that NDV only induced IFN-β production in cancer cells (Elankumaran and others 2010). A defect in IFN-α secretion is thought to be essential for successful NDV replication in these cells. This is because a defective IFN system in tumor cells could lead to the loss of antiviral host defenses and enhancement of NDV replication (Fiola and others 2006; Schirrmacher and Fournier 2009). This is further supported by the studies of Krishnamurthy and others (2006), which demonstrated that neither naturally secreted IFN-β from NDV-infected HT-1080 cancer cell lines nor exogenous IFN-β treatment was effective in preventing viral infection and replication. Here, we demonstrate for the first time that NDV AF2240 triggered only IFN-β production in RCC cells. The level of IFN-β secretion, however, is more prominent in VHL-expressing cells than in VHL-deficient cells. Since IFN-β has been shown to preferentially induce apoptosis (Chawla-Sarkar and others 2001), the increased IFN-β production presumably contributed directly toward an increased cell death in 786-VHL cells.
STAT1 is a type-I IFN intracellular signaling mediator that is involved in the regulation of cellular proliferation, modulation of the immune response, and antiviral defense (Pensa and others 2009). In this study, we examined whether the secreted IFN-β affects STAT1 activity in RCC cells following NDV infection. VHL expression in 786-VHL cells was reported to cause a slight upregulation of STAT1 protein expression compared to the parental 786-O cells (Ivanov and others 2007). However, they showed that this increase per se did not significantly affect the level of IFN-induced STAT1 phosphorylation. Phosphorylation of Y701 in STAT1 initiates its dimerization, leading to nuclear translocation and DNA binding (Ihle and others 1994). We observed a high level of phosphorylation at Y701 and S727 of STAT1 in both infected 786-O and 786-VHL cells. However, at 1.0 MOI, the phosphorylation was dramatically diminished in 786-O but not in 786-VHL. We propose that the high level of IFN-β in the culture medium of 786-VHL perhaps led to a prolonged STAT1 phosphorylation when a higher MOI was used. Our proposal is further supported by a reduction of SOCS1 and SOCS3, which are negative regulators of STAT1 (Song and Shuai 1998), in 786-VHL at 1.0 MOI, but not in 786-O (1.0 MOI) where STAT1 phosphorylation was lowered. Prolonged STAT1 phosphorylation by IFN-β is also possible since secreted IFN-β has been shown to further amplify its own expression via a positive feedback mechanism through an IFN-β-STAT1 feedback loop (Colonne and others 2011). The actual function of STAT1 Y701 phosphorylation, in the context of apoptosis, is somewhat controversial. However, increasing evidence suggests that, in conjunction with IFN type 1 responses, STAT1 phosphorylation enhances the apoptotic response (Zitzmann and others 2007). IFN-β was also shown to induce STAT1 S727 phosphorylation, resulting in apoptosis (Sanceau and others 2000). In the present study, an increased IFN-β production in 786-VHL may contribute toward a prolonged state of phosphorylation of STAT1 Y701, leading to its activation and an increase in apoptosis. We are currently investigating the detailed involvement of JAK/STAT/SOCS pathway in this event. Nevertheless, our results thus far clearly show the involvement of IFN-β signaling pathways in NDV-induced apoptosis of RCC cells.
Our initial study was designed to determine whether hypoxia, and also stable HIF-2 activity, would modulate NDV-mediated cytotoxity. Earlier, it had been reported that HIF stabilization was shown to cause resistance of RCC cells to VSV oncolysis (Hwang and others 2006). However, our results show that hypoxia enhanced NDV oncolysis in both 786-O and 786-VHL. These data suggest that hypoxia-induced stabilization of HIF-2α in 786-VHL cells failed to inhibit NDV-induced oncolytic activities; instead it was enhanced. Further, infection with NDV caused a significant decrease in the levels of exogenous wt VHL protein in the 786-VHL cells, regardless of O2 content. One consequence of this is that significant levels of HIF-2α were present in both cell lines, irrespective of the degree of hypoxia. Thus, it is unlikely that HIF-2 activity per se is responsible for the greater oncolytic activity of NDV under hypoxic conditions.
In summary, our data show that NDV AF2240 induced apoptosis in RCC cells via the IFN-β signaling, leading to STAT1 phosphorylation and perhaps the involvement of SOCS proteins. VHL restoration led to an increase in NDV-induced IFN-β signaling and apoptotic cell death. Importantly, hypoxia enhanced NDV-induced oncolysis in both 786-O and 786-VHL cells. Overall, these findings highlight the importance of our velogenic strain of NDV as a candidate for anticancer virotherapy, targeting both normoxic and particularly hypoxic cancer cells, especially for renal cell cancers that are refractory to most chemotherapeutic regimens.
Acknowledgments
This work was supported in part by the Malaysian Ministry of Science, Technology and Innovation grants 04-01-11-1159RU, 04-01-09-0802RU, 09-05-IFN-BPH-009, and 02-01-04-SF1269. C.W.C. is a MyBrain scholar under the Malaysian Ministry of Higher Education.
Author Disclosure Statement
No competing financial interests exist.
References
- Aaronson DS. Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Ahmad A. Ahmad S. Glover L. Miller SM. Shannon JM. Guo X. Franklin WA. Bridges JP. Schaack JB. Colgan SP. White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009;106(26):10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabsi AM. Bakar SA. Ali R. Omar AR. Bejo MH. Ideris A. Ali AM. Effects of Newcastle disease virus strains AF2240 and V4-UPM on cytolysis and apoptosis of leukemia cell lines. Int J Mol Sci. 2011;12(12):8645–8660. doi: 10.3390/ijms12128645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DJ. Newcastle disease virus—An avian paramyxovirus. In: Alexander DJ, editor. Newcastle disease. Boston: Kluwer Academic; 1988. pp. 11–22. [Google Scholar]
- Ali R. Alabsi AM. Ali AM. Ideris A. Omar AR. Yusoff K. Saif-Ali R. Cytolytic effects and apoptosis induction of Newcastle disease virus strain AF2240 on anaplastic astrocytoma brain tumor cell line. Neurochem Res. 2011;36(11):2051–2062. doi: 10.1007/s11064-011-0529-8. [DOI] [PubMed] [Google Scholar]
- Cassel WA. Garrett RE. Newcastle disease virus as an antineoplastic agent. Cancer. 1965;18:863–868. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M. Leaman DW. Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: Correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7(6):1821–1831. [PubMed] [Google Scholar]
- Collins JA. Schandi CA. Young KK. Vesely J. Willingham MC. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45(7):923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- Colonne PM. Eremeeva ME. Sahni SK. Beta interferon-mediated activation of signal transducer and activator of transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells. Infect Immun. 2011;79(9):3733–3743. doi: 10.1128/IAI.05008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JH. Naczki C. Koumenis C. Lyles DS. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J Virol. 2004;78(17):8960–8970. doi: 10.1128/JVI.78.17.8960-8970.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray S. Best JM. Jin L. Time-course induction of apoptosis by wild-type and attenuated strains of rubella virus. J Gen Virol. 2003;84(Pt 5):1275–1279. doi: 10.1099/vir.0.18785-0. [DOI] [PubMed] [Google Scholar]
- Cowey CL. Hutson TE. Molecularly targeted agents for renal cell carcinoma: The next generation. Clin Adv Hematol Oncol. 2010;8(5):357–364. [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Elankumaran S. Chavan V. Qiao D. Shobana R. Moorkanat G. Biswas M. Samal SK. Type I interferon-sensitive recombinant Newcastle disease virus for oncolytic virotherapy. J Virol. 2010;84(8):3835–3844. doi: 10.1128/JVI.01553-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiola C. Peeters B. Fournier P. Arnold A. Bucur M. Schirrmacher V. Tumor selective replication of Newcastle disease virus: Association with defects of tumor cells in antiviral defence. Int J Cancer. 2006;119(2):328–338. doi: 10.1002/ijc.21821. [DOI] [PubMed] [Google Scholar]
- Flint SJ. Enquist LW. Racaniello VR. Skalka AM. Principles of virology. Washington, DC: ASM Press; 2008. p. 592. [Google Scholar]
- Hwang IIL. Watson IR. Der SD. Ohh M. Loss of VHL confers hypoxia-inducible factor (HIF)-dependent resistance to vesicular stomatitis virus: Role of HIF in antiviral response. J Virol. 2006;80(21):10712–10723. doi: 10.1128/JVI.01014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN. Witthuhn BA. Quelle FW. Yamamoto K. Thierfelder WE. Kreider B. Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O. Kibel A. Gray S. Kaelin WG., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1(8):822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Ivanov SV. Salnikow K. Ivanova AV. Bai L. Lerman MI. Hypoxic repression of STAT1 and its downstream genes by a pVHL/HIF-1 target DEC1/STRA13. Oncogene. 2007;26(6):802–812. doi: 10.1038/sj.onc.1209842. [DOI] [PubMed] [Google Scholar]
- Jamal MH. Ch'ng WC. Yusoff K. Shafee N. Reduced Newcastle disease virus-induced oncolysis in a subpopulation of cisplatin-resistant MCF7 cells is associated with survivin stabilization. Cancer Cell Int. 2012;12(1):35. doi: 10.1186/1475-2867-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG., Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- Keith B. Johnson RS. Simon MC. HIF1alpha and HIF2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile BT. Alexander WS. The suppressors of cytokine signalling (SOCS) Cell Mol Life Sci. 2001;58(11):1627–1635. doi: 10.1007/PL00000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul H. Huh JS. Rove KO. Crompton L. Koul S. Meacham RB. Kim FJ. Molecular aspects of renal cell carcinoma: A review. Am J Cancer Res. 2011;1(2):240–254. [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S. Takimoto T. Scroggs RA. Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006;80(11):5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofstedt T. Fredlund E. Holmquist-Mengelbier L. Pietras A. Ovenberger M. Poellinger L. Pahlman S. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6(8):919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- Lopez-Beltran A. Carrasco JC. Cheng L. Scarpelli M. Kirkali Z. Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. 2009;16(5):432–443. doi: 10.1111/j.1442-2042.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- Lu X. Kang Y. Hypoxia and hypoxia-inducible factors: Master regulators of metastasis. Clin Cancer Res. 2010;16(24):5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclachlan NJ. Edward JD. Fenner's veterinary virology. 4th. San Diego: Academic Press; 2011. Paramyxoviridae; pp. 299–325. Chapter 17. [Google Scholar]
- Mansour M. Palese P. Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011;85(12):6015–6023. doi: 10.1128/JVI.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH. Wiesener MS. Chang GW. Clifford SC. Vaux EC. Cockman ME. Wykoff CC. Pugh CW. Maher ER. Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Pensa S. Regis G. Boselli D. Novelli F. Poli V. STAT1 and STAT3 in tumorigenesis. In: Stephanou A, editor. JAK-STAT pathway in disease. Texas: Landes Bioscience Books; 2009. pp. 100–121. [Google Scholar]
- Rawlings JS. Rosler KM. Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117(Pt 8):1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- Reichard KW. Lorence RM. Cascino CJ. Peeples ME. Walter RJ. Fernando MB. Reyes HM. Greager JA. Newcastle disease virus selectively kills human tumor cells. J Surg Res. 1992;52(5):448–453. doi: 10.1016/0022-4804(92)90310-v. [DOI] [PubMed] [Google Scholar]
- Reu FJ. Bae SI. Cherkassky L. Leaman DW. Lindner D. Beaulieu N. MacLeod AR. Borden EC. Overcoming resistance to interferon-induced apoptosis of renal carcinoma and melanoma cells by DNA demethylation. J Clin Oncol. 2006;24(23):3771–3779. doi: 10.1200/JCO.2005.03.4074. [DOI] [PubMed] [Google Scholar]
- Roos FC. Roberts AM. Hwang IIL. Moriyama EH. Evans AJ. Sybingco S. Watson IR. Carneiro LA. Gedye C. Girardin SE. Ailles LE. Jewett MA. Milosevic M. Wilson BC. Bell JC. Der SD. Ohh M. Oncolytic targeting of renal cell carcinoma via encephalomyocarditis virus. EMBO Mol Med. 2010;2(7):275–288. doi: 10.1002/emmm.201000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanceau J. Hiscott J. Delattre O. Wietzerbin J. IFN-beta induces serine phosphorylation of Stat-1 in Ewing's sarcoma cells and mediates apoptosis via induction of IRF-1 and activation of caspase-7. Oncogene. 2000;19(30):3372–3383. doi: 10.1038/sj.onc.1203670. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Fournier P. Newcastle disease virus: A promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkovics JG. Horvath JC. Newcastle disease virus (NDV): Brief history of its oncolytic strains. J Clin Virol. 2000;16(1):1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- Song MM. Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273(52):35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- Stojdl DF. Lichty B. Knowles S. Marius R. Atkins H. Sonenberg N. Bell JC. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Strauss WM. Preparation of genomic DNA from mammalian tissue. In: Ausubel FM, editor; Brent R, editor; Kingston RE, editor; Moore DD, editor; Seidman JG, editor; Smith JA, editor; Struhl K, editor. Current protocols in molecular biology. New York: John Wiley & Sons, Inc.; 1998. pp. 2.2.1–2.2.3. [Google Scholar]
- Toshchakov V. Jones BW. Perera P-Y. Thomas K. Cody MJ. Zhang S. Williams BRG. Major J. Hamilton TA. Fenton MJ. Vogel SN. TLR4, but not TLR2, mediates IFN-[beta]-induced STAT1[alpha]/[beta]-dependent gene expression in macrophages. Nat Immunol. 2002;3(4):392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- Toth CA. Thomas P. Type I interferon resistance in a colorectal cancer cell line is associated with a more aggressive phenotype in vivo. Br J Cancer. 1992;65(3):365–368. doi: 10.1038/bjc.1992.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesener MS. Turley H. Allen WE. Willam C. Eckardt KU. Talks KL. Wood SM. Gatter KC. Harris AL. Pugh CW. Ratcliffe PJ. Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: Characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92(7):2260–2268. [PubMed] [Google Scholar]
- Wong HH. Lemoine NR. Wang Y. Oncolytic viruses for cancer therapy: Overcoming the obstacles. Viruses. 2010;2(1):78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusoff K. Tan WS. Newcastle disease virus: Macromolecules and opportunities. Avian Pathol. 2001;30(5):439–455. doi: 10.1080/03079450120078626. [DOI] [PubMed] [Google Scholar]
- Yusoff K. Tan WS. Lau CH. Ng BK. Ibrahim AL. Sequence of the haemagglutinin-neuraminidase gene of the Newcastle disease virus oral vaccine strain V4(UPM) Avian Pathol. 1996;25(4):837–844. doi: 10.1080/03079459608419185. [DOI] [PubMed] [Google Scholar]
- Zitzmann K. Brand S. De Toni EN. Baehs S. Goke B. Meinecke J. Spottl G. Meyer HH. Auernhammer CJ. SOCS1 silencing enhances antitumor activity of type I IFNs by regulating apoptosis in neuroendocrine tumor cells. Cancer Res. 2007;67(10):5025–5032. doi: 10.1158/0008-5472.CAN-06-2575. [DOI] [PubMed] [Google Scholar]
- Zorn U. Dallmann I. Grosse J. Kirchner H. Poliwoda H. Atzpodien J. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus. Cancer Biother. 1994;9(3):225–235. doi: 10.1089/cbr.1994.9.225. [DOI] [PubMed] [Google Scholar]