Abstract
Purpose
Retinal ischemia-associated ocular disorders are vision threatening. This study examined whether the flavonoid baicalein is able to protect against retinal ischemia/reperfusion.
Methods
Using rats, the intraocular pressure was raised to 120 mmHg for 60 min to induce retinal ischemia. In vitro, an ischemic-like insult, namely oxidative stress, was established by incubating dissociated retinal cells with 100 μM ascorbate and 5 μM FeSO4 (iron) for 1 h. The rats or the dissociated cells had been pretreated with baicalein (in vivo: 0.05 or 0.5 nmol; in vitro: 100 μM), vehicle (1% ethanol), or trolox (in vivo: 5 nmol; in vitro: 100 μM or 1 mM). The effects of these treatments on the retina or the retinal cells were evaluated by electrophysiology, immunohistochemistry, terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling (TUNEL) staining, Western blotting, or in vitro dichlorofluorescein assay. In addition, real-time-polymerase chain reaction was used to assess the retinal expression of hypoxia-inducible factor-1α (HIF-1α), matrix metalloproteinase-9 (MMP-9), vascular endothelium growth factor (VEGF), and heme oxygenase-1 (HO-1).
Results
The retinal changes after ischemia included a decrease in the electroretinogram b-wave amplitude, a loss of choline acetyltransferase immunolabeling amacrine cell bodies/neuronal processes, an increase in vimentin immunoreactivity, which is a marker for Müller cells, an increase in apoptotic cells in the retinal ganglion cell layer linked to a decrease in the Bcl-2 protein, and changes in the mRNA levels of HIF-1α, VEGF, MMP-9, and HO-1. Of clinical importance, the ischemic detrimental effects were concentration dependently and/or significantly (0.05 nmol and/or 0.5 nmol) altered when baicalein was applied 15 min before retinal ischemia. Most of all, 0.5 nmol baicalein significantly reduced the upregulation of MMP-9; in contrast, 5 nmol trolox only had a weak attenuating effect. In dissociated retinal cells subjected to ascorbate/iron, there was an increase in the levels of reactive oxygen species, which had been significantly attenuated by 100 μM baicalein and trolox (100 μM or 1 mM; a stronger antioxidative effect at 1 mM).
Conclusions
Baicalein would seem to protect against retinal ischemia via antioxidation, antiapoptosis, upregulation of HO-1, and downregulation of HIF-1α, VEGF, and MMP-9. The antioxidative effect of baicalein would appear to play a minor role in downregulation of MMP-9.
Introduction
Central retinal artery occlusion, central retinal vein occlusion, branch retinal artery occlusion, branch retinal vein occlusion, glaucoma, and age-related macular degeneration (AMD) are all associated with retinal ischemia.1–3 All these diseases may lead to severe sequelae and therefore the management of retinal ischemia is crucial. After ischemia/reperfusion (I/R), large amounts of reactive oxygen species (ROS) are produced.1,2 These ROS attack nearby cells and cause tissue damage.1,2 Moreover, excessive release of excitatory transmitters, such as the glutamate, from ischemia-affected neurons leads to neuronal overstimulation and unwanted depolarization.1–3 Consequently, neurons that possess a high density of glutamate receptors are most at risk.1–3 This explains why neurons, such as retinal ganglion cells (RGCs) and amacrine cells, as well as their neuronal processes, which are located in the inner retina, are vulnerable to I/R.1,2 After I/R, vimentin immunoreactivity has been also shown to be increased in Müller cells.4
Ischemia induces angiogenesis.5 Furthermore, in the retina, angiogenesis is often disorganized and typically results in edema and hemorrhage; these have adverse effects on visual functioning. There is an urgent need for therapies that promote endogenous protective responses and prevent harmful angiogenesis. Increased levels of the hypoxia-inducible factor-1α (HIF-1α) have been found to be present after retinal ischemia.6 HIF-1α binds to the hypoxia response elements of hypoxia-responsive target genes, and triggers the expression of the vascular endothelium growth factor (VEGF)6 and various matrix metalloproteinases (MMPs).7–9 Liu et al.10 showed that oxidative stress in the human retinal pigment epithelium (hRPE) results in upregulation of VEGF and MMP-9. Additionally, ischemia has been proved to result in an irreversible RGC loss that is accompanied by MMP-9 upregulation.7–9 All the above evidence suggests that overexpression of HIF-1α, VEGF, and/or MMP-9 in the retina or in RGCs is directly related to ischemic insult. Heme oxygenase-1 (HO-1) has been recognized as a proangiogenic factor causing the induction of VEGF in vascular cells.11 Whether upregulation of VEGF and HO-1 coexists in the ischemic retina was included in the present investigation.
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one (Baicalein) is a natural flavonoid isolated from Scutellaria baicalensis, a commonly used traditional Chinese herbal medicine. Baicalein has many biological activities, including antioxidation in cardiomyocytes12 and inhibition of tumor-induced angiogenesis in confrontation cultures of embryoid bodies and tumor spheroids.13 The present study examines whether baicalein is able to attenuate retinal ischemic injury in terms of electrophysiology, vimentin immunoreactivity, ChAT immunoreactivity, terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling (TUNEL) staining, and Bcl-2/Bax protein analysis in addition to affecting the upregulation of HIF-1α, VEGF, MMP-9, and/or HO-1 at the mRNA level. In brain ischemia, baicalein has been recently shown to have an inhibitory effect on MMP-9 expression.14 However, up to the present, but until now, there is no evidence available exploring whether or how baicalein can suppress retinal MMP-9 upregulation induced by I/R.
Methods
Animals
All investigations involving the use of animals conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmology and Vision Research and were approved by the Institutional Review Board of Cheng Hsin General Hospital (CHGH; Taipei, Taiwan). Six-week-old Wistar rats (BioLasco) were housed in an animal house where the humidity was 40%–60% and the temperature was 19°C–23°C. They were kept on a 12-h light/12-h dark cycle with 12–15 air changes/h. The animals were provided with food and water ad libitum.
Anesthesia and euthanasia
The rats were anesthetized with an intraperitoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). They were sacrificed using a humane method (Scientific Procedures Acts 1986), namely, an overdose (at least 140 mg/kg) of i.p. sodium pentobarbitone.
Ischemia induction
The rats (200–250 g) were anesthetized and placed in a stereotaxic frame. The anterior chamber of one eye of a rat was cannulated with a 30-gauge needle connected to an elevated 0.9% saline reservoir; this caused a high intraocular pressure (HIOP) of 120 mmHg for 60 min. Eye fundus whitening confirmed the induction of an ischemic insult.1,2 A sham procedure was carried out on the controls in which the saline bottle was not elevated.3
Drug administration
Drug administration involved the preadministration (15 min before 60-min HIOP-induced retinal ischemia) of baicalein (10 or 100 μM; Sigma-Aldrich), trolox (1 mM; Sigma-Aldrich), or vehicle (1% ethanol; control). In view of the direct diffusion of an intravitreous chemical into the retina, the ischemic eye of each test rat was treated with a single intravitreous injection of 5 μL of the test compound. The paired normal eye was untreated.
In vitro, an ischemic-like insult, namely oxidative stress,15 was established by incubating dissociated retinal cells with 100 μM ascorbate and 5 μM FeSO4 for 1 h. The dissociated cells were pretreated with baicalein (100 μM), vehicle, or trolox (100 μM or 1 mM).
Electroretinogram recording
Flash electroretinogram (ERG) recordings were performed on all the rats before retinal ischemia (day 0) as well as at 1, 3, 5, and 7 days after ischemia and treatment with the defined chemicals. The animals were dark adapted for at least 8 h, and then they were anesthetized during the ERG recordings; for this, pupils were dilated with 1% tropicamide and 2.5% phenylephrine.1,2 A strobe was placed 2 cm in front of the animal to provide a stimulus of 0.5 Hz. Fifteen consecutive responses were recorded at 2-second intervals and at 10 kHz; the responses were amplified and averaged using an amplifier P511/regulated power supply RPS 107/stimulator PS22 (Grass-Telefactor; Astro-Med, Inc.). For comparative purposes, the b-wave ratio, namely, the b-wave amplitude of the treated ischemic eye when compared with that of the untreated contralateral normal eye, was calculated.1,2
Immunofluorescence analysis
The rats were sacrificed and intracardially perfused with 0.9% normal saline (w/v); then, the rat retinas were removed, fixed with 4% (w/v) paraformaldehyde for 45 min, and transferred to 30% sucrose for cryosectioning.1,2 Sampling was carried out one and 7 days after induction of retinal ischemia and pretreatment with 0.5 nmol baicalein or vehicle, or the sham operation. The retinal sections from the eyes were incubated overnight with primary antibodies, namely, the goat anti-ChAT polyclonal antibody (1:100; AB144p; Chemicon) or the mouse anti-vimentin monoclonal antibody (1:100; V6630; Sigma-Aldrich). Next, the retinal sections were incubated with appropriate secondary antibodies, the rhodamine-conjugated rabbit anti-goat antibody (1:500; AP106R; Chemicon) or the fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:500; AP124F; Millipore). At the same time, the nuclei of the cells were labeled by 4,6-diamidine-2-phenylindole dihydrochloride (DAPI; 30 nM; Molecular Probes). The retinal sections were examined using a fluorescence microscope (Olympus BX61).
The TUNEL assay
At 1 and 7 days after I/R, the eyes were enucleated for TUNEL staining (In situ Cell Death Detection Kit, Fluorescein; Roche) to detect cell apoptosis.3 The tissue was then fixed with 10% formaldehyde for 24 h. The retinal sections were treated with proteinase K (25 μg/mL), and incubated in H2O2/methanol for 5 min at room temperature to inactivate endogenous peroxidases. Corresponding negative (without dUTP) and positive control [3 U/mL deoxyribonuclease (DNase) I-treated] sections were also measured. After rinsing with Tris-buffered saline, the samples were incubated in a terminal deoxynucleotidyl-transferase (TdT) enzyme/labeling reaction mix at 37°C for 90 min. This reaction was based on the binding of digoxigenin-dUTP to the 3′-OH end of DNA by TdT, followed by incubation with an anti-digoxigenin antibody conjugated with peroxidase. Following termination of the labeling reaction in a stop buffer, the sections were processed in a standard streptavidin-horseradish peroxidase (HRP) reaction with 3,3′ diaminobenzidine as the chromogenic peroxidase substrate, and also counterstained with methyl green. Assessment of the sections was undertaken at 40×magnification (Zeiss). Six microscopic fields from each eye, consisting of 3 adjacent areas on both sides of the optic nerve head (ONH; 1 mm away from ONH), were used to count the TUNEL-positive cells in the RGC layer. The average number of TUNEL-positive cells per field was used for the analysis.3
Western blotting analysis
At 24 h after retinal ischemia and pretreatment with the defined chemicals or a sham operation, the rats were killed. The retinas were removed and sonicated in the lysis buffer, namely, the mammalian protein extraction reagent (Pierce). Equal amounts of denatured protein (30 μg/20 μL/well) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad) using 12% separating and 5% stacking gels containing 0.1% SDS.16 The separated proteins were transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech.).16 The membranes were then blocked for 1 h at 4°C with 5% fat-free skimmed milk in Tris-buffered saline. Next, the blots were incubated individually overnight at 4°C with one of the following 3 primary antibodies, namely, the rabbit monoclonal anti-Bcl-2 antibody (1:1,000; Cell Signaling Technology), the mouse monoclonal antibody [6A7] to Bax (ab5714) (1:1,000; Abcam, Inc.), or the mouse monoclonal [AC-15] anti-β-actin antibody (1:5,000; Abcam, Inc.). The blots were then incubated in the secondary antibody, either the HRP-conjugated goat anti-rabbit or anti-mouse IgG (1:5,000, 1:2,000, or 1:25,000; Amersham) as appropriate at 37°C for 1 h. The primary/secondary antibodies were diluted in 1% fat-free skimmed milk. Finally, the membranes were developed using the enhanced chemiluminescent analysis system (SuperSignal West Pico Chemiluminescent Substrate; Pierce), and exposed to an X-ray film. Scanning densitometry was used to analyze the amount of each protein present.
ROS assay
The assay is as described elsewhere.15 This involves the use of 2′,7′-dichlorofluorescein diacetate (DCFH-DA), which is a stable nonfluorescent molecule that readily crosses cell membranes and is hydrolyzed by intracellular esterases to nonfluorescent 2′,7′-dichlorofluorescein (DCFH). DCFH is then rapidly oxidized in the presence of ROS (e.g., H2O2) to highly fluorescent 2′,7′-dichlorofluorescein (DCF).
The rat retinas were dissociated enzymatically by incubating retinas in the physiological saline Hank's balanced salt solution (HBSS; Gibco, Paisley) containing 4 mg/mL dispase (2 retinas/2 mL) at 37°C for 10 min. Retinal tissues were dissociated mechanically by repetitive pipetting with fire-molded Pasteur pipettes. Cellular debris and undissociated tissues were removed until a homogenous suspension was obtained. The cells were washed with the HBSS medium and centrifuged at 3,000 g at 4°C for 5 min. The pellet was suspended in fresh HBSS containing 40 μM DCFH-DA (added from a stock solution of 20 mM DCFH-DA in ethanol) at 37°C for 30 min. After loading with DCFH-DA, the cellular suspension was centrifuged at 3,000 g at 4°C for 5 min. The pellet was resuspended in fresh HBSS and divided in 50-μL aliquots in each well (104/well) of a 96-well plate and incubated at 37°C for 1 h. ROS formation was stimulated by 100 μL of FeSO4 5 μM combined with ascorbate 100 μM for 1 h at 37°C; untreated DCFH-DA-loaded cells incubated in HBSS were defined as control. In some instances, 50 μL of various concentrations of baicalein (final concentration: 100 μM) or trolox (final concentration: 100 μM or 1 mM), or vehicle (1% ethanol) were added 15 min before an oxidative stress. The fluorescence was measured at intervals of 0 (before stimulant addition) or 30 min after stimulation using a Hitachi F-4500 spectrofluorometer (with excitation and emission wavelengths 488 nm and 535 nm, respectively, and band widths 5 nm). About 20 μL from each fraction was taken for protein determination by the bicinchoninic acid assay. The instrument was adjusted to zero with 200 μL HBSS. The results were expressed in nmol DCF/hour/mg of protein and analytically expressed as optical intensity relative to the control group (normalized to 100%).
Assessment of the levels of various retinal mRNAs by real-time polymerase chain reaction
The levels of Thy-1, HIF-1α, VEGF, MMP-9, and HO-1 mRNAs present in the retinas were determined using a real-time polymerase chain reaction (PCR) technique.1 Twenty four hours after retinal ischemia and pretreatment with the defined chemicals or a sham operation, the rats were killed and the retinas were removed. This was followed by sonication in TriReagent (Sigma Chemical). Total retinal RNA was isolated and first-strand complementary DNA (cDNA) synthesis was performed on 2 μg RQ1 RNase-Free DNase (0.05 U/μL; Promega)-treated RNA using High Capacity RNA-to-cDNA Master Mix. The first-strand cDNA then underwent real-time PCR using Fast SYBR Green Master Mix. The PCR was initiated by incubation at 95°C for 20 s; then, 40 cycles of 95°C for 3 s and 60°C for 30 s were performed. Cycling was carried out on a StepOnePlus™ Real-Time PCR System. Relative quantification (a comparative method) was performed using the housekeeping gene β-actin as the internal standard. This process allows the normalized quantification of the target mRNA (Ct) and takes into account the differences in the amount of total RNA added to each reaction (ΔCt=Cttarget − Ctβ-actin; cycle threshold, Ct). The relative Thy-1/HIF-1α/VEGF/MMP-9/HO-1 expression changes induced by ischemia or sham procedure were calculated as fold changes relative to the control normal retina with respect to the calibrator (ΔΔCt=ΔCtinduced − ΔCtnormal), which was represented by the control retina. Relative quantification of gene expression was calculated as fold changes according to the 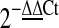 method, as described in the manufacturer's instructions, and was carried out by the accompanying software (RQ, ver. 1.3). The PCR reagents, software, and machine were purchased from AB Applied Biosystems. The data obtained for each treatment were pooled, and a total percentage change relative to the control was calculated. The PCR oligonucleotide primers were obtained from MISSION BIOTECH as follows:
method, as described in the manufacturer's instructions, and was carried out by the accompanying software (RQ, ver. 1.3). The PCR reagents, software, and machine were purchased from AB Applied Biosystems. The data obtained for each treatment were pooled, and a total percentage change relative to the control was calculated. The PCR oligonucleotide primers were obtained from MISSION BIOTECH as follows:
β-actin forward primer 5′-GAACCGCTCATTGCCGATAGTG-3′;
reverse primer 5′-TTGTCCCTGTATGCCTCTGGTCG-3′;
Thy-1 forward primer 5′-ACCAAGGATGAGGGCGACTA-3′;
reverse primer, 5′-CAGGCTTATGCCACCACACTT-3′;
HIF-1α forward primer: 5′-ACAGCTCCCCAGCATTTCAC-3′;
reverse primer: 5′-GGACAAACTCCCTCACCAAAAA-3′;
MMP-9 forward primer 5′-TGCGCTGGGCTTAGATCATT -3′;
reverse primer 5′-TGGATGCCTTTTATGTCGTCTTC-3′;
VEGF forward primer: 5′-GCGGGCTGCTGCAATG-3′;
reverse primer: 5′-TGCAACGCGAGTCTGTGTTT-3′;
HO-1 forward primer 5′-CAGGTGTCCAGAGAAGGCTTT-3′;
reverse primer 5′-TCTTCCAGGGCCGTGTAGAT-3′;
Statistical analysis
One-way analysis of variance (ANOVA) was performed to compare 3 or more independent groups. Following the one-way ANOVA, the Tukey multiple-comparison test1 was utilized to compare the control (such as vehicle-treated ischemic retinas) with all other groups (such as baicalein-treated ischemic retinas). A value of P<0.05 was considered significant.
Results
The effect of baicalein on b-wave amplitude
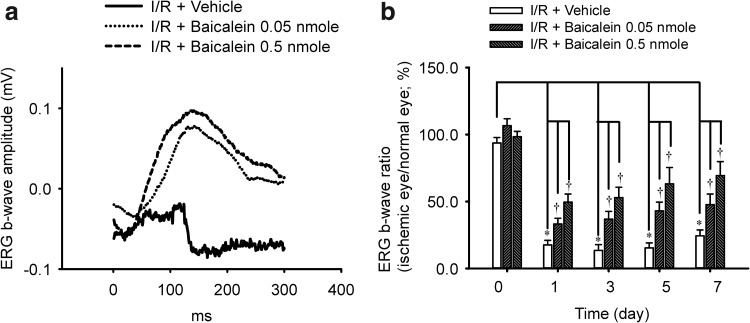
As shown in Fig. 1, in the sham operation eye (sham; Fig. 1a), the ERG b-wave amplitude was measured at 0.12 mV. After induction of retinal ischemia and reperfusion for 1 day, the b-wave amplitude was drastically reduced. This reduction was not affected by pretreatment with vehicle (I/R+Vehicle: 0.04 mV; Fig. 1a). However, pretreatment with baicalein (I/R+baicalein 0.05 nmol; I/R+baicalein 0.5 nmol; Fig. 1a) dose dependently attenuated the ischemia-induced reduction in b-wave amplitude resulting in values of 0.12 and 0.16 mV, respectively, 1 day after I/R. In Fig. 1b (n=7), when compared to the preischemia b-wave ratio (baseline, day 0: 94%±4%), at 1, 3, 5, and 7 days after I/R and pretreatment with vehicle (I/R+Vehicle), there is a significant (P<0.05) b-wave ratio reduction (day 1: 18%±3%; day 3: 13%±4%; day 5: 15%±4%; day 7: 24%±4%). However, after pretreatment with baicalein (I/R+baicalein), there was a concentration-dependent (0.05 vs. 0.5 nmol) and significant (P<0.05; at 0.05 and 0.5 nmol) improvement in the ischemia-reduced b-wave ratio on day 1 (33%±4% vs. 50%±6%), day 3 (37%±6% vs. 53%±8%), day 5 (43%±7% vs. 63%±12%), and day 7 (48%±8% vs. 69%±10%) after I/R. In this context, the preischemia b-wave ratio (day 0) was found to be 107%±5% versus 98%±4%. When the ERG b-wave ratios of the sham operation eye versus the fellow normal eye were compared [n=4; presham operation (day 0): 97%±4%; postoperation day 1: 99%±7%; day 3: 102%±10%; day 5: 93%±6%; day 7: 108%±8%], there was no significant difference between the ERG b-wave ratio before the sham operation (day 0) and that on day 1, 3, 5, or 7 after the sham operation.
FIG. 1.
Electroretinogram (ERG). (a) The b-wave amplitude was drastically decreased after ischemia/reperfusion (I/R) and pretreatment with vehicle in 1 rat from the I/R+vehicle group. This decrease was dose dependently attenuated by pretreatment with baicalein in 1 rat from the I/R+baicalein 0.05/0.5 nmol group. (b) As compared to the preischemic b-wave ratio (0 day), there was a significant reduction (*P<0.05) in the vehicle-pretreated ischemic retina at 1, 3, 5, and 7 days after I/R. This significant reduction was dose dependently and significantly alleviated by pretreatment with baicalein (†P<0.05). The results are the mean±SEM (n=7).
The effect of baicalein on ChAT immunoreactivity
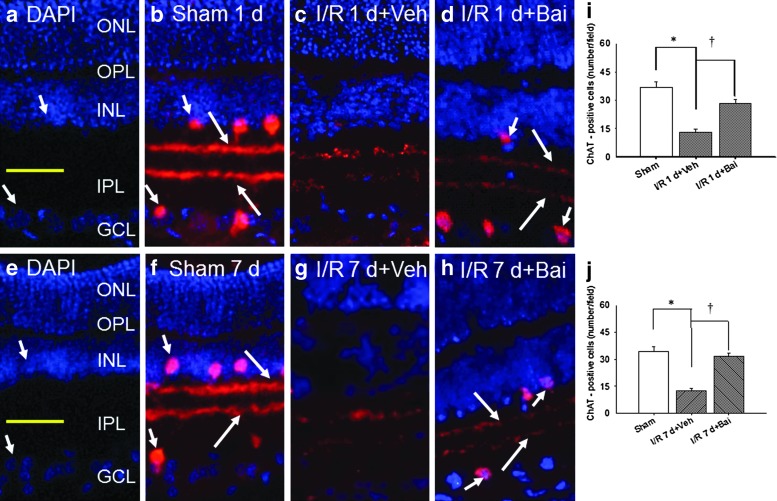
As shown in Fig. 2, at 1 and 7 days after the operation, when the sham operation retinas (control: Fig 2b or i and for j) were examined, ChAT immunoreactivity (red) was found to be associated with the amacrine cell bodies [short arrows; sham 1 day (Fig. 2b, i: 36.83±2.72); sham 7 days (Fig. 2f, j: 34.33±2.69/field); n=6], which are found in the inner nuclear layer (INL) and the ganglion cell layer (GCL); furthermore, the neuronal processes can be seen to have a 2-band pattern in the inner plexiform layer (IPL; long arrows). In the ischemic retinas that were pretreated with vehicle, the immunolabeling of the amacrine cell bodies [I/R 1d+Veh (Fig. 2c, i: 13.17±1.67); I/R 7d+Veh (Fig. 2g, j: 12.67±1.15/field); n=6] was obviously or significantly (P<0.05) decreased at 1 and 7 days after I/R; furthermore, labeling in the IPL was hardly visible. Importantly, this effect of I/R was obviously or significantly (P<0.05) counteracted by pretreatment with 0.5 nmol baicalein [I/R 1d+Bai (Fig. 2d, i: 28.50±1.88); I/R 7d+Bai (Fig. 2h, j: 31.67±1.58); n=6] and the results after treatment with baicalein show retinas that are quite similar to those of the control retinas.
FIG. 2.
ChAT immunoreactivity. In the sham operation retina (sham 1d, b; sham 7d, f), ChAT immunoreactivity (red) is associated with amacrine perikarya (short arrows) in the INL and the GCL as well as their neuronal processes in the IPL, which can be seen as 2 clearly defined strata of immunoreactivity (long arrows). I/R caused an almost complete obliteration of the ChAT immunoreactivity in the IPL and the number of ChAT immunopositive amacrine cell bodies was drastically reduced; the ischemia-induced alterations were not affected by pretreatment with vehicle (I/R 1 d+Veh, c; I/R 7 d+Veh, g). However, the effect of I/R was obviously nullified by pretreatment with 0.5 nmol baicalein (I/R 1 d+Bai, d; I/R 7 d+Bai, h). DAPI (blue) was used to counterstain the nuclei in the sham operation retina (a, e). The merge images of ChAT and DAPI labeling are shown in pictures (b–d) and (f–h). Each bar represents the mean±SEM (n=6) at 1 (i) and 7 days after the operation (j). */†, respectively, represents significant differences (P<0.05) between the vehicle-pretreated ischemic group and the sham operation group and between the vehicle-pretreated ischemic group and the baicalein-pretreated ischemic group. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; DAPI, 4,6-diamidine-2-phenylindole dihydrochloride; ChAT, choline acetyltransferase. Scale bar=25 μm. Sham, sham-operation eye; sham 1d, sham 1 day; sham 7d, sham 7 days.
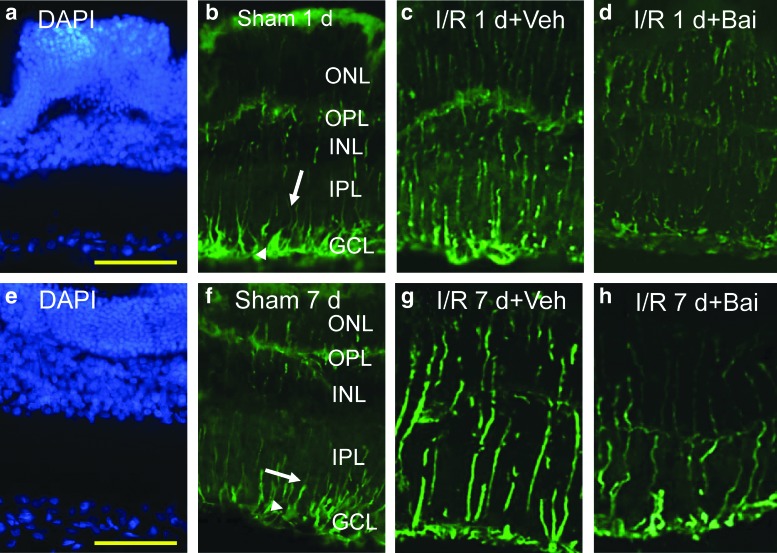
The effect of baicalein on vimentin immunoreactivity
As shown in Fig. 3, in the sham operation retina at 1 and 7 days after the operation (control; sham 1 day, Fig. 3b; sham 7 days, Fig. 3f), the vimentin immunolabeled Müller cell processes can be seen to extend from the end foot region into the IPL as well as into the INL and into the outer nuclear layer. Immunoreactivity was increased after I/R and pretreatment with vehicle (I/R 1d+Veh, Fig. 3c; I/R 7d+Veh, Fig. 3g). However, importantly, the ischemic effect was attenuated by pretreatment with 0.5 nmol baicalein (I/R 1d+Bai, Fig. 3d; I/R 7d+Bai, Fig. 3h) and the results for the retinas after treatment with baicalein are quite similar to those of the control retinas.
FIG. 3.
Vimentin immunoreactivity. At 1 and 7 days after the sham operation (sham 1d, b; Sham 7d, f), anti-vimentin (green) immunolabeled Müller cell end feet (arrow heads) as well as processes are visible in the IPL (arrows), INL, and ONL. As compared to the sham operation retina (b, f), anti-vimentin immunolabeling was increased after I/R and pretreatment with vehicle (I/R 1 d+Veh, c; I/R 7 d+Veh, g). This increase was counteracted at 1 and 7 days after I/R by pretreatment with 0.5 nmol baicalein (I/R 1 d+Bai, d; I/R 7 d+Bai, h). DAPI (blue) was used to counterstain the nuclei in the sham operation retina (a, e). Scale bar=25 μm. Color images available online at www.liebertpub.com/jop
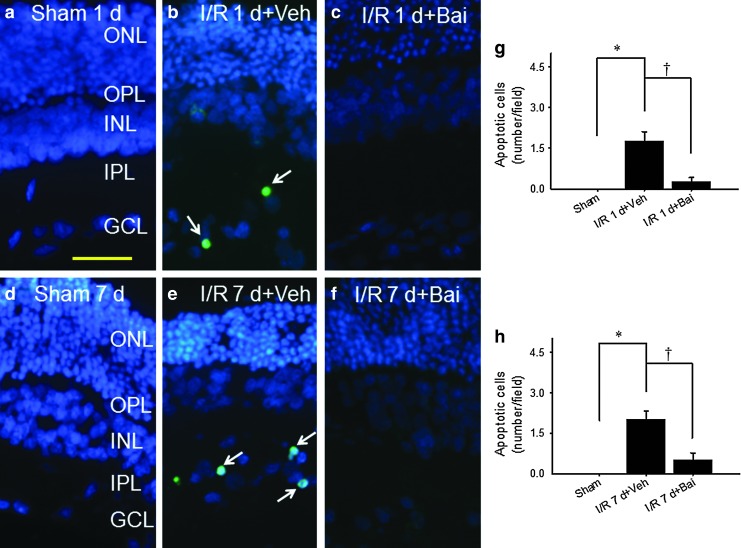
The effect of baicalein on the presence of apoptotic cells in the RGC layer
As shown in Fig. 4, when compared with the sham operation group (Fig. 4a, g; d, h: no TUNEL-positive cells; n=8), at 1 or 7 days following ischemia and pretreatment with vehicle, there was a significant increase (Fig. 4b, g: 1.75±0.37; Fig. 4e, h: 2.00±0.33 cells/field; n=8) in TUNEL-positive cells in the RGC layer. This increase was attenuated by pretreatment with 0.5 nmol baicalein (Fig. 4c, g: 0.25±0.16; Fig. 4f, h: 0.50±0.27 cells/field; n=8).
FIG. 4.
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL). No TUNEL-positive cell was observed at 1 and 7 days after the sham operation (sham 1d, a; Sham 7d, d). After ischemia and pretreatment with vehicle (I/R 1 d+Veh, b; I/R 7 d+Veh, e) or 0.5 nmol baicalein (I/R 1 d+Bai, c; I/R 7 d+Bai, f), the number of apoptotic cells (arrows; green) was, respectively, obviously increased relative to the sham operation control or decreased in the retinal ganglion cell layer relative to the ischemia and pretreatment with vehicle control. The merged images for TUNEL and DAPI (blue) labeling are shown (a–f). Each bar represents the mean±SEM (n=8) at 1 (g) and 7 days after the operation (h). */†, respectively, represents significant differences (P<0.05) between the vehicle-pretreated ischemic group and the sham operation group and between the vehicle-pretreated ischemic group and the baicalein-pretreated ischemic group. Scale bars=25 μm.
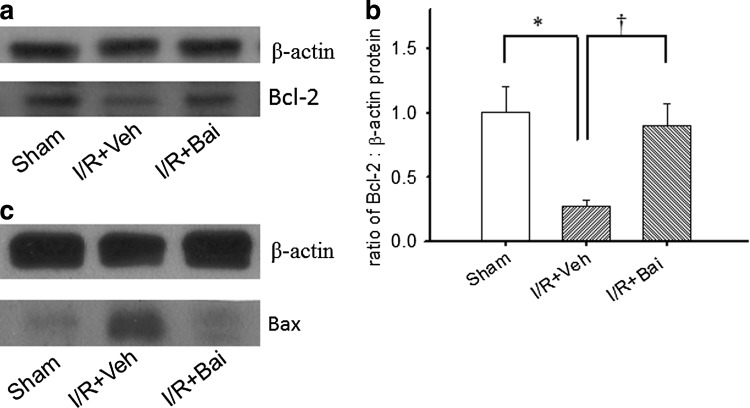
Measurement of the levels of Bcl-2 in the rat retina by Western blotting
As shown in Fig. 5, as compared with the sham operation group (sham; Fig. 5a, b: band volume of Bcl-2/β-actin as 1.00±0.20; n=3), ischemia and pretreatment with vehicle significantly (P<0.05) decreased Bcl-2 levels (I/R+Veh; Fig. 5a, b: 0.28±0.05; n=3); however, this downregulation was significantly (P<0.05) attenuated by pretreatment with 0.05 nmol baicalein (I/R+Bai; Fig. 5a, b: 0.90±0.17; n=3).
FIG. 5.
Western blotting. Using the lysed retinal cells, the antibodies against Bcl-2 (a), Bax (c), and β-actin are detected as 26-kDa, 20–22 kDa, and 42-kDa bands, respectively. The blot is a representative 1 of 3 independent experiments. Each bar represents the ratio of Bcl-2 to β-actin (b). */†, respectively, represents significant differences (P<0.05) between the vehicle-pretreated ischemic group and the sham operation group and between the vehicle-pretreated ischemic group and the 0.5 nmol baicalein-pretreated ischemic group. The results are mean±SEM (n=3).
Additionally (Fig. 5c), when compared with the sham operation group (control: band volume of Bax/β-actin as 1.00±0.54; n=3), ischemia and pretreatment with vehicle obviously 2-fold increased Bax levels (2.05±0.48; n=3); however, this upregulation was 2-fold attenuated by pretreatment with 0.05 nmol baicalein (1.05±0.60; n=3).
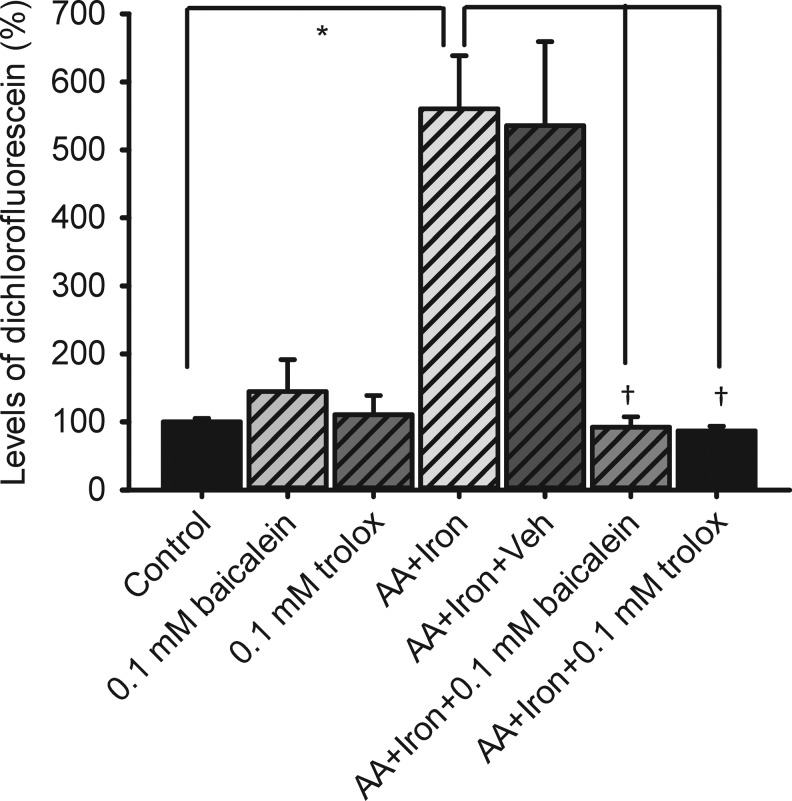
Measurement of the ROS levels
As shown in Fig. 6, in the present study, as compared to dissociated retinal cells incubated with HBSS (control: 14,380.61±733.17 DCF/hour/μg protein as 100%±5.29%; n=5), an hour of incubation of the dissociated retinal cells with 100 μM ascorbate/5 μM iron sulfate significantly (P<0.05) stimulated the production of ROS (560.42%±78.22%; n=5). Furthermore, the present results demonstrated that pretreatment with 0.5 nmol 100 μM trolox (86.58%±6.98%; n=5) or baicalein (92.18%±15.26%; n=5) significantly (P<0.05) blunted the ascorbate/iron-induced significant production of ROS, which was not affected by pretreatment with vehicle (535.67%±123.79%; n=3). A significant effect on reducing ascorbate/iron-induced significant production of ROS had been also created by 1 mM trolox (40.99%±9.13%; n=3 not shown in Fig. 6). Trolox (110.59%±28.13%; n=3) or baicalein (144.73%±46.81%; n=3) alone did not significantly increase the DCF level.
FIG. 6.
Production of reactive oxygen species quantified as the amount of DCF formed in dissociated rat retinal cells. Oxidative stress was induced by a combined treatment of 100 (M ascorbate and 5 (M FeSO4 (iron). */†, respectively, represents a significant difference (P<0.05) between the oxidative stress group and the control group (untreated DCFH-DA-loaded cells in the Hank's balanced salt solution only), or between the oxidative stress group and the 0.1 mM baicalein-/trolox-pretreated oxidative stress group. DCFH-DA, 2′,7′-dichlorofluorescein diacetate.
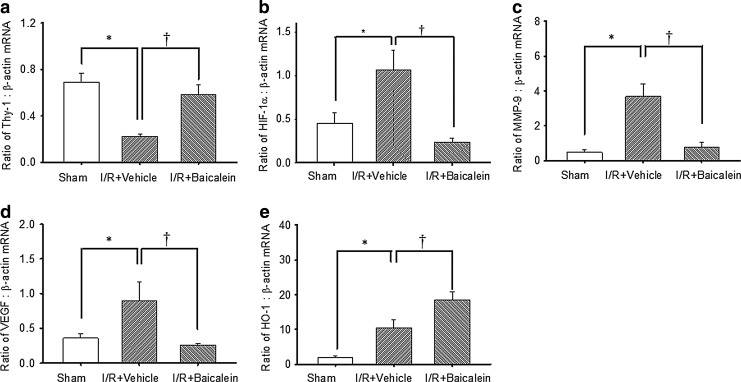
The effect of baicalein on the retinal mRNA levels of Thy-1, HIF-1α, MMP-9, VEGF, or HO-1
As shown in Fig. 7 (n=6), as compared to the control retina [Thy-1 (0.69±0.08; Fig. 7a), HIF-1α (0.45±0.12; Fig. 7b), MMP-9 (0.46±0.15; Fig. 7c), VEGF (0.36±0.07; Fig. 7d), and HO-1 (1.90±0.58; Fig. 7e)], the ratios for Thy-1 (0.22±0.02; Fig. 7a), HIF-1α (1.07±0.22; Fig. 7b), MMP-9 (3.68±0.72; Fig. 7c), VEGF (0.90±0.28; Fig. 7d), and HO-1 (10.41±2.50; Fig. 7e) in the vehicle-pretreated ischemic retina were significantly (P<0.05) changed after I/R. When compared with the vehicle-pretreated ischemic retina, the ischemic retina pretreated with baicalein (0.5 nmol) showed a significant (P<0.05) attenuation in the upregulation of HIF-1α (0.23±0.05; Fig. 7b), MMP-9 (0.78±0.25; Fig. 7c), and VEGF (0.25±0.03; Fig. 7d) as well as a significant (P<0.05) attenuation in the downregulation of Thy-1 (0.58±0.09; Fig. 7a). This was complemented by a further significant upregulation of HO-1 (18.54±2.27; Fig. 7e).
FIG. 7.
Real-time polymerase chain reaction analysis of the expression of Thy-1 (a), HIF-1α (b), MMP-9 (c), VEGF (d), HO-1 (e), and β-actin. Twenty four hours after ischemia plus reperfusion (I/R), whole retinal extracts were isolated from the sham operation eyes (control), the ischemic eyes with preadministration of vehicle (1% ethanol), or the ischemic eyes with preadministration of baicalein (0.5 nmol). The effect of the treatments on the ratio of Thy-1/HIF-1α/MMP-9/VEGF/HO-1: β-actin mRNA was determined compared to a control, namely, β-actin, a housekeeping gene. */†, respectively, represent significant differences (P<0.05) between the vehicle-pretreated ischemic group and the sham operation group and significant differences (P<0.05) between the vehicle-pretreated ischemic group and the baicalein-pretreated ischemic group, respectively. The abbreviations are HIF-1α, hypoxia-inducible factor-1α; MMP-9, matrix metalloproteinase-9; VEGF, vascular endothelium growth factor; HO-1, heme oxygenase-1. The results are the mean±SEM (n=6).
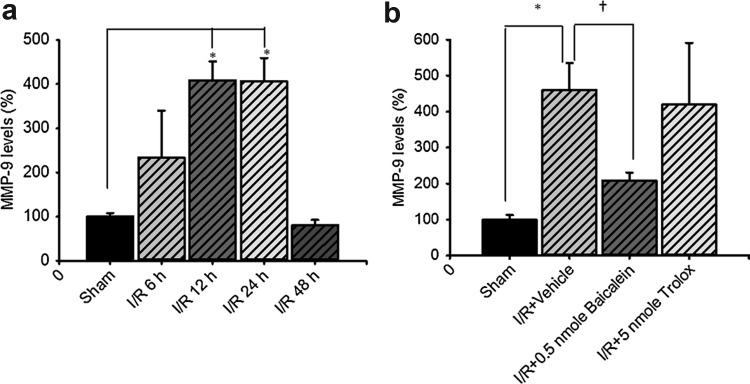
Assessment of retinal MMP-9 mRNA levels
As shown in Fig. 8, the real-time PCR quantitative analysis (a; n=3) showed that when the ratio of MMP-9 mRNA to β-actin mRNA was compared between the sham-operation group (100.01%±7.71%) and the ischemic retina subjected to 60-min ischemia plus various periods of reperfusion; MMP-9 mRNA was gradually upregulated and then downregulated in 60-min ischemia plus 6 h (233.21%±106.28%), 12 h (408.44%±42.62%), 24 h (406.76%±52.51%), and then 48 h of reperfusion (80.21%±12.27%). Moreover, there is a significant difference (p<0.05) between the sham operation group and the 12- or 24-h reperfusion group.
FIG. 8.
(a) Time-dependent levels of MMP-9 mRNA in the rat retina subjected to sham operation or high intraocular pressure-induced retinal ischemia plus various periods of reperfusion (n=3). * represents a significant difference (P<0.05) between the sham operation group and the 12- or 24-h reperfusion group. (b) The effect of preadministered baicalein (0.5 nmol) or trolox (5 nmol) on the levels of MMP-9 mRNA in the retina subjected to ischemia plus 24 h of reperfusion (n=4). */†, respectively, represents a significant difference (P<0.05) between the sham operation group and the vehicle-pretreated ischemic group, as well as the vehicle-pretreated ischemic group and the baicalein-/trolox-pretreated ischemic group.
Furthermore (b; n=4), the inhibitory effects of pretreated 100 μM 0.5 nmol baicalein (208.79%±22.12%) significantly inhibited the significant upregulation of the ratio of MMP-9/β-actin mRNA (460.30%±75.02%) induced by ischemia plus 24 h of reperfusion. In contrast, 100 μM 5 nmol trolox (419.93%±171.06%) only to a relatively small extent inhibited this significant upregulation. The ratio of MMP-9 mRNA to β-actin mRNA was also measured in the sham operation group (100.00%±12.69%). As mentioned in the ROS assay results, 1 mM trolox was presently used because it had around a 2-fold stronger antioxidative effect compared with 100 μM trolox or baicalein.
Discussion
Vimentin immunoreactivity has been shown to be enhanced in Müller cells after I/R4 and this is confirmed after I/R and pretreatment with vehicle in the present study (Fig. 3c, g). The dysfunctional Müller cells will have an influence on the normal b-wave1,2 and such an effect is also found in the present result (Fig. 1). When the ischemic retinas were pretreated with vehicle, the increase in vimentin immunoreactivity (Fig. 3c, g) parallels the reduction in b-wave (Fig. 1). Of clinical importance, we found that these ischemia-induced alterations were ameliorated by pretreatment with 0.5 nmol baicalein (Figs. 1 and 3).
It is widely acknowledged that RGC death plays a major role in retinal ischemia or experimental glaucoma.1,2 Since the inner retina is mainly injured during retinal ischemia, both RGCs and neighboring cells, namely, amacrine cells, are directly or indirectly damaged.1,2 Amacrine cells are neurons that use various neurotransmitters and make lateral synaptic contacts onto other amacrine cells as well as with RGCs and bipolar cells. Cholinergic amacrine cells use acetylcholine as a neurotransmitter and these are localized in the INL and the GCL. In the present study, we have found the number of cholinergic amacrine cells was reduced by retinal ischemia and pretreatment with vehicle (Fig. 2c or i, g or j). The same observations in relation to the ChAT immunoreactivity reduction have been described previously for the ischemic rat retina.1,2 It has been previously reported that functionality of the RGCs is compromised when there is a loss of cholinergic signaling in a laser ablated retina.17 In this study of retinal ischemia, we have also clearly shown that both RGCs and amacrine cells are affected and these findings are based on the Thy-1 mRNA (Fig. 7a) and ChAT immunoreactivity results (Fig. 2). Consistent with the results of Dong et al.,18 it would seem that highly selectively synaptic connections between starburst amacrine cells and direction-selective ganglion cells are necessary for functionality. Importantly, our results also show that the ischemia induced decrease in ChAT immunoreactivity and Thy-1 mRNA was obviously and significantly attenuated by pretreatment with baicalein (Figs. 2d or i, h or j, and 7a).
As compared to the sham operation retinas (Figs. 7a and 4a or g, d or h), after I/R, the ischemic retinas pretreated with vehicle showed significantly fewer RGCs (indexed by Thy-1 mRNA; Fig. 7a) together with more apoptotic cells in the RGC layer (Fig. 4b or g, e or h). These findings should be compared to retinas treated with 0.5 nmol baicalein, which showed significantly more RGCs (Fig. 7a) and significantly fewer apoptotic cells in the RGC layer (Fig. 4c or g, f or h). Thus, the protective effect of baicalein on retinal cells such as RGCs appears to be related, at least in part, to a modulation of the apoptotic pathway. This is further supported by the finding that pretreatment of the ischemic retina with baicalein significantly induced overexpression of the Bcl-2 protein that acts as a brake on apoptosis (Fig. 5a or b) and, though not significantly induced, did also seem to induce downregulation of the proapoptotic protein Bax (Fig. 5c). As shown by a recent study,19 baicalein administration to diabetic rats ameliorated diabetes-induced Müller cell GFAP upregulation, RGC loss, and VEGF overexpression within the ischemic retina. This is consistent with the present results. Baicaein would seem to protect against retinal ischemic injury by attenuating Müller cell vimentin upregulation (Fig. 3d, h), by decreasing the number of apoptotic cells in the RGC layer (Fig. 4c or g, f or h) and by downregulating VEGF expression (Fig. 7d).
Recently, the antioxidant baicalein has been found to exert significant neuroprotective effects against oxidative stress in vitro, specifically, when hRPEs are pretreated with baicalein, and then challenged with H2O2 for 24 h.10 As shown by the present DCF assay results (Fig. 6), the protective effect of baicalein on ischemia-injured sensory retinal cells such as RGCs and amacrine cells (Figs. 2, 4, and 7) would also, at least in part, seem to be associated with its antioxidative effect like that of trolox.
The MMP-9 mRNA level was significantly upregulated following 60-min ischemia plus 24 h of reperfusion (Fig. 8a). This significant MMP-9 mRNA upregulation was significantly attenuated by pretreated baicalein; however, the strong antioxidant trolox only attenuated the MMP-9 mRNA upregulation to an obviously lesser degree than baicalein. Both these changes contrast with the fact that this upregulation was not affected by vehicle (figure 8b). Thus, the underlying mechanism of the inhibitory effect of baicalein on retinal ischemia-induced MMP-9 upregulation appears to be only “partially” related to its anti-oxidative effect. As mentioned, MMP-9 is upregulated in the ischemic retina;9 this upregulation has been further demonstrated to be induced by IL-1β and mitogen-activated protein kinases (MAPKs). Whether other factors, such as HIF-1α, IL-1β, or MAPKs, might play a major role in the inhibitory effect of baicalein on MMP-9 upregulation in the ischemic retina needs further investigation.
Ischemia/hypoxia has been proved to induce an overexpression of HIF-1α, VEGF, and MMP-9.6–10 In the present study, HIF-1α, MMP-9, and VEGF mRNA levels (Fig. 7b–d) were found to be significantly upregulated in the ischemic retinas. Furthermore, this significant upregulation was significantly attenuated by pretreatment with baicalein, but not by vehicle. This is further supported by a report of Al-Shabrawey et al.20 indicating that the lipo-oxygenase pathway inhibitor baicalein abrogated new vessel growth and downregulated VEGF expression in the ischemic or diabetic retina. Their results also indicate that baicalein might modulate the expression of HIF-1α and VEGF at the mRNA level.
HO-1 can exert a potent indirect antioxidative function by degrading heme to biliverdin, bilirubin, and CO.21 Moreover, these by-products have their own significance with essential cellular metabolism and further contribute to the suppression of oxidative stress. In this context, a higher expression of HO-1 in macular RPE has been detected in eyes with choroidal neovascularization suggesting that there is an increased level of oxidative stress,22 which is strongly related to ischemia.5 What is more, strategies boosting HO-1 expression, either by stimulation of endogenous production as preconditioning or by exogenous administration of chemical agents/gene transfer, have indicated the benefits of higher HO-1 expression in terms of protecting against retinal ischemia/reperfusion injury.23,24 The present results provide evidence that, after retinal ischemia and pretreatment with baicalein, the upregulation of HO-1 appears to be one of the mechanisms by which baicalein protects against retinal ischemic injury (Fig. 7e). In addition, retinal ischemia and neovascular AMD (nvAMD) are presumably associated with an upregulation of VEGF and MMP-9, which are the downstream mediators of an ischemia-related increase in HIF-1α.5–10 When taken together, we have shown that baicalein seems to have the potential to manage problematic retinal ischemia and nvAMD.
In conclusion, various types of ischemic changes in the rat retina can be observed by electrophysiology (b-wave), immunohistochemistry (ChAT immunoreactivity and vimentin immunoreactivity), histopathology (TUNEL-positive cells in the RGC layer), Western blotting (Bcl-2/Bax), and real-time PCR analysis (HIF-1α, VEGF, MMP-9, and HO-1 expression). Oxidative stress, an ischemic cascade, can be also detected by the present in vitro DCF assay. Clinically, it is important to note that all of these changes were found to be significantly affected by baicalein treatment before I/R or oxidative stress.
This study supports the hypothesis that baicalein is able to protect the retina against ischemia by an antioxidative effect as well as via an antiapoptotic effect; furthermore, baicalein also seems to upregulate HO-1 and to suppress the upregulation of HIF-1α, VEGF, and MMP-9.
Acknowledgments
The authors thank CHGH for the grant (101-46).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chen Y.Q. Pan W.H.T. Liu J.H., et al. The effects and underlying mechanisms of S-allyl L-cysteine treatment of the retina after ischemia/reperfusion. J. Ocul. Pharmacol. Ther. 2012;28:110–117. doi: 10.1089/jop.2011.0099. [DOI] [PubMed] [Google Scholar]
- 2.Osborne N.N. Casson R.J. Wood J.P.M., et al. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Peng P.H. Chao H.M. Juan S.H., et al. Pharmacological preconditioning by low dose cobalt protoporphyrin induces heme oxygenase-1 overexpression and alleviates retinal ischemia-reperfusion injury in rats. Curr. Eye Res. 2011;36:238–246. doi: 10.3109/02713683.2010.539760. [DOI] [PubMed] [Google Scholar]
- 4.Wurm A. Iandiev I. Uhlmann S., et al. Effects of ischemia-reperfusion on physiological properties of Müller glial cells in the porcine retina. Invest. Ophthalmol. Vis. Sci. 2011;52:3360–3367. doi: 10.1167/iovs.10-6901. [DOI] [PubMed] [Google Scholar]
- 5.Zarbin M.A. Current concepts in the pathogenesis of age related macular degeneration. Arch. Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 6.Håkansson G. Gesslein B. Gustafsson L.L., et al. Hypoxia-inducible factor and vascular endothelial growth factor in the neuroretina and retinal blood vessels after retinal ischemia. J. Ocul. Biol. Dis. Inform. 2010;3:20–29. doi: 10.1007/s12177-010-9050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aktas Z. Gurelik G. Göcün P.U., et al. Matrix metalloproteinase-9 expression in retinal ganglion cell layer and effect of topically applied brimonidine tartrate 0.2% therapy on this expression in an endothelin-1-induced optic nerve ischemia model. Int. Ophthalmol. 2010;30:253–259. doi: 10.1007/s10792-009-9316-9. [DOI] [PubMed] [Google Scholar]
- 8.Guo L. Moss S.E. Alexander R.A., et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X. Chintala S.K. Influence of interleukin-1 beta induction and mitogen-activated protein kinase phosphorylation on optic nerve ligation-induced matrix metalloproteinase-9 activation in the retina. Exp. Eye Res. 2004;78(4):849–60. doi: 10.1016/j.exer.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Liu J.H. Wann H. Chen M.M., et al. Baicalein significantly protects human retinal pigment epithelium cells against H2O2-induced oxidative stress by scavenging reactive oxygen species and down-regulating the expression of matrix metalloproteinase-9 and vascular endothelial growth factor. J. Ocul. Pharmacol. Ther. 2010;26:421–429. doi: 10.1089/jop.2010.0063. [DOI] [PubMed] [Google Scholar]
- 11.Dulak J. Deshane J. Jozkowicz A., et al. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang W.T. Shao Z.H. Yin J.J., et al. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur. J. Pharmacol. 2007;566:58–66. doi: 10.1016/j.ejphar.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wartenberg M. Budde P. De Marees M., et al. Inhibition of tumor-induced angiogenesis and matrix-metalloproteinase expression in confrontation cultures of embryoid bodies and tumor spheroids by plant ingredients used in traditional Chinese medicine. Lab. Invest. 2003;83:87–98. doi: 10.1097/01.lab.0000049348.51663.2f. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.H. Lee S.R. The effect of baicalein on hippocampal neuronal damage and metalloproteinase activity following transient global cerebral ischaemia. Phytother. Res. 2012;26:1614–1619. doi: 10.1002/ptr.4644. [DOI] [PubMed] [Google Scholar]
- 15.Chao H.M. Chidlow G. Melena J. Wood J.P.M. Osborne N.N, et al. An investigation into the potential mechanisms underlying the neuroprotective effect of clonidine in the retina. Brain Res. 2000;877:47–57. doi: 10.1016/s0006-8993(00)02592-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu J.H. Chen M.M. Huang J.W., et al. Therapeutic effects and mechanisms of action of mannitol during H2O2-induced oxidative stress in human retinal pigment epithelium cells. J. Ocul. Pharmacol. Ther. 2010;26:249–257. doi: 10.1089/jop.2009.0127. [DOI] [PubMed] [Google Scholar]
- 17.He S. Masland R.H. Retinal direction selectivity after targeted laser ablation of starburst amacrine cell. Nature. 1997;389:378–382. doi: 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- 18.Dong W. Sun W. Zhang Y., et al. Dendritic relationship between starburst amacrine cells and direction-selective ganglion cells in the rabbit retina. J. Physiol. 2004;556((Pt 1)):11–17. doi: 10.1113/jphysiol.2004.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L.P. Sun H.L. Wu L.M., et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2009;50:2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shabrawey M. Mussell R. Kahook K., et al. Increased expression and activity of 12-lipooxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implication in retinal neovascularization. Diabetes. 2011;60:614–624. doi: 10.2337/db10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J. Tan S. Liu F., et al. Heme oxygenase and ocular disease: a review of the literature. Curr. Eye Res. 2012;37:955–960. doi: 10.3109/02713683.2012.700753. [DOI] [PubMed] [Google Scholar]
- 22.Frank R.N. Amin R.H. Puklin J.E. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am. J. Ophthalmol. 1999;127:694–709. doi: 10.1016/s0002-9394(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 23.Sun M.H. Pang J.H. Chen S.L., et al. Retinal protection from acute glaucoma-induced ischemia-reperfusion injury through pharmacologic induction of heme oxygenase-1. Invest. Ophthalmol. Vis. Sci. 2010;51:4798–808. doi: 10.1167/iovs.09-4086. [DOI] [PubMed] [Google Scholar]
- 24.Liu X.Q. Wu B.J. Pan W.H.T., et al. Resveratrol mitigates rat retinal ischemic injury: roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J. Ocul. Pharmcol. Ther. 2013;29(1):33–40. doi: 10.1089/jop.2012.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]