Abstract
Kaposi’s sarcoma (KS) was first described in 1872 by Moritz Kaposi. In 1994, Chang et al. first identified DNA sequences corresponding to human herpesvirus-8 (HHV-8) in AIDS-associated Kaposi sarcoma biopsies. It is now believed that presence of HHV-8 is necessary but not sufficient to cause KS. Other factors like immunosuppressive therapy also play a role. We describe an HIV-negative elderly patient who developed KS of skin and mucous membrane after prolonged use of corticosteroids for knee pain. The patient was positive for HHV-8.
Keywords: Kaposi sarcoma, corticosteroids, HHV-8, HIV/AIDS
Introduction
Kaposi’s sarcoma (KS) is one of the malignancies that is most seen in HIV-positive or immunocompromised patients. The use of immunosuppressant drugs is also associated with the risk of developing KS, especially in kidney transplant patients.1 Immunosuppressant drugs may also enhance the risk of KS in other patients. In 1981, Leung et al. described the first case of steroid induced KS in a temporal arteritis patient after 6 months of prednisolone therapy.2
Case description
A 65-year-old Kashmiri male from a rural area presented with a 1-year history of multiple violaceous skin lesions and dependant edema of lower extremities. The patient’s history included bilateral knee pain for which he had been receiving oral and injectable corticosteroid (in a dose varying from 5–40 mg/day) for the previous 8 years. The patient’s history included 3 episodes of melena one year prior to presentation and mild hypertension for the previous year, possibly steroid-induced. There was no history of fever, cough, chest pain, breathlessness, hemoptysis, stiffness or swelling of joints or convulsions. Physical examination revealed multiple small, discrete, violaceous patches, plaques and nodules distributed bilaterally over the lower and upper extremities with scattered lesions over the shoulder and buttocks (Figures 1, 2, 3). Some of the small papules and nodules had coalesced to form large plaques (Figure 2). Most of the lesions were non-scaly and non-blanching, and they did not bleed on pinprick. Some of the lesions were tender. Lymphedema was noted in the lower extremities and hands (Figure 3). Mucous membrane of the oral cavity showed characteristic patches on the hard and soft palate (Figure 4). Penile mucosa showed purple plaques, and anal mucosa showed two small pink patches on the left lateral wall. There was no lymphadenopathy or organomegaly.
Figure 1.
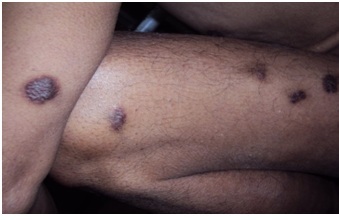
Discrete, violaceous lesions of Kaposi’s sarcoma on the arm and thigh.
Figure 2.
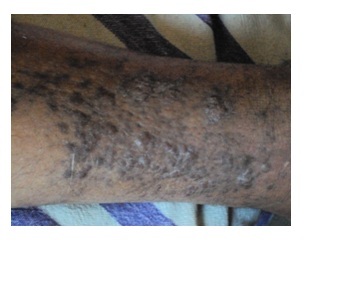
Lesions of Kaposi’s sarcoma on the lower limb, showing confluence.
Figure 3.
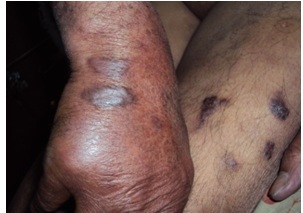
Violaceous nodules and plaques of Kaposi’s sarcoma. Note also edema of the hand.
Figure 4.
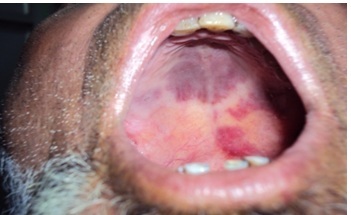
Characteristic patches of Kaposi’s sarcoma on the hard and soft palate.
Laboratory studies, including a complete blood cell count, urinalysis, liver function tests, kidney function tests, blood glucose, electrolytes and coagulogram, were within normal ranges. The serological tests for syphilis and human immunodeficiency virus (HIV) were negative or nonreactive. Tests for rheumatoid factor and antinuclear antibodies were also negative. Chest radiography, ultrasonography of abdomen and computed tomography (CT) of chest and abdomen were normal. Proctoscopy and colonoscopy showed 1° hemorrhoid at 12 o’clock position and two small pink patches at the lateral wall of anus.
Histopathologic examination of skin biopsy from one of the patches (Figure 5) showed neovascularization of the entire dermis with small irregular vascular channels intercommunicating and surrounding preexisting normal vascular channels. These neovascularised channels were of capillary nature with incomplete walls and showed extravasation of red blood cells. The vessels were seen dissecting the collagen bundles and adnexal structures. Section from a nodule (Figure 6) showed fascicles of spindle cells with interweaving free red cells. There were focal hemosiderin deposits and perivascular round cell inflammatory infiltrates. No mitotic figures were identified.
Figure 5.
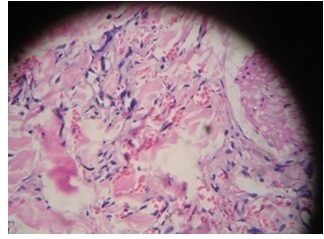
Micro-section from plaque shows dilated, irregular new vessels and pre-existing vessels in the dermis. Also seen are few scattered spindle cells (H & E× 400).
Figure 6.
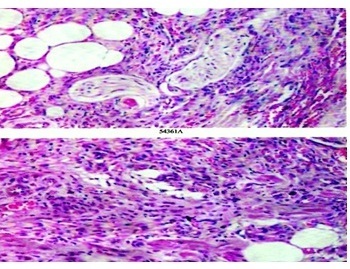
Micro-section from nodule shows fascicles of spindle cells in the dermis and superficial subcutis. Between the spindle cells are seen red blood cells in pseudo vascular channels. Spindle cells are seen encircling adipocytes and nerve fascicles (H & E×400).
On immunohistochemistry, the tumor cells were positive for CD31 (Figure 7) and CD34. HHV-8 stain showed weak granular positivity.
Figure 7.
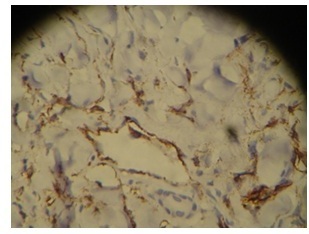
Immunohistochemistry. The section which is immune-stained for CD31 highlights tumor cells in the vessels and in the stroma.
After confirming the diagnosis of KS by biopsy and immunohistochemistry, the patient was treated with 7 courses of systemic chemotherapy of pegylated liposomal doxorubicin, 40 mg/m2, administered every 2 weeks over a 4-month period. There was a significant decrease in the edema of his legs and flattening of the KS lesions after the fourth course of chemotherapy. In addition, oral corticosteroids were stopped.
Discussion
Kaposi’s sarcoma (KS) typically involves the skin and manifests as violaceous lesions that enlarge from patches to plaques and nodules. Four types of KS are recognized: classic KS, HIV-related KS, African endemic KS and iatrogenic KS.3 Classic KS occurs in elderly men involving the skin of lower extremities and is indolent. Endemic KS occurs in Sub-Saharan Africa. Epidemic KS refers to KS in HIV-infected individuals. It is aggressive and involves the skin and gastrointestinal and respiratory tracts. Iatrogenic KS occurs in individuals who are immunosuppressed because of organ transplantation and tends to be aggressive.4,5
Excess use of immunosuppressive drugs (including corticosteroids) has also been associated with high prevalence of iatrogenic KS.6,7 Onset of the disease after administration of the triggering drug in previously reported studies ranged from less than one month to more than 20 years. The dose of corticosteroid ranged from 5 to 125 mg/day. There is no evident correlation between the development of KS and dose or duration of steroid therapy.7 In vitro evidence supports the hypothesis that glucocorticoids have a direct role in stimulating tumor development and growth. They cause upregulation of Kaposi cell proliferation and the activation of HHV-8.8
Chang et al. first reported a link between HHV-8 and KS in 1994.9 The virus was found in more than 90% of KS samples from HIV sero-positive patients, but it had low prevalence in healthy controls. HHV-8 DNA persists in endothelial cells and spindle cells of KS.10 Furthermore, HHV-8 virus alone is not sufficient to cause KS, but it may be an important cofactor in the development of disease.11 HHV-8 has been isolated from all types of KS lesions, including corticosteroid induced KS.12
Treatment modalities comprise local therapy for-example surgery, radiotherapy and local chemotherapy such as injections of vinca alkaloids or local immune therapy by interferon, 9 cis retinoic acid or imiquimod. Patients with extensive skin or mucosal lesions may need chemotherapeutic agents.13 Positive results have been found for pegylated liposomal doxorubicin, danaurabucin, paclitaxel and interferon-α.14 In patients with iatrogenic KS, immunosuppressive medication may be reduced or modified with the considerate possibility of grafts being rejected with insufficient immunosuppression.4 Although corticosteroids act to induce or trigger the disease, it is only in the case of the autoimmune diseases that KS resolves after suspension of treatment with corticosteroids.7
Our patient is an elderly male who had received oral steroids for the past 8 years in a dose varying from 5–40 mg per day. He developed typical KS lesions in the skin of lower extremities and other parts of his body. They also developed in the mucous membrane, which is a rare presentation. After stopping corticosteroids, our patient showed good response to the pegylated liposomal doxorubicin chemotherapy. To the best of our knowledge, this is the first case of steroid induced Kaposi’s sarcoma to be reported from Kashmir valley and adds one more case to the rare entity, steroid-induced Kaposi’s sarcoma in non-HIV patients with significant mucous membrane involvement.
Acknowledgments
The authors are grateful to DR Nirmala A Jambhekar, Professor and Head, Department of Pathology, Tata Memorial Hospital, Mumbai, India for doing immunohistochemistry on paraffin blocks of skin biopsy.
References
- 1.Bencini PL, Montagnino G, Tarantino A, et al. Kaposi’s sarcoma in kidney transplant recipients. Arch Dermatol. 1993;129:248–50. http://doi.org/fnj7tb. [PubMed] [Google Scholar]
- 2.Leung F, Fam AG, Osoba D. Kaposi’s sarcoma complicating corticosteroid therapy for temporal arteritis. Am J Med. 1981;71:320–2. doi: 10.1016/0002-9343(81)90135-2. http://doi.org/c6tgx3. [DOI] [PubMed] [Google Scholar]
- 3.Martin RW, 3rd, Hood AF, Farmer ER. Kaposi sarcoma. Medicine (Baltimore) 1993;72:245–61. doi: 10.1097/00005792-199307000-00004. http://doi.org/d9r4dh. [DOI] [PubMed] [Google Scholar]
- 4.Montagnino G, Bencini PL, Tarantino A, et al. Clinical features and course of Kaposi’s sarcoma in kidney transplant patients: report of 13 cases. Am J Nephrol. 1994;14:121–6. doi: 10.1159/000168700. http://dx.doi.org/10.1159/000168700. [DOI] [PubMed] [Google Scholar]
- 5.Abbasi F, Fardkhani SK, Sha’bani M. Steroid induced Kaposi’s sarcoma in an HIV negative patient. Iran J Dermatol. 2008;11:171–2. http://iranjd.ir/abstract.asp?articleID=3165. [Google Scholar]
- 6.Francès C. Kaposi’s sarcoma after renal transplantation. Nephrol Dial Transplant. 1998;13:2768–73. doi: 10.1093/ndt/13.11.2768. http://doi.org/dzfvpk. [DOI] [PubMed] [Google Scholar]
- 7.Trattner A, Hodak E, David M, et al. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer. 1993;72:1779–83. doi: 10.1002/1097-0142(19930901)72:5<1779::aid-cncr2820720543>3.0.co;2-m. http://doi.org/fn4bhq. [DOI] [PubMed] [Google Scholar]
- 8.Guo WX, Antakly T. AIDS-related Kaposi’s sarcoma: evidence for direct stimulatory effect of glucocorticoid on cell proliferation. Am J Pathol. 1995;146:727–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. http://doi.org/bzfgz2. [DOI] [PubMed] [Google Scholar]
- 10.Kemény L, Gyulai R, Kiss M, et al. Kaposi’s sarcoma associated herpesvirus/human herpesvirus-8: a new virus in human pathology. J Am Acad Dermatol. 1997;37:107–13. doi: 10.1016/s0190-9622(97)70220-2. http://doi.org/cr5wh9. [DOI] [PubMed] [Google Scholar]
- 11.Bursics A, Morvag K, Ábrahám K, et al. HHV-8 positive, HIV negative disseminated Kaposi’s sarcoma complicating steroid dependent ulcerative colitis: a successfully treated case. Gut. 2005;54:1049–50. doi: 10.1136/gut.2005.069500. http://doi.org/c2hbw6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rady PL, Hodak E, Yen A, et al. Detection of human herpesvirus-8 DNA in Kaposi’s sarcoma from iatrogenically immunosuppressed patients. J Am Acad Dermatol. 1998;38:429–37. doi: 10.1016/s0190-9622(98)70501-8. http://doi.org/bj6wr7. [DOI] [PubMed] [Google Scholar]
- 13.Toschi E, Sgadari C, Monini P, et al. Treatment of Kaposi’s sarcoma--an update. Anticancer Drugs. 2002;13:977–87. doi: 10.1097/00001813-200211000-00001. http://doi.org/dqbmfm. [DOI] [PubMed] [Google Scholar]
- 14.Di Lorenzo G, Kreuter A, Di Trollo R, et al. Activity and safety of pegylated liposomal doxorubicin as first-line therapy in the treatment of non-visceral classic Kaposi’s sarcoma: a multicenter study. J Invest Dermatol. 2008;128:1578–80. doi: 10.1038/sj.jid.5701215. http://doi.org/fpztsp. [DOI] [PubMed] [Google Scholar]


