Abstract
Persistently elevated oxidative stress and inflammation precede or occur during the development of type 1 or type 2 diabetes mellitus and precipitate devastating complications. Given the rapidly increasing incidence of diabetes mellitus and obesity in the space of a few decades, new genetic mutations are unlikely to be the cause, instead pointing to environmental initiators. A hallmark of contemporary culture is a preference for thermally processed foods, replete with pro-oxidant advanced glycation endproducts (AGEs). These molecules are appetite-increasing and, thus, efficient enhancers of overnutrition (which promotes obesity) and oxidant overload (which promotes inflammation). Studies of genetic and nongenetic animal models of diabetes mellitus suggest that suppression of host defenses, under sustained pressure from food-derived AGEs, may potentially shift homeostasis towards a higher basal level of oxidative stress, inflammation and injury of both insulin-producing and insulin-responsive cells. This sequence promotes both types of diabetes mellitus. Reducing basal oxidative stress by AGE restriction in mice, without energy or nutrient change, reinstates host defenses, alleviates inflammation, prevents diabetes mellitus, vascular and renal complications and extends normal lifespan. Studies in healthy humans and in those with diabetes mellitus show that consumption of high amounts of food-related AGEs is a determinant of insulin resistance and inflammation and that AGE restriction improves both. This Review focuses on AGEs as novel initiators of oxidative stress that precedes, rather than results from, diabetes mellitus. Therapeutic gains from AGE restriction constitute a paradigm shift.
Introduction
The incidence of type 2 diabetes mellitus (T2DM), and increasingly T1DM, continues to surge despite many therapeutic advances. Diabetes mellitus is now the leading cause of cardiovascular, renal and other serious comorbidities in old but also young adults.1–5 The pandemic proportions of the problem make it imperative that new etiologic factors and effective, low-cost therapeutic interventions are identified. The pathogenesis and demographics of diabetes mellitus are complex, but mounting evidence suggests that the environment, namely socioeconomic and behavioral etiologies, potentiates or even supersedes genetic susceptibility.6 Elevated oxidative stress appears to precede the development of both T1DM and T2DM and their sequelae, which indicates a weakening of host defense mechanisms over time.7 Hyperglycemia was long thought to be the single major cause of oxidative-stress-driven diabetic complications.8 However, once diabetes mellitus is established, strict control of hyperglycemia was beneficial for some comorbidities, such as retinopathy and nephropathy, but proved less effective against macrovascular disease, with a high mortality caused by macrovascular complications.8–11
Major antioxidant trials have yet to markedly reduce the incidence of diabetes mellitus or conditions that precede this disorder, such as obesity and the metabolic syndrome,12 which suggests that the presence of high pre-existing (or basal) oxidative stress may be underestimated in both severity and duration. This hypothesis is strongly supported by evidence that reveals a high prevalence of cardiometabolic risk factors clustered in individuals with a normal phenotype but with unexplained high oxidative stress and inflammation.13,14
A comprehensive search for new initiators of oxidative stress has led to re-evaluation of the environment and revealed a crucial link between a positive energy balance and deleterious outcomes, such as obesity and diabetes mellitus. Although the modern diet is thought to underlie both types of diabetes mellitus, as well as prediabetes and cardiovascular disease, the diabetogenic culprits within the diet remain a subject of debate.15–18 We have proposed that the modern (Western) nutritional environment, although it provides adequate energy, is replete with oxidants that promote an abnormal oxidative stress state.19–21 Advanced glycation endproducts (AGEs) and advanced lipoxidation endproducts (ALEs) represent a class of pro-oxidants in foods, the presence of which is promoted by food processing at high temperatures.22–27 A major factor that accounts for the widespread use of thermal food processing, aside from concerns on safety and storage, is that AGEs significantly enhance flavor, smell and appearance of foodstuffs.24,28 Thus, pro-oxidant AGEs also serve as ‘appetite-enhancing’ agents that simultaneously spur overnutrition, inflammation, obesity and diabetes mellitus.
In this Review, insights from studies of humans and mice are discussed with an emphasis on the effects of exogenous AGEs and the suppression of specific factors of host defense mechanisms. The loss of these defenses is proposed to be the driving force behind the increased oxidative stress and the pathogenesis of both T1DM and T2DM and their complications. New cell-protective liaisons between cellular AGE receptors (AGER1) and the NAD+-dependent deacetylase sirtuin 1 (SIRT1)—two components of a complex and powerful homeostasis system—are highlighted. An imbalance between host defenses and increased oxidant challenges from the environment may form the basis of the incipient cell injury that underlies diabetes mellitus. AGE restriction is introduced as a novel cost-efficient strategy in the prevention and treatment of the current diabetes epidemic.
Origin of AGEs and ALEs
AGEs are known to modulate a multitude of intracellular and extracellular structural and pro-oxidant effects, including inflammation, cell proliferation or apoptosis, as well as insulin signaling in organs as varied as islet β cells, blood vessels, kidneys and brain.16,17,29–31 AGEs are pro-oxidant metabolic derivatives of nonenzymatic reactions between reducing sugars and free amines of proteins, largely α-NH2 or ε-NH2 groups of proteins, as well as of aminolipids and nucleic acids.29,32–34 Extracellular and intracellular reactive carbonyl precursors, such as glyoxal, 3-deoxyglucosone or methylglyoxal derivatives,29 readily react with proteins or lipids to generate oxidants, namely Nε-carboxymethyllysine (CML), or crosslink-forming endproducts, for example, pentosidine.29,32,35,36 These processes permanently alter connective tissue proteins, plasma lipoproteins, cell membrane phospholipids or DNA.16,17,37–39 The chemical process of glycation, also known as Maillard reaction,40 is exquisitely sensitive to pH, temperature, hydration, type of sugar and acid or base buffering conditions; it is slow and strictly regulated in vivo. However, under super-physiological conditions, AGEs form at vastly accelerated rates, dependent on source (animal or plant), time and temperature applied.22,25,26,41–43
AGEs in nutrients are similar to those found in animal or human tissues and comprise mixtures of reactive and nonreactive AGEs that share biochemical, immunochemical and molecular properties.23,44,45 Reactive AGEs can promote the generation of new AGEs and reactive oxygen species (ROS), deplete antioxidant systems and elicit secretion of inflammatory mediators.23 Assessment of AGEs now relies on sensitive immunoassays for certain AGEs which have been characterized by high-pressure liquid chromatography, gas chromatography and mass spectrometry, such as CML or the methylglyoxal-derived hydroimidazolone.23,28,32,35 These products are neither unique nor the most prevalent AGEs, but they are common in vivo and correlate well with native oxidants, such as 8-isoprostanes, or their bioactivities.20,21,23 CML and methylglyoxal-derived hydroimidazolone are, therefore, currently among the clinically accepted biomarkers for AGEs in vivo and in nutrients.20,21,46,47
The role of fatty acids in the pathogenesis of diabetes mellitus and insulin resistance has been well-established;48 however, AGEs formed from lipids have not been widely recognized as a source of ROS. Glucose-derived glycolytic intermediates can readily form on amine-containing lipids, such as phosphatidylethanolamine, which generates ALEs, ROS and peroxidized fatty acids, that is, 4-hydroxynonenal and malondialdehyde.37,49,50 Thus, AGE modification of the apoprotein or the lipid particle of apolipoprotein B (ApoB) can delay receptor-mediated LDL cholesterol removal,49 which may increase levels of highly atherogenic AGE–LDL in plasma or the blood vessel wall, even if the plasma lipid profile appears normal.51
Over the past decade, it has become apparent that the interactions between AGEs and ALEs are far more prevalent in vivo than previously estimated. Substantial amounts of nonenzymatically oxidized lipids are orally absorbed52,53 or generated from AGE precursors.54 Unsaturated fatty acids, mostly from exogenous sources, can act as major donors of reactive carbonyls and are more efficient catalysts of AGE or ALE production than is glucose. Paradoxically, these facts have escaped serious attention. Given that ALEs also interact with AGE receptors, these compounds could underlie phenomena currently attributed to free fatty acids, such as insulin resistance and atherosclerosis. AGEs may also play a major part in atherosclerosis,54,55 as the very low affinity of fatty acids for receptors (that is, toll-like receptor 4 [TLR4]) does not suggest a role for fatty acids at circulating levels.56–58 Intracellular AGE formation is naturally slow, partly regulated by the balance between nascent oxidants and antioxidants59 and partly by the glyoxalase system.60–62 Extracellular AGE-modified proteins are sequestered by cellular or soluble AGE receptors,30 degraded by proteolytic digestion to soluble peptides, and are normally excreted by the kidneys.63,64 One reason for delayed AGE detoxification is that crosslinks of AGEs and proteins are resistant to degradation, which delays their turnover and interferes with tissue repair.35,36 Anti-AGE host defenses include suppression of signaling via the AGE receptor AGER1 and renal AGE elimination or detoxification.65,66 The link between decreased renal AGE excretion and chronic diabetes mellitus is poorly understood and may in part be a result of reduced renal AGER1 levels.65,66 Although native defense mechanisms are enormously adaptive, the high level of oxidants in the current nutritional environment may potentially exceed their efficiency and limits, especially in the presence of diabetes mellitus.19
AGEs in nutrients: a modern Trojan horse
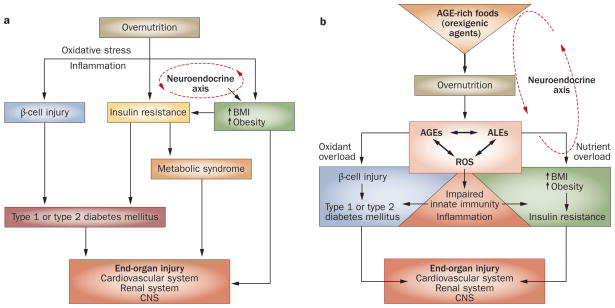
Obesity, a condition found to be inflammatory,56 is associated with an ‘imbalance’ in native defenses,59,67 defined as a surplus of unopposed ROS. Food is quantitatively the largest environmental factor in direct contact with host defenses. Deviating from nutrient-based views that propose that excess nutrients can cause increased oxidative stress (Figure 1a), it has been suggested that ROS-promoting substances enter the body with processed foods in the form of embedded AGEs (Figure 1b).19–21,23,25,27,28 The burgeoning presence of toxic AGEs in modern foodstuffs has resulted from vast socioeconomic changes in the past 50 years and new technologies employed in the mass production of food.43,68,69 At the simplest level, this change involves heat and dehydration, as well as ionization or irradiation. These processes, though intended to improve safety and transportability of foods, amplify glycoxidation reactions and the formation of AGEs and ALEs. Initially, an increased amount of AGEs in food was considered to be desirable, given the effect of AGEs on food flavor.68,70
Figure 1.
Detrimental effects of overnutrition. a | The classic scheme: high levels of pro-oxidant AGEs result from chronic hyperglycemia and contribute to diabetic complications. Overnutrition promotes inflammation and insulin resistance. Excess nutrient availability (via neuroendocrine networks) stimulates food intake, which promotes weight gain and obesity. These conditions are associated with increased inflammation, known to underlie insulin sensitivity, β-cell injury and diabetes mellitus. Chronic hyperglycemia increases intracellular oxidative stress (that is, from mitochondria or the endoplasmic reticulum) and increases the risk of complications, including cardiovascular disease, retinopathy, chronic kidney disease and neuropathy, involving the central and peripheral nervous systems. b | A paradigm shift: AGEs precede diabetes mellitus. Modern food contains appetite-enhancing AGEs, which promote food consumption and overnutrition, leading to increased BMI, obesity and diabetes mellitus, as well as oxidant overload. Sustained influx of nutrient AGEs and ALEs leads to suppression of innate host defenses and a surplus of intracellular ROS, which increases the basal oxidant stress and inflammation. The combination of these processes can simultaneously cause β-cell dysfunction, impaired insulin secretion and insulin resistance, as well as diabetic complications. Restriction of food-derived AGEs reduces oxidative stress and prevents or improves type 1 and type 2 diabetes mellitus in mice. Abbreviations: AGE, advanced glycation endproduct; ALE, advanced lipoxidation endproduct; CNS, central nervous system; ROS, reactive oxygen species.
Approximately 10% of a single AGE-rich protein preparation is orally absorbed by humans.19 This proportion was confirmed by kinetic studies in normal rats, using orally administered double-labeled AGE tracers (121I–14C-glucose-derived AGE–ovalbumin), to separate exogenous from native AGEs, and an AGE inhibitor, aminoguanidine, to block interference from AGEs formed de novo.71 AGEs were absorbed mostly as a single peptide, dipeptides or tripeptides, and approximately two-thirds remained in contact with tissues for >72 h, whereas the rest was rapidly excreted by the kidneys. Based on these kinetic studies, the rate of tissue AGE accumulation due to carbonyls in nutrients was estimated to be an order of magnitude greater than that due to endogenous metabolites, glucose or lipids.
The actual effect of sustained exposure to food-derived AGEs was evaluated in a series of studies in mice. Diabetic, ApoE-deficient, hyperlipidemic or aging mice were exposed to either a regular diet or a diet with a lower amount of AGEs (referred to as an AGE-restricted diet), while both groups of mice were kept under normal energy balance. Importantly, mice on the AGE-restricted diet did not develop severe diabetic tissue injury despite an excess of metabolites, such as glucose or lipids, either as single or as combined entities (Box 1).72–76 Namely, development of diabetic vasculopathy and nephropathy appeared to be dependent on the glycoxidants, including ALEs, present in regular animal food. This provocative interpretation was confirmed by more direct studies in which a single synthetic pre-AGE, methylglyoxal-modified albumin, was added to an AGE-restricted mouse diet.73 The significant increases in systemic AGE levels and oxidative stress in methylglyoxal-fed mice supported the postulate that chronic ingestion of excessive amounts of AGEs influences oxidative stress balance and alters the cellular and metabolic phenotype of these animals, as well as that of their progeny.
Box 1. AGE restriction in mice.
Prevents or improves
Extends
Healthy lifespan73
Studies in rodents have been conducted for extended intervals, entire lifespan (>3 years) and for consecutive generations (F0–F5). Genomic and/or proteomic analyses are pending.
These data may have direct relevance to humans, given the marked expansion of processed foods in the past 50 years and the close relationship of AGEs to food flavor. It is safe to conclude that the consumption of thermally altered AGE-rich foods has also greatly increased during this time. This behavioral change may potentially have contributed to both overnutrition, causing obesity, and to oxidant overload, leading to inflammation (Figure 1a,b), and may partially explain the paradoxical coexistence of good energy balance (positive) and inflammation (negative) that precede diabetes mellitus and related cardiometabolic disturbances.
Host defenses
The host defense system employs numerous mechanisms to restrict AGE toxicity. Along with degradation of AGEs and antioxidant systems, AGE receptors are a major part of the innate and adaptive responses utilized to avoid or regulate the predominantly adverse effects of these molecules.17,30 At least two classes of AGE receptors exist: AGER1, which suppresses AGEs and AGE-related ROS and inflammation, and RAGE, which propagates these effects.77–80 Ideally, AGER1 opposes or intercepts the proinflammatory effects of RAGE and maintains AGE homeostasis.79–80 This defense mechanism is lost in patients with diabetes mellitus, namely AGER1 activity is suppressed, which contributes to the production of further AGEs, ROS and hence tissue injury.
Protection against diabetes mellitus is among the suggested properties of AGER1, based on findings in AGER1 transgenic mice or wild-type mice fed AGE-restricted, AGE-supplemented diets or calorie-restricted mice.72–81 Increased AGER1 expression is associated with extended lifespan in mice,72 a finding which may be due to a synergism with SIRT1, a deacetylase with strong links to both anti-inflammatory and proinsulin actions.82,83 AGER1 and SIRT1 are suppressed in conditions linked to the oxidant overload that is present in diabetes mellitus, overnutrition and aging.72,84–86 The coordinated downregulation of these two cell-protective systems, as well as of downstream positive effector pathways, may be the result of a single cause, a sustained and largely unopposed supply of oxidants. Once these first order host defenses are impaired, basal oxidative stress would be expected to increase above the level required for normal cellular functions, and the surplus ROS could trigger inflammation in the pancreas, β-cell injury and peripheral insulin resistance. This view is supported by human and animal data showing that sustained and long-term reduction of surplus ROS—that is, by lowering food-derived AGE consumption without altering energy or nutritional balance—restores host defenses,21 basal oxidative stress and ameliorates abnormal transcriptional factor activity (NFκB or forkhead box protein O [FOXO]).87 The net effect in vivo is increased tissue resistance to injury and resetting of the threshold for normal insulin actions.72,73
AGE receptors
Elucidation of AGE interactions with AGE receptors has proven complicated. First, the receptors have a relatively low binding affinity. Second, the ligands have a high versatility in terms of structure or charge. Third, AGEs can signal through non-AGE receptors, such as scavenger receptors, G-protein-coupled receptors, pattern recognition receptors and TLRs, as well as via receptor-independent pathways. In addition, their mode of engagement is not completely known. Finally, under sustained AGE ligand excess, levels of AGE receptors are differentially regulated. For instance, AGER1, along with other antioxidant systems and host defenses, is found to be depleted, suppressed or unresponsive, whereas RAGE is upregulated in high oxidative stress states.
In addition to the better known AGE receptors discussed below, many other receptors interact with AGEs, including scavenger receptor class A type 2 (SCARB2), platelet glycoprotein 4 (CD36), AGER3 (encoded by the gene LGALS3) or soluble proteins known to be parts of antimicrobial defenses, such as lysozyme and defensins.30,89,116,117 The evidence reviewed below emphasizes the complexity and abundance of pathways that are responsive to, regulated by and possibly obscured by chronic exposure to excessive amounts of environmental AGEs.
AGER1—defense against AGE toxicity
AGER1, which is encoded by the gene DDOST, is an evolutionarily conserved type 1 transmembrane protein, critically involved in protein synthesis, AGE turnover, regulation of ROS and cell survival.79,80,87,88 This ~50 kD AGE-binding protein is present on most cells, including macrophage and mononuclear cells, and mediates the uptake, degradation and disposal of AGEs from cells and tissues.79,89,90 Although first identified in the endoplasmic reticulum (as OST48),91,92 AGER1 is present on the plasma membrane and in subcellular compartments. By sequestering and degrading AGEs, AGER1 prevents the accumulation of these molecules in the extracellular milieu and the cytoplasm, which blocks the generation of ROS and new AGEs promoted by other receptors (RAGE, TLR4, EGFR, SCARB2, CD36) or by nonreceptor mechanisms.16,30,67,93–96
In humans, AGER1 levels inversely correlate with intracellular AGEs and directly with urine AGEs.21 This relationship curtails a series of AGE-induced cell-destabilizing processes. Thus, AGER1 inhibits the activation of NADPH oxidases p47phox (also known as neutrophil cytosol factor 1) and gp91 by suppressing Tyr311 and Tyr332 phosphorylation of PKCδ.97 This action prevents the activation and nuclear translocation of NFκB p6597 and of AGE responses that are promoted by RAGE.98 As AGER1 also prevents AGE-initiated transactivation of EGFR in the presence of high oxidative stress,80,97 it may serve to restrict kinase hyperactivity of other G-protein-coupled receptors.99,100 Of note, AGER1 inhibits AGE-induced Ser36 phosphorylation of the p66Shc isoform of SHC-transforming protein 1, a major oxidative stress and apoptosis-promoting adaptor protein.87 This process merits attention, as increased levels of p66Shc are linked to diabetes mellitus, atherosclerosis and kidney disease.101 In addition, the deletion or mutation of the gene encoding p66Shc extends lifespan in mice.101,102 Consistent with its role in the negative regulation of p66Shc activity, AGER1 also inhibits AGE-mediated suppression of the antioxidant effect of FOXO3 on superoxide dismutase 2 (SOD2) expression.87
Given that AGER1 deletion is lethal in yeast, the biological properties of AGER1 were confirmed by in vitro gene transfer and gene silencing studies.79,87,92 Furthermore, in transgenic mice, high AGER1 levels prevented formation of occlusive atheromas caused by wire-injury, a high fat-diet or T2DM.81 These studies helped unravel the potential in vivo significance of this anti-AGE mechanism in diabetes mellitus, given that it is suppressed in conditions of chronically elevated oxidative stress.
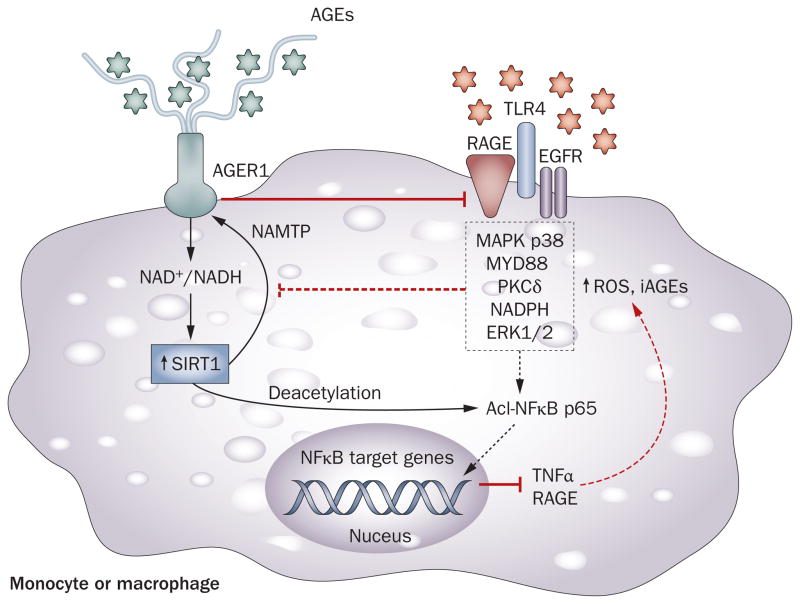
A potentially important protective synergism between AGER1 and the SIRT1 has been identified.88 Of the numerous sirtuins, SIRT1 is thought to play a major part in insulin signaling and secretion, insulin resistance, inflammation and lifespan.82,83 SIRT1, like AGER1, is suppressed in diabetes mellitus, high-fat feeding and aging.82,84,86 These conditions are also characterized by high levels of AGEs and oxidative stress. Of note, AGER1 overexpression blocks AGE-induced suppression of SIRT1 via NAD+/NADH (H. Vlassara, unpublished work),88 inhibiting NFκB p65 hyperacetylation and inflammatory events in monocytes and tryptophan 5-hydroxylase 1 (TPH1) positive cells. Furthermore, AGER1 prevents AGE-induced impaired signaling via the insulin receptor and insulin receptor substrate 1 (IRS1) in adipocytes.88 This action prevents an AGE-induced decrease in glucose uptake by adipocytes in vitro and in vivo. These data suggest that AGER1 provides a shield to SIRT1 against a high external oxidant load, thereby helping mitigate inflammation and preserve the metabolic actions of insulin (Figures 2 and 3). The fact that AGER1 protein levels in peripheral blood mononuclear cells isolated from healthy humans are responsive to and correlate with circulating AGE levels supports this view.21
Figure 2.
Synergism between AGER1 and SIRT1. Innate host defense cells, monocytes and macrophages normally bind, endocytose and degrade AGEs via the cell-surface AGER1 (also known as DDOST), the levels of which normally correlate inversely with intracellular levels of AGEs. Prolonged supply of external AGEs, however, depletes AGER1. Ensuing surplus ROS promotes inflammation via RAGE, TLR4, EGFR and other receptors regulating the activities of NFκB, AP-1, FOXO3 and other factors, via numerous pathways. These increase AGEs, ROS and inflammatory mediators, including RAGE and its ligands. AGEs, acting via ROS, are potent suppressors of NAD+, partly by reducing NAMPT and SIRT1 levels. Decreased SIRT1 levels promote NFκB p65 hyperacetylation and enhanced transcription of inflammatory genes consistent with an M1 macrophage profile. For instance, TNF induces an overlapping set of target genes, which contributes to insulin resistance. AGER1, by blocking AGEs, controls many of these effects. The protective effects of AGER1 may stem from its long extracellular tail with high-affinity AGE-binding, which competitively interferes with AGE–cell-surface interactions leading to ROS. This property may constitute the essence of the positive synergism between AGER1 and SIRT1. Though suppressed in humans and animals with chronic diabetes mellitus, monocyte/macrophage AGER1 and SIRT1 levels can be restored to normal following AGE restriction. Abbreviations: AGE, advanced glycation endproduct; AGER1, AGE receptor 1; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated kinase 1/2; FOXO3, forkhead box protein O3; MAPK, mitogen-activated protein kinase; MYD88, myeloid differentiation primary response protein MyD88; NAMPT, nicotinamide phosphoribosyltransferase; NFκB, nuclear factor κB; PKCδ, protein kinase C δ type; RAGE, receptor for advanced glycosylation endproducts; ROS, reactive oxygen species; SIRT1, NAD-dependent deacetylase sirtuin-1; TLR4, toll-like receptor 4; TNF, tumor necrosis factor.
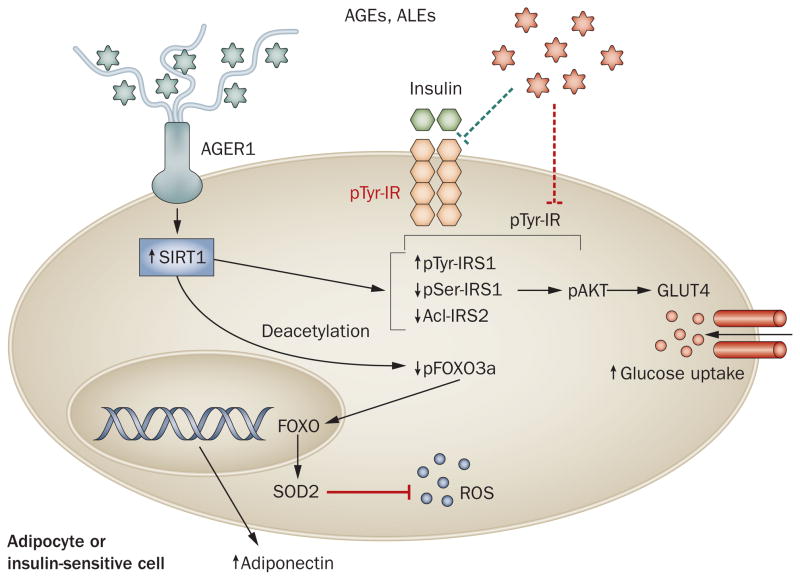
Figure 3.
Excessive AGEs impair insulin sensitivity in insulin-target tissues. In adipocytes, as in immune cells, AGE-mediated effects are normally controlled by AGER1, via ROS suppression, protecting SIRT1-dependent insulin functions. Excessive food-derived protein and lipid AGEs can suppress AGER1 and other host defenses, reversing this balance. For instance, exposure to AGEs reduces insulin receptor and IRS1 tyrosine phosphorylation and increases IRS1 serine phosphorylation, resulting in impaired insulin signaling and decreased glucose uptake. Enhanced NFκB p65 hyperacetylation of preadipocytes, endothelial cells and immune cells residing in adipose tissue can also promote inflammatory cytokine production, impair FOXO3α activity and further ROS production. Restored AGER1 and SIRT1, following AGE restriction in individuals with diabetes mellitus, coincides with markedly lower plasma insulin levels and higher adiponectin levels —evidence of a crucial crosstalk between host defenses and metabolic pathways in diverse tissues and cells. Abbreviations: Acl, acetylation; AGE, advanced glycation endproduct; AGER1, AGE receptor 1; ALE, advanced lipoxidation endproduct; FOXO3α, forkhead box protein O3 α; GLUT4, glucose transporter type 4; IR, insulin receptor; IRS, insulin receptor substrate; NFκB, nuclear factor κB; pAkt, phosphorylated serine/threonine-protein kinase AKT; pSer, serine phosphorylation; pTyr, tyrosine phosphorylation; ROS, reactive oxygen species; SIRT1, NAD-dependent deacetylase sirtuin-1; SOD2, mitochondrial superoxide dismutase 2 [Mn].
After extended periods of exposure to high levels of AGEs, as in patients with diabetes mellitus and chronic kidney disease, AGER1 levels are suppressed.20,21,85,87 As AGER1 levels decrease, AGEs and ROS accumulate, feeding into a cycle of formation of new ROS and AGEs. Although the cause of AGER1 downregulation in diabetes mellitus is not evident, this effect is reversible by consumption of AGE-restricted foods in both humans and mice.21,72,73,88 SIRT1 levels are also restored by AGE restriction, a fact which underscores the interdependence of these two pathways. The anti-AGE activities of another AGE-binding molecule, serum lysozyme, resemble those of AGER1.103 Lysozyme, an antibacterial protein, binds AGEs within a short cysteine-bounded (ABCD) motif. Notably, both the anti-AGE activity and antibacterial function of lysozyme are also decreased in patients with chronic diabetes mellitus. In summary, AGER1 expression levels correlate positively with levels of other intracellular antioxidant mechanisms (for example, SIRT1, NAMPT, SOD2, GSH) and negatively with pro-oxidant pathways (that is, RAGE, NADPH oxidase, p66shc). If AGER1 is important in the maintenance of normal homeostasis, then reduced AGER1 expression levels may signal a compromise in host defenses (Box 2). Similarly, the timely restoration of decreased AGER1 levels following a medical intervention may also further the recovery of other important native defenses, a hypothesis which remains to be established.
Box 2. AGER1 in the host defense against glycoxidants.
The early loss of function of AGER1 caused by sustained external AGE pressure is proposed as the basis of compromised host defenses and increased susceptibility to maladaptive inflammatory processes underlying diabetes mellitus and its complications.
AGER1 promotes
AGE removal
SIRT1, NFκB p65 deacetylation
NAMPT, NAD+/NADH levels
Insulin receptor and IRS1 tyrosine phosphorylation
Glucose uptake
FOXO3α activity, SOD2 expression
AGER1 inhibits
Intracellular AGEs and ROS production
NAPDH oxidase, p47, gp91 activity
p66shc activity
RAGE expression and signaling, EGFR activity
PKCδ activity
NFκB p65 activity
IRS1 serine phosphorylation
AKT activity
RAGE—passive enabler of inflammation
RAGE action propagates ROS and inflammation in both acute and chronic diseases, including diabetes mellitus.104–107 RAGE is a member of a family of low-affinity, pattern-recognition receptors that function at the interface of innate and adaptive immunity and bind multiple ligands, including high mobility group protein B1 (HMGB1), amyloid β protein and members of the calcium-binding S100 protein group.108–110 The binding conditions of AGEs by RAGE relative to these other ligands, in the initiation of signaling events in vivo, are not well-understood and even debated.111 Whereas the full-length molecule does not contribute to endocytosis or removal of AGEs, the extracellular domains of RAGE may be shed as soluble variants and contribute to AGE clearance.112 On the other hand, activation of full-length cell-associated RAGE induces an array of signaling events, including MAPK p38–JNK and JAK–STAT, CDC42–RAC and others, many of which are the result as well as the cause of ROS. This apparent redundancy could be partly owing to the diversity of putative RAGE ligands or cell types involved and partly owing to the interaction of RAGE with other receptors, especially TLR4. A series of reports suggest that TLR2 and TLR4 levels are elevated in patients with diabetes mellitus and that they interact with AGEs, either directly or via RAGE and other RAGE ligands, such as HMGB1.55,109,110,113 Of note, inflammatory and vascular cells with either absent or defective TLR4s lack the typical inflammatory response to AGEs (H. Vlassara, unpublished work). Although numerous studies report an association between RAGE and diabetic complications, with or without the involvement of TLR4, it has been difficult to assign a primary role to this receptor other than that of a broad-spectrum ROS transducer. Ambient or intracellular oxidative stress appears to be an overarching biomodulator of RAGE except when RAGE is genetically manipulated.114,115 Thus, a decrease in basal oxidative stress by simply restricting the availability of external AGEs is sufficient to suppress both RAGE mRNA and protein levels in diabetic or aging mice.72,73 Similarly, AGE restriction reduces RAGE levels in peripheral blood mononuclear cells of healthy humans and of those with diabetes mellitus to levels markedly below their baseline (>60%), which indicates that RAGE is readily regulated by AGEs in the external environment.21,88 Of note, RAGE mRNA and protein levels in peripheral blood mono-nuclear cells from healthy individuals directly correlate with serum levels of AGEs and oxidative stress, as well as with ingested AGE levels,21 whereas they are only modestly elevated in patients with diabetes mellitus without comorbidities. These findings in animals and humans may offer new perspectives on the role of RAGE in diabetes mellitus. Namely, as with other signal transduction molecules that regulate proinflammatory events, the in vivo upregulation of RAGE may be a result rather than a cause of increased oxidative stress. Once host defenses, including AGER1, are compromised and basal oxidative stress rises, upregulated RAGE may amplify and perpetuate this condition.
Kidneys and AGE clearance
The main ‘entry’ for food-derived AGEs is the gastrointestinal tract and their main ‘exit’ is the kidney. Owing to its ability to conserve and also distinguish useful from toxic products—whether from the external or internal environment—the kidney has long been known as a key component of host defenses. The kidney is undoubtedly a major player in the detoxification of AGEs, both by filtration or by active uptake and secretion, two processes that result in the net excretion of AGEs in urine.19,63,71,118 As a consequence of their large blood supply, the kidneys are directly exposed to a larger amount of circulating AGEs than many other organs, a fact which may make them vulnerable to injury by circulating reactive carbonyls and ROS.119 This process may underlie the early reduction of AGE clearance documented in mice and in patients with diabetes mellitus, before recognizable impairments in renal function occur.19,72,73,75 Any reduction in renal AGE clearance may potentially cause a build-up of prerenal AGEs and an increase in the formation of new AGEs and ROS within both renal and extrarenal tissues. An early consequence of this process could be the suppression of AGER1 levels, which results in further ROS generation and inflammation.42 In fact, serum levels of AGEs rise long before any clinical evidence of renal disease manifests.20,120 This factor becomes especially relevant following the onset of diabetes mellitus, given that increased levels of AGEs mediated by hyperglycemia further augment oxidative stress and its pathogenic consequences.75,121
Several studies show that chronic ingestion of excess amounts of AGEs can predispose the human kidney to chronic injury in the absence of diabetes mellitus by suppressing local anti-AGE defenses, that is, AGER1, and inducing oxidative stress and inflammatory responses.20,21 The presence of diabetes mellitus may aggravate these changes.19,21 In support of this view, AGE restriction in mice prevented diabetic or age-related kidney disease, in spite of persistent hyperglycemia.72,73,75 AGE restriction in humans with diabetic or nondiabetic kidney disease led to decreased serum levels of AGEs, restoration of AGER1 levels and significantly decreased systemic oxidative stress and inflammation.21,122 Although functional renal end points are not yet available in humans, a common thread across animal and human studies is emerging: a pre-existing oxidant overload may reduce host defenses before the onset of diabetes mellitus and set the stage for diabetic renal disease.
AGEs and diabetes mellitus: a paradigm shift
Animal studies
The unique pathogenic role of AGEs as environmental toxins has been amply supported by studies showing that the parenteral or oral administration of chemically designed AGEs leads to a cycle of increased cell and tissue AGEs, oxidative stress and inflammation in healthy mice.119,123 These changes were associated with tissue injury that resembled diabetic vascular or renal complications. Of note, these effects were induced without hyperglycemia and could be interrupted by pharmacological inhibitors of AGEs. Indeed, although a number of anti-AGE and antioxidant agents are effective against diabetic complications in mice,124–127 it has yet to be determined whether they can reliably alter the course of sustained glycoxidant overload in humans.
Several studies in humans and animals have suggested the emergence of a paradigm shift in that pathogenic levels of AGEs can be acquired from ingested AGEs, which may be an antecedent rather than the result of hyperglycemia. These studies advanced the postulate that AGEs cause abnormal oxidative stress in the prediabetic state and that processed nutrients are the principal source of these AGEs.20,21,72,73 They also demonstrate that oxidant overload and insulin resistance can be diminished21 and that tissue injury and the development of diabetes mellitus can be prevented in several mouse models by restricting the intake of food AGEs without reducing energy intake.72,73,75,128–130 As briefly described below, these studies have provided a new framework and an experimental setting from which to revisit the pathogenesis and treatment of diabetes mellitus and its complications.
Type 1 diabetes mellitus
Exposure to exogenous AGEs begins in utero. The accrual of AGEs is cumulative with time and could have a role in disease susceptibility to autoimmune T1DM, as well as T2DM. This hypothesis was first tested in a model of autoimmune T1DM, the nonobese diabetic (NOD) mouse.129 Compared with female NOD controls, over 90% of which became diabetic at ~4–5 months of age, less than one-third of NOD mice fed an AGE-restricted diet developed T1DM.129 Two-thirds remained healthy until study end (12 months). The incidence of T1DM decreased to less than 15% in the next two generations, as long as an AGE-restricted diet was maintained in both dams and offspring. The AGE-restricted NOD mice had minimal insulitis consistent with reduced stimulation of cytotoxic T cells and macrophages, possibly owing to the restriction of oxidant AGEs in the diet. NOD mice fed a standard chow, which is enriched in AGEs, had the typical hyper-responsive, interferon-γ-positive, but interleukin-4-negative islet T cells that were unresponsive to common islet antigens, such as glutamate decarboxylase or insulin. The blockade of islet β-cell injury in AGE-restricted mice suggested that the levels of oxidants in the diet were sufficient to trigger diabetes mellitus, and this effect was unrelated to the nutrient composition of the diet. Given that diabetes mellitus recurred when offspring were returned to the standard diet, the strong antidiabetic effects of AGE restriction appear to result from retention of a lower basal oxidant stress and, thus, of a normal host defense in this mouse strain that is otherwise genetically susceptible to diabetes mellitus. These findings have been confirmed in vitro and in rodents parenterally administered AGEs and anti-AGE agents or an AGE-restricted diet (J. M. Forbes, personal communication).
Type 2 diabetes mellitus
The study on AGE restriction in NOD mice was repeated in different animal models of T2DM (leptin-receptor-deficient db/db mice, C57B6 mice fed a high-fat diet and in C57B6 mice with age-related T2DM), with analogous results.72,73,128,130 In each model, AGE restriction led to a decrease in oxidative stress and improved insulin resistance, even when other traditional risk factors, such as hyperglycemia, obesity, high-fat intake, caloric intake or advanced age, were present. Furthermore, AGE restriction inhibited diabetic renal or vascular complications.74,75 The direct toxicity of food-derived AGEs was further supported by heat-treating or by supplementing a low-AGE diet with methylglyoxal-albumin: both cohorts of mice had an early-onset of age-associated insulin resistance and renal disease (H. Vlassara, unpublished work). Mice fed an AGE-rich diet, as well as mice fed regular mouse chow, had suppressed tissue expression of AGER1, SIRT1, and plasma adiponectin, as well as impaired insulin receptor signaling and low insulin-stimulated glucose uptake.
This area of research is currently evolving; however, a unifying observation from these animal models, in which diabetes mellitus was induced by different ‘diabetogenic’ agents or which had a strong genetic propensity to diabetes mellitus, was that simply reducing the basal (pre-existing) oxidative stress by dietary AGE restriction sharply reduced the risk of diabetes mellitus and its complications.
From mice to humans
The recognition of early glucose–protein interactions were initially focused on HbA1c, seen as a prototype that reflects glycemic control in vivo.8,29 This observation was followed by the discovery of the toxicity of late derivatives of these interactions, or AGEs, in the extracellular and intracellular space of individuals with diabetic complications.29 The efficacy of several AGE inhibitors in reducing diabetic complications in animal models supports the role of these molecules as toxins.29,132 However, these comorbidities were predominantly viewed as the consequence of hyperglycemia or aging.29,124–127,132,133 The conceptual framework of the toxicity of AGEs underwent a new expansion with the advent of data pointing to the external environment as a source of preformed tissue-reactive carbonyls. This new evidence hinted at processes not previously associated with AGEs, such as insulin production and insulin sensitivity, which are distinct from and can precede hyperglycemia.72,73,128–130
Increased oxidative stress, which precedes diabetes mellitus, is evidently a mediator of inflammatory events. This cycle of events, however, could not persist without the prior saturation and neutralization of critical host defenses, such as AGER1. Once AGER1, under external oxidant pressure, is suppressed in tissues, including adipocytes as well as peripheral blood mononuclear cells, unopposed AGEs may attenuate other key protective mechanisms. Importantly, decreased SIRT1 levels result in continued hyperacetylation of NFκB p65, enhancement of inflammation and a reduction in the metabolic actions of insulin (Figures 2 and 3).82,83
The postulate that AGEs can influence insulin sensitivity was tested in insulin-resistant patients with T2DM exposed to AGE restriction. Compared to the control group, patients on an AGE-restricted diet for 4 months had substantial reductions in plasma insulin, leptin, tumor necrosis factor (TNF) and RAGE concentrations and marked increases in adiponectin, AGER1 and SIRT1 levels. These responses were accentuated by monocytic NFκB p65 hyperacetylation, as a result of SIRT1 suppression.88 In addition, gene transfer and silencing studies in monocyte-like THP1-positve cells provided confirmation that SIRT1 actions are under the control of AGER1 in monocyte and macrophages, where SIRT1 enhances anti-inflammatory functions, as well as in adipocytes, where SIRT1 regulates glucose uptake and utilization.82,134
Studies on obesity have revealed that changes in insulin target cells are partly owing to inflammatory events arising in adipocytes or from locally recruited immune cells.134–137 Tissue macrophages were reported to switch from the common anti-inflammatory (M2) phenotype to the proinflammatory phenotype (M1),138 secreting a variety of chemokines that induce additional inflammatory mediators by insulin target cells via paracrine action. In addition, responses to ‘unique’ antigens by expanded subpopulations of activated T-helper-1 (TH1) cells that are present in muscle and adipose tissue were shown to promote insulin resistance.134,138–140 On the other hand, anti-inflammatory regulatory T cells, which normally attenuate insulin resistance, were found to be decreased in response to these antigens.140–142
AGEs are well-known to be a potent trigger for monocytes and macrophages,93,143–145 promoting cytokine release and reducing insulin sensitivity. AGEs can also induce T-cell activation and secretion of interferon γ via surface AGE receptors.145,146 Thus, a ‘preconditioning’ of T cells and monocyte populations by orally absorbed AGEs can occur in the circulation before full in situ activation,140,146–148 setting the stage for inflammation and insulin resistance.149
Indeed, the fact that adipose tissue in obesity contains large numbers of macrophages indicates that recruitment may begin long before insulin resistance is clinically recognizable.134 Macrophages, prompted by regulatory T cells, might initially be recruited to adipose tissue for the purpose of clearing stored ALEs and of containing undue inflammation.141,142 However, prolonged stimulation by AGEs of macrophages resident in adipose tissue may result in suppression of AGER1 in macrophages and a shift to the M1 phenotype. This change would promote further ROS formation and a feed-forward process of chronic inflammation and insulin resistance, which could lead to diabetes mellitus.
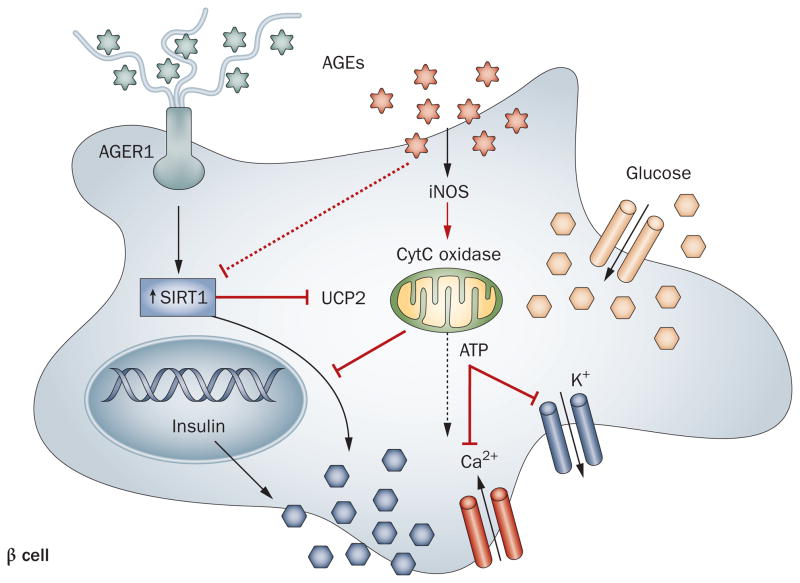
New data suggest that AGEs can also directly impair insulin secretion in pancreatic islet β cells via a number of pathways. For instance, AGE-induced inducible nitric oxide synthase (iNOS) dependent mitochondrial ROS can suppress cytochrome C oxidase and ATP generation, thereby impairing membrane depolarization and impeding insulin release.31 Given that AGEs suppress SIRT1, which is also prominent in pancreatic β cells,150 they may intercept SIRT1 regulation of mitochondrial uncoupling protein 2 (UCP2) and Ca2+ channel function in β cells, which could suppress insulin secretion (Figure 4).82 Alternatively, as AGEs promote macrophage chemotaxis144 and secretion of TNF and IL-1,143 they may recruit inflammatory cells to islets. Increased IL-1 levels are thought to contribute to impaired insulin secretion, decreased cell proliferation and apoptosis in β cells.151 This view is supported by findings that show a recombinant IL-1-receptor antagonist improves glycemia and β-cell secretory function in patients with T2DM.152
Figure 4.
AGEs impair insulin secretion in pancreatic islet β cells. This process can occur via several pathways: by iNOS induction, and through the generation of mitochondrial ROS, AGEs suppress cytochrome C oxidase levels and ATP generation, reducing membrane depolarization and insulin release; by suppressing insulin gene promoter activity; by suppressing SIRT1, which regulates UCP2, impairing β-cell depolarization and secretory function; by promoting immune cell (T cell, macrophage) recruitment, activation and β-cell cytotoxicity, apoptosis or cell death. AGER1 normally suppresses the effects of AGEs and ROS and may enhance SIRT1 expression and function in β cells, positively regulating insulin secretion. Under chronic high-level AGE conditions, AGER1 is downregulated, which may contribute to β-cell dysfunction or destruction. Abbreviations: AGE, advanced glycation endproduct; AGER1, AGE receptor 1; CytC, cytochrome c; iNOS, inducible nitric oxide synthase; SIRT1, NAD-dependent deacetylase sirtuin-1; UCP2, mitochondrial uncoupling protein 2.
As these lines of evidence continue to evolve, they remain consistent with the emerging argument that a sustained influx of AGE-rich nutrients has the potential to suppress innate defense mechanisms and of setting the stage for unopposed oxidative stress in otherwise healthy individuals. The result could be an increase in the basal levels of oxidative stress, which can cause a shift from a normal phenotype to one of depleted antioxidant reserves and, thus, a ‘hypersensitivity’ to subsequent oxidant challenges. This scenario is particularly pertinent to individuals who have a positive energy balance (that is, overnutrition, which promotes obesity) but also applies to nonobese individuals with risk factors for diabetes mellitus.7,14,15,18,153 This newly appreciated link between AGEs and nutrients may help in understanding how the environment can reduce host defenses and lead to the growing epidemics of the metabolic syndrome and diabetes mellitus.
AGEs: a case for epigenetic change?
The rates of increase in obesity and diabetes mellitus point to a complex and markedly altered interplay between genes and the changing food environment. Whether epigenetic changes could be traced to the increased AGE content of modern food and whether this could play a part in the high incidence of diabetes mellitus was tested in transgenerational studies in C57B6 mice exposed to an AGE-restricted or methylglyoxal-supplemented diet and monitored for five generations (F0 to F5).118,154 A striking observation was made in that by the fifth generation, methylglyoxal-fed F5-mice developed insulin resistance followed by diabetes mellitus at 16–18 months of age, whereas AGE-restricted F5-mice did not develop these changes until the age of 36 months or more (H. Vlassara, unpublished work). This acceleration of T2DM onset in methylglyoxal-fed mice over five generations (by more than one-third of a normal life cycle) could not be attributed to genetic effects, an argument similar to that raised by the doubling of obesity in humans within one generation.155 Similarly, this dramatic effect could not be attributed to energy or nutrient intake, as all cohorts were pair-fed a nutritionally balanced diet. Rather, the changes represented the effect of a known AGE (methylglyoxal) added to food that otherwise had a low AGE content. It was noteworthy that, as tissue AGER1 decreased in a stepwise manner, by the fifth generation serum AGEs in methylglyoxal-fed mice were about threefold higher than those in AGE-restricted mice, consistent with a gradual rise in the basal oxidative stress transmitted between generations. Of note, in the AGE-restricted group, the delay or absence of insulin resistance was linked to lower oxidative stress and higher AGER1 levels. Together with data from the transgenerational NOD studies,129 these findings suggest that the increasing oxidant burden in the offspring is, on the one hand, determined maternally and, on the other hand, augmented by food oxidants in the postnatal period.
These findings are in agreement with reports that maternal diet influences disease development, such as obesity and hypertension in humans and animals.156–162 The crossgenerational loss of function in anti-AGE and oxidative stress-regulatory genes could to some degree reflect epigenetic changes owing to the gradual increase in oxidant levels over several generations. Although these data clearly warrant further investigation, it seems probable that impaired host defenses can gradually result in hyper-responsiveness to inflammatory stimuli and increased susceptibility to disease. The present studies suggest that changes that result from a disproportionate increase in oxidants contained in current nutrients may involve heritable changes in gene function, such as those for AGE receptors and sirtuins. The fact that these changes can be modified in mice raises optimism that they may also be controlled in both healthy individuals and in those with diabetes mellitus.
In this context, clinical evidence indicates that circulating AGEs in healthy infants may closely correlate with, and even exceed, maternal or adult AGE levels within the first year of life,163 which is in line with the above findings in mice. Furthermore, infants born to mothers with high serum AGEs had higher plasma insulin and lower adiponectin levels, findings which may be the result of the AGE content of heat-treated, commercial infant foods, which was found to be ~100-fold higher than in maternal breast milk.164,165 These alarming facts indicate that, as a result of an excessive AGE influx, children today may be at risk of altered host defense and immune systems, which may, at least in part, explain the diabetes epidemic.18,166–168 It remains to be seen whether exposure of children to processed foods accounts for the increased incidence of T1DM or T2DM and whether this can be prevented by AGE restriction.
A reversible trend?
Epidemiologic studies show that increased oxidative stress among clinically normal individuals precedes insulin resistance, T1DM and T2DM.7,14,15,18,153 Studies also link high circulating levels of AGEs with prediabetic conditions, such as the metabolic syndrome, cardiac or chronic kidney disease, or frailty of aging.46,47,169–172 New data have led to practical tools for assessing dietary AGE intake in humans (currently estimated at ~15 Eq or 15 × 106 AGE units per day).42,173 AGE restriction proved possible and effective based on the simple avoidance of dry heat, namely frying, roasting or broiling methods in food processing, which was accomplished without altering caloric intake. A diet given to healthy individuals that contained ~30–50% of the average AGE intake was achieved by employing lower temperature and high-moisture methods during the manufacturing process. This approach was effective in lowering circulating AGEs, as well as inflammation and insulin resistance.
Individuals across the age spectrum who consume an AGE-rich diet may have elevated levels of high-sensitivity C-reactive protein (hsCRP), TNF, fibrinogen, vascular cell adhesion protein 1 (VCAM1) and HOMA (homeostatic model assessment), a predictor of insulin resistance (Box 3).20,21,174 One interpretation of these associations is that modern foods, replete with appetite-enhancing AGEs, undermine host defenses, while promoting inflammation. A further interpretation is that the ‘healthy’ human baseline has shifted to a phenotype which displays substantial overlap with ‘prediabetes’. In these individuals, the best determinants of systemic AGEs and oxidative stress are diet-derived AGEs, rather than glucose, age, sex, calories or nutrient intake.20,21 The fact that simply restricting AGE intake, without altering nutrient or caloric intake, can lead to a significant reduction in oxidative stress and inflammatory markers supports the conclusion that AGEs in food are toxins for patients with diabetes mellitus and, importantly, also for healthy individuals.
Box 3. AGE restriction in humans.
Healthy individuals21,27,28,164
Reduced basal oxidative stress
Reduced inflammation (TNF, VCAM1, hsCRP)
Improved markers of insulin resistance
Improvement of markers of vascular dysfunction
Lower levels of serum AGEs
Lower levels of AGER1, RAGE and SIRT1
Lower intracellular AGE levels in peripheral blood mononuclear cells
Patients with diabetes mellitus*19,54,88,122
; Reduced oxidative stress
Reduced inflammation (TNF, hsCRP, VCAM1)
Lower plasma insulin levels
Improved insulin resistance
Lower leptin levels
Increased adiponectin levels
Increased AGER1, SIRT1 levels
Decreased acetylation of NFκB p65
Decreased RAGE, p66shc levels
Patients with kidney disease21,172
Lower oxidative stress
Reduced AGEs
Improved inflammation, p66shc, NADPH p47phox
Restored AGER1, SIRT1 levels
Decreased RAGE levels
Unlike animal studies, human interventional studies on AGE restriction have been brief (~6 weeks up to 4 months); thus, except for improved insulin resistance and inflammation, no major clinical end points have been reported. *H. Vlassara, unpublished work.
Serum levels of AGEs are elevated in patients with diabetes mellitus, and their levels predict morbidity and mortality. This concept is not entirely new,19,47,63,175–177 but this realization and the data from multiple animal studies prompted new clinical studies that focus on food-derived AGEs and their role in the pathogenesis of insulin resistance and diabetes mellitus (Box 1 and 3). A link between food AGEs, inflammation and human diabetes mellitus was initially suggested by the finding that brief AGE restriction (4–6 weeks) in patients with T1DM and T2DM lowered systemic AGE levels, oxidative stress and inflammation, including TNF, hsCRP and VCAM1 concentrations.122 A 4-month trial of AGE restriction in patients with well-controlled T2DM revealed a ~40% decrease of hyperinsulinemia.88 The study also reported reduced oxidative stress and inflammation, including lower plasma leptin, TNF and RAGE levels, and acetylated NFκB p65 in mononuclear cells. A marked recovery was noted in levels of AGER1, SIRT1 and adiponectin, three factors with anti-inflammatory properties that were decreased at baseline. As the return of innate defenses to normal levels resulted in decreased inflammation and insulin resistance,134 this strategy carries promise as an efficient, low-cost treatment for those with diabetes mellitus or with prediabetes.6,7,15–18,88,153,166
Importantly, sevelamer—an oral nonabsorbable negatively charged polymer that, in addition to phosphates, can sequester AGEs in the gut—offers critical support to the benefits of AGE restriction. Within 2 months, sevelamer, but not CaCO3—a phosphate-binder that does not bind AGEs—effectively lowered serum AGE levels, markers of oxidative stress and inflammation, and restored levels of AGER1 and SIRT1 in patients with early diabetic nephropathy (H. Vlassara and G. E. Striker, unpublished work). This alternative approach confirms the significance of reducing oral AGE absorption.
Modification of dietary habits requires education and legislative action in order to restrict foods rich in AGEs, label foods with AGE content and promote AGE restriction as a strategy to help stem the diabetes epidemic and its complications. A strategy aimed at screening for and advising those with high levels of AGE markers or dietary consumption may be a first move in this direction.
Conclusions
The current diabetes epidemic is unarguably a result of environmental changes. A major shift over the past half century is the enrichment of nutrients by AGEs, highly palatable and appetite-enhancing, pro-oxidant substances that simultaneously drive overnutrition and oxidant overload. Sustained oxidant overload may overwhelm host defenses and raise basal levels of oxidative stress, presaging chronic inflammation. These states can impair both insulin production and insulin sensitivity and lead to diabetes mellitus. Evidence from transgenerational animal studies and human trials indicates that high basal oxidative stress, which precedes both T1DM and T2DM, is the result of externally derived, as well as maternally transmitted, AGEs. Altered host defenses may amount to epigenetic changes—a possibility that is worthy of in-depth investigation.
Efforts to curtail the current diabetes epidemic must address and reduce the pre-existing elevated basal oxidative stress and inflammation in order to prevent, improve or reverse diabetes mellitus. Randomized, controlled clinical studies are needed to confirm and expand on the notion that AGE restriction is a promising, cost-effective clinical intervention to achieve these goals.
Key points.
The current epidemics of diabetes mellitus and aging-related diseases may largely be due to environmental factors, as industrial and societal changes have led to the production and consumption of foods rich in advanced glycation endproducts (AGEs)
The sustained influx of AGEs leads to suppression of host defenses and a surplus of intracellular reactive oxygen species, which can shift basal oxidative stress and lead to inflammation and obesity
The combination of these processes can simultaneously cause β-cell dysfunction, impaired insulin secretion and insulin resistance, as well as diabetic complications
Restriction of food-related AGEs helps reduce basal oxidative stress, restore host defenses and prevents or improves type 1 and type 2 diabetes mellitus in mice, regardless of genetic susceptibility
AGE restriction in humans is emerging as a promising, cost-effective, broadly applicable intervention
Acknowledgments
Work described in this Review was supported in part by MERIT grant AG-23,188, AG-09,453 and DK091231 (H. Vlassara) and RR-00071.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors researched the data for the article, provided a substantial contribution to discussions of the content, contributed equally to writing the article and reviewed and/or edited the manuscript before submission.
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14 (Suppl 5):S1–S85. [PubMed] [Google Scholar]
- 2.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, et al. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006;113:2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 5.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 7.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371:736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACCORD Study Group. et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [No authors listed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skyler JS, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Parthasarathy S, Khan-Merchant N, Penumetcha M, Khan BV, Santanam N. Did the antioxidant trials fail to validate the oxidation hypothesis? Curr Atheroscler Rep. 2001;3:392–398. doi: 10.1007/s11883-001-0077-9. [DOI] [PubMed] [Google Scholar]
- 13.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 14.Wildman RP, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 15.Knip M, et al. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54 (Suppl 2):S125–S136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 16.Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29:1420–1432. doi: 10.2337/dc05-2096. [DOI] [PubMed] [Google Scholar]
- 17.Vlassara H, Striker G. Glycotoxins in the diet promote diabetes and diabetic complications. Curr Diab Rep. 2007;7:235–241. doi: 10.1007/s11892-007-0037-z. [DOI] [PubMed] [Google Scholar]
- 18.Wentworth JM, Fourlanos S, Harrison LC. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat Rev Endocrinol. 2009;5:483–489. doi: 10.1038/nrendo.2009.149. [DOI] [PubMed] [Google Scholar]
- 19.Koschinsky T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uribarri J, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlassara H, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brands CM, Alink GM, van Boekel MA, Jongen WM. Mutagenicity of heated sugar-casein systems: effect of the Maillard reaction. J Agric Food Chem. 2000;48:2271–2275. doi: 10.1021/jf9907586. [DOI] [PubMed] [Google Scholar]
- 23.Cai W, et al. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002;8:337–346. [PMC free article] [PubMed] [Google Scholar]
- 24.Finot PA. Historical perspective of the Maillard reaction in food science. Ann NY Acad Sci. 2005;1043:1–8. doi: 10.1196/annals.1333.001. [DOI] [PubMed] [Google Scholar]
- 25.Finot PA. The absorption and metabolism of modified amino acids in processed foods. J AOAC Int. 2005;88:894–903. [PubMed] [Google Scholar]
- 26.Ames JM. Determination of N epsilon-(carboxymethyl)lysine in foods and related systems. Ann NY Acad Sci. 2008;1126:20–24. doi: 10.1196/annals.1433.030. [DOI] [PubMed] [Google Scholar]
- 27.Pouillart P, et al. Strategy for the study of the health impact of dietary Maillard products in clinical studies: the example of the ICARE clinical study on healthy adults. Ann NY Acad Sci. 2008;1126:173–176. doi: 10.1196/annals.1433.040. [DOI] [PubMed] [Google Scholar]
- 28.Birlouez-Aragon I, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91:1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436–443. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, et al. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology. 2009;150:2569–2576. doi: 10.1210/en.2008-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu MX, et al. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 33.Schalkwijk CG, Stehouwer CD, van Hinsbergh VW. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab Res Rev. 2004;20:369–382. doi: 10.1002/dmrr.488. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Ames JM, Smith RD, Baynes JW, Metz TO. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res. 2009;8:754–769. doi: 10.1021/pr800858h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Monnier VM, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group Diabetes Control and Complications Trial. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA. 1993;90:6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification—a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani N, Thornalley PJ. The dicarbonyl proteome: proteins susceptible to dicarbonyl glycation at functional sites in health, aging, and disease. Ann NY Acad Sci. 2008;1126:124–127. doi: 10.1196/annals.1433.043. [DOI] [PubMed] [Google Scholar]
- 40.Maillard LC. Action des acides anines sur les sucres: formation des melanoidines par voie methodique. Crit Rev Acad Sci. 1912;154:1653–1671. [Google Scholar]
- 41.Uribarri J, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2009;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg T, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien J, Morrissey PA. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit Rev Food Sci Nutr. 1989;28:211–248. doi: 10.1080/10408398909527499. [DOI] [PubMed] [Google Scholar]
- 44.Makita Z, Vlassara H, Cerami A, Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- 45.Mitsuhashi T, Vlassara H, Founds HW, Li YM. Standardizing the immunological measurement of advanced glycation endproducts using normal human serum. J Immunol Methods. 1997;207:79–88. doi: 10.1016/s0022-1759(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 46.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilhovd BK, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2007;50:1409–1417. doi: 10.1007/s00125-007-0687-z. [DOI] [PubMed] [Google Scholar]
- 48.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bucala R, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Januszewski AS, Alderson NL, Jenkins AJ, Thorpe SR, Baynes JW. Chemical modification of proteins during peroxidation of phospholipids. J Lipid Res. 2005;46:1440–1449. doi: 10.1194/jlr.M400442-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Stitt AW, et al. Elevated AGE-modified ApoB in sera of euglycemic, normolipidemic patients with atherosclerosis: relationships to tissue AGEs. Mol Med. 1997;3:617–627. [PMC free article] [PubMed] [Google Scholar]
- 52.Staprans I, Rapp JH, Pan XM, Feingold KR. Oxidized lipids in the diet are incorporated by the liver into very low density lipoprotein in rats. J Lipid Res. 1996;37:420–430. [PubMed] [Google Scholar]
- 53.Staprans I, Pan XM, Rapp JH, Feingold KR. Oxidized cholesterol in the diet is a source of oxidized lipoproteins in human serum. J Lipid Res. 2003;44:705–715. doi: 10.1194/jlr.M200266-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Cai W, et al. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;110:285–291. doi: 10.1161/01.CIR.0000135587.92455.0D. [DOI] [PubMed] [Google Scholar]
- 55.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2275–2281. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 56.Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14:485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 58.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adachi T, Inoue M, Hara H, Maehata E, Suzuki S. Relationship of plasma extracellular-superoxide dismutase level with insulin resistance in type 2 diabetic patients. J Endocrinol. 2004;181:413–417. doi: 10.1677/joe.0.1810413. [DOI] [PubMed] [Google Scholar]
- 60.Shinohara M, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142–1147. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson MM, Heinecke JW. Production of Nε-(carboxymethyl)lysine is impaired in mice deficient in NADPH oxidase: a role for phagocyte-derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes. 2003;52:2137–2143. doi: 10.2337/diabetes.52.8.2137. [DOI] [PubMed] [Google Scholar]
- 62.Thornalley PJ. Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–1348. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 63.Makita Z, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 64.Makita Z, et al. Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994;343:1519–1522. doi: 10.1016/s0140-6736(94)92935-1. [DOI] [PubMed] [Google Scholar]
- 65.Skolnik EY, et al. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991;174:931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He CJ, et al. Differential expression of renal AGE-receptor genes in NOD mouse kidneys: possible role in non-obese diabetic renal disease. Kidney Int. 2000;58:1931–1940. doi: 10.1111/j.1523-1755.2000.00365.x. [DOI] [PubMed] [Google Scholar]
- 67.Sindhu RK, Koo JR, Roberts CK, Vaziri ND. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens. 2004;26:43–53. doi: 10.1081/ceh-120027330. [DOI] [PubMed] [Google Scholar]
- 68.van Boekel MA. Formation of flavour compounds in the Maillard reaction. Biotechnol Adv. 2006;24:230–233. doi: 10.1016/j.biotechadv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Zamora R, Hidalgo FJ. Coordinate contribution of lipiod oxidation and maillard reaction to the nonenzymatic food browning. Crit Rev Food Sci Nutr. 2005;45:49–59. doi: 10.1080/10408690590900117. [DOI] [PubMed] [Google Scholar]
- 70.Tessier FJ, Niquet C. The metabolic, nutritional and toxicological consequences of ingested dietary Maillard reaction products: a literature review [French] J Soc Biol. 2007;201:199–207. doi: 10.1051/jbio:2007025. [DOI] [PubMed] [Google Scholar]
- 71.He C, Sabol J, Mitsuhashi T, Vlassara H. Dietary glycotoxins: inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes. 1999;48:1308–1315. doi: 10.2337/diabetes.48.6.1308. [DOI] [PubMed] [Google Scholar]
- 72.Cai W, et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol. 2007;170:1893–1902. doi: 10.2353/ajpath.2007.061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai W, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–336. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin RY, et al. Lowering of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis. 2002;163:303–311. doi: 10.1016/s0021-9150(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 75.Zheng F, et al. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002;18:224–237. doi: 10.1002/dmrr.283. [DOI] [PubMed] [Google Scholar]
- 76.Lin RY, et al. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2003;168:213–220. doi: 10.1016/s0021-9150(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt AM, Stern DM. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci. 2001;6:D1151–D1160. doi: 10.2741/schmidt. [DOI] [PubMed] [Google Scholar]
- 78.Coughlan MT, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu C, et al. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci USA. 2004;101:11767–11772. doi: 10.1073/pnas.0401588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci USA. 2006;103:13801–13806. doi: 10.1073/pnas.0600362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torreggianni M, et al. Advanced glycation end product receptor-1 transgenic mice are resistant to inflammation, oxidative stress, and post-injury intimal hyperplasia. Am J Pathol. 2009;175:1722–1732. doi: 10.2353/ajpath.2009.090138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 83.Yoshizaki T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Kreutzenberg SV, et al. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He CJ, Koschinsky T, Buenting C, Vlassara H. Presence of diabetic complications in type 1 diabetic patients correlates with low expression of mononuclear cell AGE-receptor-1 and elevated serum AGE. Mol Med. 2001;7:159–168. [PMC free article] [PubMed] [Google Scholar]
- 86.Menghini R, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 87.Cai W, et al. AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol. 2008;294:C145–C152. doi: 10.1152/ajpcell.00350.2007. [DOI] [PubMed] [Google Scholar]
- 88.Urribarri J, et al. Improved insulin resistance in human type 2 diabetes by AGE-restriction; potential role of AGER1 and SIRT1. Diabetes. doi: 10.2337/DC11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vlassara H, Brownlee M, Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986;164:1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vlassara H, Brownlee M, Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci USA. 1985;82:5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamagata T, et al. Genome organization of human 48-kDa oligosaccharyltransferase (DDOST) Genomics. 1997;45:535–540. doi: 10.1006/geno.1997.4966. [DOI] [PubMed] [Google Scholar]
- 92.Silberstein S, Kelleher DJ, Gilmore R. The 48-kDa subunit of the mammalian oligosaccharyltransferase complex is homologous to the essential yeast protein WBP1. J Biol Chem. 1992;267:23658–23663. [PubMed] [Google Scholar]
- 93.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 94.Kislinger T, et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21:905–910. doi: 10.1161/01.atv.21.6.905. [DOI] [PubMed] [Google Scholar]
- 95.Ohgami N, et al. CD36, a member of class B scavenger receptor family, is a receptor for advanced glycation end products. Ann NY Acad Sci. 2001;947:350–355. doi: 10.1111/j.1749-6632.2001.tb03961.x. [DOI] [PubMed] [Google Scholar]
- 96.Kislinger T, et al. Nε-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 97.Cai W, et al. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-delta: implications for vascular disease. Am J Physiol Cell Physiol. 2009;298:C624–C634. doi: 10.1152/ajpcell.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wautier MP, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 99.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR) Novartis Found Symp. 2002;248:171–6. discussion 176–180, 277–282. [PubMed] [Google Scholar]
- 100.Fukuhara S, Chikumi H, Gutkind JS. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene. 2001;20:1661–1668. doi: 10.1038/sj.onc.1204182. [DOI] [PubMed] [Google Scholar]
- 101.Napoli C, et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Migliaccio E, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 103.Li YM, Tan AX, Vlassara H. Antibacterial activity of lysozyme and lactoferrin is inhibited by binding of advanced glycation-modified proteins to a conserved motif. Nat Med. 1995;1:1057–1061. doi: 10.1038/nm1095-1057. [DOI] [PubMed] [Google Scholar]
- 104.Gao X, Zhang H, Schmidt AM, Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2008;295:H491–H498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deane R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 106.Bierhaus A, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 107.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Branco-Madeira F, Lambrecht BN. High mobility group box-1 recognition: the beginning of a RAGEless era? EMBO Mol Med. 2010;2:193–195. doi: 10.1002/emmm.201000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 110.Qin YH, et al. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol. 2009;183:6244–6250. doi: 10.4049/jimmunol.0900390. [DOI] [PubMed] [Google Scholar]
- 111.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, et al. sRAGE induces human monocyte survival and differentiation. J Immunol. 2010;185:1822–1835. doi: 10.4049/jimmunol.0903398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 114.Sourris KC, et al. Receptor for AGEs (RAGE) blockade may exert its renoprotective effects in patients with diabetic nephropathy via induction of the angiotensin II type 2 (AT2) receptor. Diabetologia. 53:2442–2451. doi: 10.1007/s00125-010-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tikellis C, et al. Cardiac inflammation associated with a Western diet is mediated via activation of RAGE by AGEs. Am J Physiol Endocrinol Metab. 2008;295:E323–E330. doi: 10.1152/ajpendo.00024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vlassara H, Brownlee M, Cerami A. Specific macrophage receptor activity for advanced glycosylation end products inversely correlates with insulin levels in vivo. Diabetes. 1988;37:456–461. doi: 10.2337/diab.37.4.456. [DOI] [PubMed] [Google Scholar]
- 117.Mitsuhashi T, Li YM, Fishbane S, Vlassara H. Depletion of reactive advanced glycation endproducts from diabetic uremic sera using a lysozyme-linked matrix. J Clin Invest. 1997;100:847–854. doi: 10.1172/JCI119600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vlassara H, et al. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl. 2009;114:S3–S11. doi: 10.1038/ki.2009.401. [DOI] [PubMed] [Google Scholar]
- 119.Vlassara H, et al. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci USA. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oberg BP, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 121.Wu J, et al. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol. 2010;176:2163–2176. doi: 10.2353/ajpath.2010.090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vlassara H, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci USA. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J Biol Chem. 2000;275:21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- 125.Metz TOAN, Chahich ME, Thorpe SR, Baynes JW. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J Biol Chem. 2003;278:42012–42019. doi: 10.1074/jbc.M304292200. [DOI] [PubMed] [Google Scholar]
- 126.Wolffenbuttel BH, et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Degenhardt TP, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–950. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 128.Hofmann SM, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–2089. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- 129.Peppa M, et al. Fetal or neonatal low-glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes. 2003;52:1441–1448. doi: 10.2337/diabetes.52.6.1441. [DOI] [PubMed] [Google Scholar]
- 130.Sandu O, et al. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54:2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 131.Odegaard AO, Pereira MA. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr Rev. 2006;64:364–372. doi: 10.1111/j.1753-4887.2006.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 132.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 133.Stitt AW, et al. Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–794. doi: 10.2337/diabetes.54.3.785. [DOI] [PubMed] [Google Scholar]
- 134.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 135.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 136.Scherer PE. Adipose tissue: from lipid storage to compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 137.Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the phyriology of white adipose tissue. J Cell Physiol. 2008;216:3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- 138.Nguyen MT, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2008;282:357279–357292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 139.Patsouris D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 141.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Winer S, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 143.Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 144.Kirstein M, et al. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci USA. 1990;87:9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Y, et al. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–4278. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Imani F, et al. Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon gamma: role in tissue remodeling. J Exp Med. 1993;178:2165–2172. doi: 10.1084/jem.178.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kinkhabwala M, et al. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med. 1990;171:941–946. doi: 10.1084/jem.171.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen A, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13:1311–1320. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 150.Bordone L, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Böni-Schnetzler M, et al. Increased interleukin (IL)-1β messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 153.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 154.Vlassara H, et al. Identifying advanced glycation end products as a major source of oxidants in aging: implications for the management and/or prevention of reduced renal function in elderly persons. Semin Nephrol. 2009;29:594–603. doi: 10.1016/j.semnephrol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 156.Dabelea D. The accelerating epidemic of childhood diabetes. Lancet. 2009;373:1999–2000. doi: 10.1016/S0140-6736(09)60874-6. [DOI] [PubMed] [Google Scholar]
- 157.Dabelea D, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case–Control Study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alexander BT, Ojeda NB. Prenatal inflammation and the early origins of hypertension. Clin Exp Pharmacol Physiol. 2008;35:1403–1404. doi: 10.1111/j.1440-1681.2008.05074.x. [DOI] [PubMed] [Google Scholar]
- 159.Ojeda NB, Grigore D, Alexander BT. Developmental programming of hypertension: insight from animal models of nutritional manipulation. Hypertension. 2008;52:44–50. doi: 10.1161/HYPERTENSIONAHA.107.092890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nivoit P, et al. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia. 2009;52:1133–1142. doi: 10.1007/s00125-009-1316-9. [DOI] [PubMed] [Google Scholar]
- 161.Ling C, et al. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia. 2008;51:615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–2725. doi: 10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mericq V, et al. Maternally transmitted and food-derived glycotoxins: a factor preconditioning the young to diabetes? Diabetes Care. 2010;33:2232–2237. doi: 10.2337/dc10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dittrich R, et al. Concentrations of Nepsilon-carboxymethyllysine in human breast milk, infant formulas, and urine of infants. J Agric Food Chem. 2006;54:6924–6928. doi: 10.1021/jf060905h. [DOI] [PubMed] [Google Scholar]
- 165.Birlouez-Aragon I, et al. Assessment of protein glycation markers in infant formulas. Food Chemistry. 2004;87:253–259. [Google Scholar]
- 166.Wilkin TJ. Diabetes: 1 and 2, or one and the same? Progress with the accelerator hypothesis. Pediatr Diabetes. 2008;9:23–32. doi: 10.1111/j.1399-5448.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 167.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 168.Couper JJ, et al. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care. 2009;32:94–99. doi: 10.2337/dc08-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009;57:1874–1880. doi: 10.1111/j.1532-5415.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22:74–79. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Semba RD, Fink JC, Sun K, Windham BG, Ferrucci L. Serum carboxymethyl-lysine, a dominant advanced glycation end product, is associated with chronic kidney disease: the Baltimore longitudinal study of aging. J Ren Nutr. 2010;20:74–81. doi: 10.1053/j.jrn.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Uribarri J, et al. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol. 2003;14:728–731. doi: 10.1097/01.asn.0000051593.41395.b9. [DOI] [PubMed] [Google Scholar]
- 173.Uribarri J, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2009;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akkarachaneeyakorn S, et al. Optimization of combined microwave-hot air roasting of malt based on energy consumption and neo-formed contaminants content. J Food Sci. 2010;75:E201–E207. doi: 10.1111/j.1750-3841.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 175.Miura J, et al. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with type 1 diabetes. J Diabetes Complications. 2003;17:16–21. doi: 10.1016/s1056-8727(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 176.Gul A, Rahman MA, Salim A, Simjee SU. Advanced glycation end products in senile diabetic and nondiabetic patients with cataract. J Diabetes Complications. 2009;23:343–348. doi: 10.1016/j.jdiacomp.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 177.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]