Abstract
Rationale
Endocannabinoid, opioid, and dopamine systems interact to exhibit cannabinoid receptor neuromodulation of opioid peptides and D4 dopamine receptor gene expression in CB1-cannabinoid-deficient mouse striatum.
Objective
Using CB1-transgenic mice, we examine primary age–sex influences and interactions on opioid and dopamine system members’ gene expression in striatum.
Materials and methods
Real-time quantitative polymerase chain reaction was used to analyze gene expression of opioid peptides [preproenkephalin (PPENK); preprodynorphin (PPDYN)], opioid receptors [delta-opioid receptor (δ-OR); mu-opioid receptor (μ-OR)] and dopamine receptor subtypes (D1 through D5) in male/female CB1(+/+)/CB1(−/−) mice striata at two adult ages [young (60–90 days); old (140–300 days)].
Results
(1) Increased PPENK and PPDYN, owing to genotype [CB1(+/+) vs. CB1(−/−)], depended on sex. When genotype-independent, they depended on sex (PPENK) or age (PPDYN). (2) δ-OR was age-dependent (higher in old). (3) μ-OR, owing to genotype, was age-dependent [higher in old CB1(−/−) males]. When genotype-independent, it depended on sex (higher in females). (4) Female D1 was genotype-independent and age-dependent, while male D1 was higher in old over young CB1(+/+) mice. (5) D5, owing to genotype, was sex-dependent [higher in young female CB1(−/−) mice]. (6) D2, genotype-independent, was higher in old over young male mice. (7) Young female D3 was higher in CB1(−/−) over CB1(+/+) mice. Male D3 was age-dependent (higher in old mice). (8) D4, owing to genotype, was sex-dependent [higher in CB1(−/−) over CB1(+/+) females]. Genotype-independent D4 was sex-dependent in young mice (higher in females) and age-dependent in males (higher in old).
Conclusions
Greater striatal expression is genotype-dependent in females (opioid-peptides, D3, D4, D5) and genotype-independent in both females (PPENK, μ-OR, D4) and old males (PPDYN, δ-OR, D2, D3, D4).
Keywords: Real-time polymerase chain reaction, δ-opioid receptors (DOR), μ-opioid receptors (MOR), κ-opioid receptors (KOR), Enkephalin, Dynorphin, Dopamine receptors, Caudate-putamen, Basal ganglia, C57BL/6 mice
Introduction
The endocannabinoid system in the brain is comprised of CB1, CB2, and additional putative cannabinoid receptors that respond to a family of eicosanoid mediators referred to as endocannabinoids, notably anandamide and 2-arachidonoylglycerol. The function of the endocannabinoid system includes the retrograde transmission of endocannabinoids and activation of presynaptic CB1 receptors to regulate neurotransmitter release (Chevaleyre et al. 2006). CB1 receptors are highly expressed in the striatum (Herkenham et al. 1991), and they affect directly or indirectly several functions that involve the basal ganglia (i.e., judgment, memory and reward, and the controls for planning and modulation of movement; Gerdeman et al. 2003; van der Stelt and Di Marzo 2003). There is a growing body of evidence that the endogenous cannabinoid system might participate in the motivational and dopamine-releasing effects of several drugs of abuse (Basavarajappa and Hungund 2005; Laviolette and Grace 2006; Parolaro et al. 2005). This may be mediated in part by activation of dopaminergic mesolimbic neurons. For example, stimulation of the CB1 receptors by the cannabinoid agonist Δ9-THC caused dopamine release and changes in gene expression in the striatum (Laviolette and Grace 2006). In CB1 knockout mice [CB1 (−/−)], responses to Δ9-THC are absent (Zimmer et al. 1999), and reinforcement effects of alcohol and opioid drugs are dramatically reduced (Hungund et al. 2003; Ledent et al. 1999). Moreover, CB1 antagonism by SR141716 (rimonabant) reduced opioid self-administration and conditioned place preference in rodents (Ledent et al. 1999; Martin et al. 2000).
In humans, age and sex differences have been reported when the biological and behavioral effects of drugs of abuse were examined (Greenfield et al. 2003; Randall et al. 1999; von Sydow et al. 2001; Young et al. 2002). These and other studies indicate that the onset and progression to drug addiction and the substances abused differ based on age and sex (Degenhardt et al. 2007). It also suggests the need to identify the neuromodulator mechanisms that are responsible for these differences.
Recent studies in animals indicate that ablation of the CB1 receptor causes significant differences in the gene expression of the endogenous opioid peptides, preproenkephalin (PPENK) and preprodynorphin (PPDYN), as well as D4 dopamine receptors in the striatum of CB1(−/−) mice (Gerald et al. 2006; Steiner et al. 1999) and reduction of D2 dopamine receptor binding (Houchi et al. 2005). In addition, human genetic variation (polymorphisms) in the cannabinoid, dopamine, and opioid receptor systems are, to varying degrees, linked with neuropathologies including those related to drug addiction (Chakrabarti et al. 2006; Hoenicka et al. 2007; Hopfer et al. 2006; Saxon et al. 2005; van den Wildenberg et al. 2007). In this present study, the evaluation of gene expression differences in CB1(−/−) transgenic mice was furthered by including, in our examination, the primary effects and interactions of sex and age on the gene expression of five members of the opioid receptor system and five dopamine receptor subtypes in the striatum.
Materials and methods
Animals
CB1 transgenic mice studies Zimmer et al. (1999) generated CB1(−/−) mice by replacing the coding region in the mouse CB1 receptor gene between amino acids 32 and 448 with a PGK-neo cassette to create chimeric mice which were bred with C57BL/6J animals. In the present study, CB1(+/+) and CB1(−/−) mice were produced by inbreeding the CB1(+/−) mice at North Carolina Central University or the University of North Carolina-Chapel Hill. All animals used in this study were treated in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 90–23) revised 1996. Animals were anesthetized and decapitated, as approved by institutional Animal Care and Use Committees. Adult mice of both sexes at two different ages [60–90 days (young) and 140–300 days (old)] were used. The number of animals used were males: CB1(+/+) young (6–8), old (8–10); CB1(−/−) young (7–8), old (5–8) and females: CB1(+/+) young (4–5), old (4); CB1(−/−) young (4–5), old (3).
Reverse transcription and qPCR
Both striata were dissected free hand from each mouse brain and immediately stored at −80°C until extraction of total RNA. Total RNA was isolated from striata of all mice using Trizol Reagent (Invitrogen) stored at −80°C. RNA was purified for reverse transcription and quantitative real-time polymerase chain reaction (qPCR) using the RNeasy MinElute Cleanup Kit (Qiagen) and registered a 260/280 nm absorbance ratio of >2.0.
Total purified RNA (1 μg) was reversed transcribed into complementary DNA (cDNA) using a high-capacity cDNA archive kit (Applied Biosystems). Quantitative real-time PCR was performed using a 7500 real-time PCR system (Applied Biosystems) utilizing the TaqMan gene expression assays specific for 18S (human), opioid peptides (mouse preprodynorphin and preproenkephalin), five dopamine receptor subtypes (mouse D1–D5), and three opioid receptors (mouse δ-OR, μ-OR, κ-OR). Gene expression was normalized to 18S cDNA. Relative quantitation of gene expression levels was performed using the Delta Delta Ct method (2−ΔΔCtmethod; Livak and Schmittgen 2001).
Statistics and data analysis
Relative expression data from qPCR for each gene examined was tested for normality using the Anderson–Darling normality test and for homogeneity of variance in group using Levene’s test. If data for a particular gene did not comply with the assumption of normality and/or homogeneity of variance, the relative expression ratios for that gene were subjected to a logarithmic transformation. Both transformed and non-transformed relative gene expression ratios were treated as the response in a general linear model (GLM) univariate analysis of variance (ANOVA). The model utilized a 2×2×2 factorial design by which genotype [CB1(+/+), CB1(−/−)], sex (male, female), and age (young, old) were fixed (i.e., each factor level represented a discrete value). The factors were crossed such that the main effects (genotype, sex, and age), primary interactions (genotype × sex, genotype × age, and sex × age) and the second-order interaction (genotype × sex × age) could be evaluated. A subsequent pairwise comparison of all means was performed using Tukey–Kramer and Bonferroni multiple comparison tests. To further examine the effect of CB1 ablation, both transformed and non-transformed data from CB1(+/+) and (CB1(−/−) mice of the same age and sex were compared using a two-sample t test. For this t test, equal variance was not assumed. For all data in this study, differences at the p≤0.05 level were considered significant. All statistical calculations and analyses were performed using the MINITAB 13.32 statistical software package (Minitab Inc., State College, PA, USA).
Results
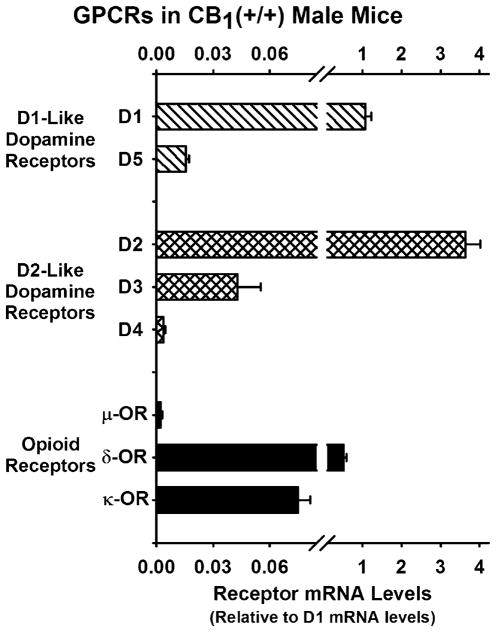
The amount of messenger RNA (mRNA) for eight G-protein coupled receptors (GPCRs) were examined in striata from young adult male CB1(+/+) mice, and the results are presented in Fig. 1 relative to D1 mRNA levels. The receptors examined included the D1-like dopamine receptor subtypes for which D1 was approximately 100-fold greater than D5. For the D2-like dopamine receptor subtypes, the D2 was approximately 100-fold greater than D3 and 1,000-fold greater than D4. For the opioid receptors, δ-OR was 200-fold greater than μ-OR and approximately tenfold greater than kappa opioid receptor (κ-OR).
Fig. 1.
G-protein coupled receptor (GPCR) mRNA levels in striata of young adult male CB1(+/+) mice. Relative mRNA levels for GPCRs (D1-like, D2-like, and opioid receptors) were determined in total RNA extracts from striata isolated from four to eight young CB1(+/+) mice brains. Analysis for D1-like (D1 and D5) and D2-like (D2, D3, D4) dopamine receptors and for opioid receptors (μ-OR, δ-OR, and κ-OR) were performed using RT-qPCR as described in “Materials and methods”. Levels of mRNA were quantitated using the Delta Delta Ct method (2−ΔΔCtmethod; Livak and Schmittgen 2001), and the results are presented relative to D1 dopamine receptor subtype mRNA levels in the striatum
Striatal opioid peptide gene expression
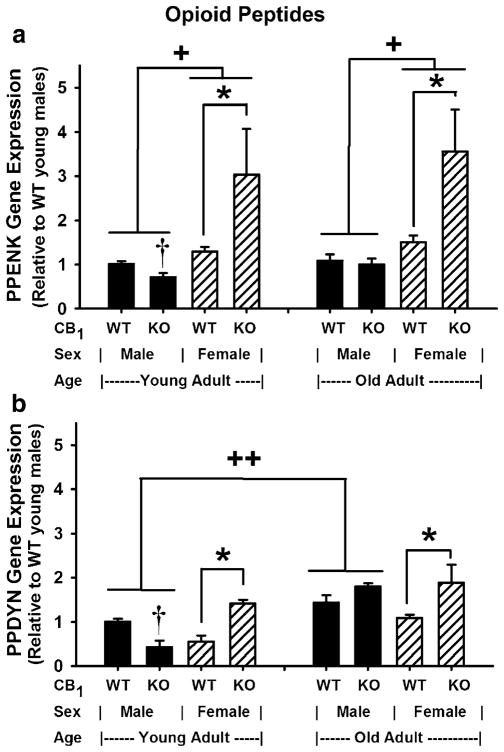
Evaluation of the data, in Fig. 2a, using a GLM ANOVA revealed that PPENK gene expression was dependent on interactions between sex and genotype [F(1,39) =11.29, p= 0.002] and on the main effect of sex [F(1,39)=31.94, p< 0.0005]. The apparent interactions between genotype and sex can be attributed to the influence from sex (female) on genotype (CB1 ablation). Subsequent pairwise comparisons of all means revealed significantly higher gene expression in CB1(−/−) over CB1(+/+) female mice (p<0.03) and higher gene expression in females over males independent of age and genotype (p<0.03). There were no main effects due to genotype [F(1,39)=2.85, p=0.099] or age [F(1,39)= 2.62, p=0.114], no primary interactions between sex and age [F(1,39) =0.10, p=0.751] nor between genotype and age [F(1,39) =1.15, p=0.290], and no second-order interactions among genotype, sex, and age [F(1,39) =0.03, p=0.859]. Although GLM ANOVA did not reveal an effect of genotype in males, a two-sample t test showed that PPENK gene expression in young males was reduced in CB1(−/−) mice when compared with CB1(+/+) mice (t =2.79, df=9, p=0.021).
Fig. 2.
Striatal opioid peptide gene expression in mice: The influence of CB1 ablation, sex, and age. a PPENK gene expression, b PPDYN gene expression. Gene expression levels were determined in total RNA extracts from brain striata of young and old adult CB1(+/+) [wild-type (WT)] and CB1(−/−) [mice with CB1 receptor ablation (KO)] mice (n=3–10) using RT-qPCR. All data are presented relative to expression levels in young adult male CB1(+/+) mice. Data were evaluated using a general linear model (GLM) univariate ANOVA. The model utilized a 2×2×2 factorial design where genotype, sex, and age were fixed. Subsequent differences due to genotype, sex, and age were determined using Tukey–Kramer and Bonferroni multiple comparison tests. In mice of the same age and sex, differences due to genotype (WT vs. KO mice) were also determined using a two-sample t test. a (PPENK gene expression): *differs due to genotype (WT vs. KO), p<0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. +differs due to sex, p<0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. †WT vs. KO mice of the same age and sex differ with a two-sample t test comparison; p=0.021. b (PPDYN gene expression): *differs due to genotype (WT vs. KO), p<0.001; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. ++differs due to age, p<0.002; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. †WT vs. KO mice of the same age and sex differ with a two-sample t test comparison; p=0.006
Evaluation of the data in Fig. 2b showed significant differences in PPDYN gene expression due to second-order interactions among genotype, sex, and age [F(1,38)=4.4, p= 0.043]; differences due to primary interactions between genotype and sex [F(1,38) =14.59, p<0.0005]; and significant main effects due to age [F(1,38) =33.37, p<0.0005] and genotype [F(1,38)=8.82, p=0.005]. There were no effects due to interactions between sex and age [F(1,38)=2.70, p= 0.109] nor between genotype and age [F(1,38) =3.45, p=0.071]. Unlike PPENK gene expression, which was higher in female over males, there was no main effect due to sex [F(1,38) =0.29, p=0.594]. Therefore, the significant second-order interactions among genotype, sex, and age were due mainly to the effect of age. The primary interaction between genotype and sex appeared to be mainly due to the effect of genotype, and subsequent pairwise comparisons of all means support this interpretation. Post hoc test revealed significantly higher PPDYN gene expression in old over young male mice regardless of genotype (p<0.002) and that CB1 ablation increased PPDYN gene expression in female mice regardless of age (p<0.001). When young males were evaluated separately, PPDYN gene expression appeared to be reduced in CB1 (−/−) mice when compared to their CB1(+/+) counterparts, and this was confirmed using a two-sample t test (t=3.63, df=9, p=0.006).
Striatal δ-OR and μ-OR gene expression in mice: influence of age and sex
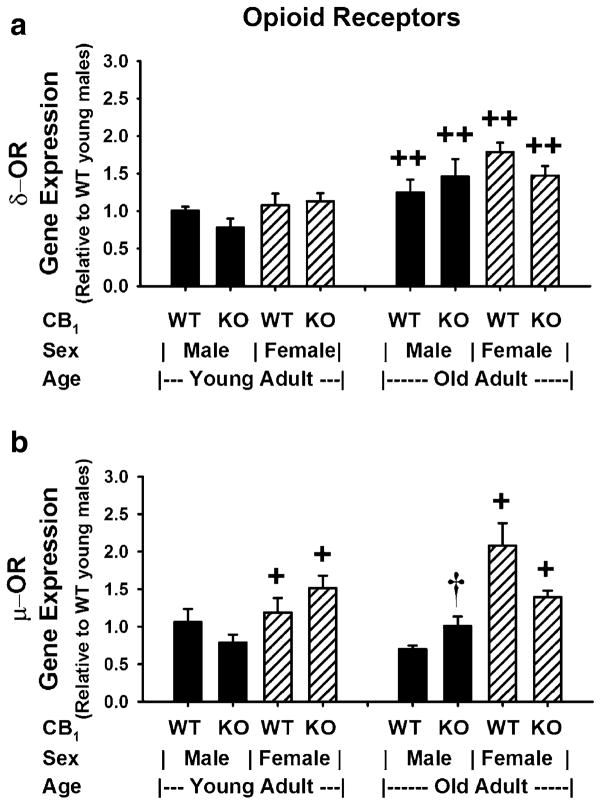
Evaluation of the data from Fig. 3a using a GLM ANOVA revealed significant differences in δ-OR gene expression due to the main effect of age [F(1,40) =14.91, p<0.0005]. Subsequent pairwise comparisons of all means confirmed significantly higher δ-OR gene expression in old over young mice when animals of the same genotype and sex were compared (p<0.05). There were no main effects due to genotype [F(1,40)=0.29, p=0.592] or sex [F(1,40) =3.74, p=0.06] nor were there primary or second-order interactions between groups: genotype and sex [F(1,40)=0.25, p= 0.621]; genotype and age [F(1,40) =0.02, p=0.878]; sex and age [F(1,40) =0.06, p=0.800]; or genotype, sex, and age [F(1,40) =2.52, p=0.121].
Fig. 3.
Striatal δ-OR and μ-OR in mice: Influence of CB1 ablation, sex, and age. a δ-OR (delta opioid receptor) and b μ-OR (mu opioid receptor). Gene expression levels were determined in total RNA extracts from brain striata of young and old adult CB1(+/+) and CB1(−/−) mice (n=3–10) and subjected to analysis as described in Fig. 2. a (δ-OR gene expression): ++differs due to age, p<0.05; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. b (μ-OR gene expression): +differs due to sex, p<0.002; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. †WT vs. KO mice of the same age and sex differ with a two-sample t test comparison; p=0.05
Data from Fig. 3b were evaluated using a GLM ANOVA, and it showed significant differences in μ-OR gene expression due to second-order interactions among genotype, sex, and age [F(1,37) =9.42, p=0.004] and a significant main effect due to sex [F(1,37) =29.19, p< 0.0005]. Therefore, the interactions among genotype, sex, and age were due mainly to the effects of sex. There were no main effects due to genotype [F(1,37) =0.05, p=0.830] or age [F(1,37) =1.05, p=0.313] and no primary interactions between genotype and sex [F(1,37) =0.07, p=0.798], genotype and age [F(1,37) =0.00, p=0.993], or sex and age [F(1,37) =2.04, p=0.162]. Subsequent pairwise comparisons of all means confirmed significantly higher μ-OR gene expression in female over male mice when expression was examined in mice of the same age and genotype (p<0.002). When CB1 (+/+) mice were compared to CB1(−/−) mice of the same age and sex, using a two-sample t test, a significant increase in μ-OR gene expression was observed in CB1(−/−) over CB1(+/+) old male mice (t=−2.27, df=9, p=0.05).
In a preliminary study with a limited number of samples, the influence of CB1 ablation, sex, and age on κ-OR gene expression was evaluated using a GLM ANOVA. The results revealed significant differences in κ-OR gene expression due to the effect of age [F(1,25)=5.95, p=0.022], and there were significant interactions between genotype and sex [F(1,25)=4.25, p=0.05]. No other interaction between groups (genotype and age; sex and age; genotype, sex, and age) were observed. Subsequent pairwise comparisons of all means using Tukey–Kramer and Bonferroni multiple comparison tests revealed significantly higher κ-OR gene expression in old over young male mice (p=0.043).
Dopamine D1-like receptor subtype gene expression: influence of CB1 ablation, sex, and age
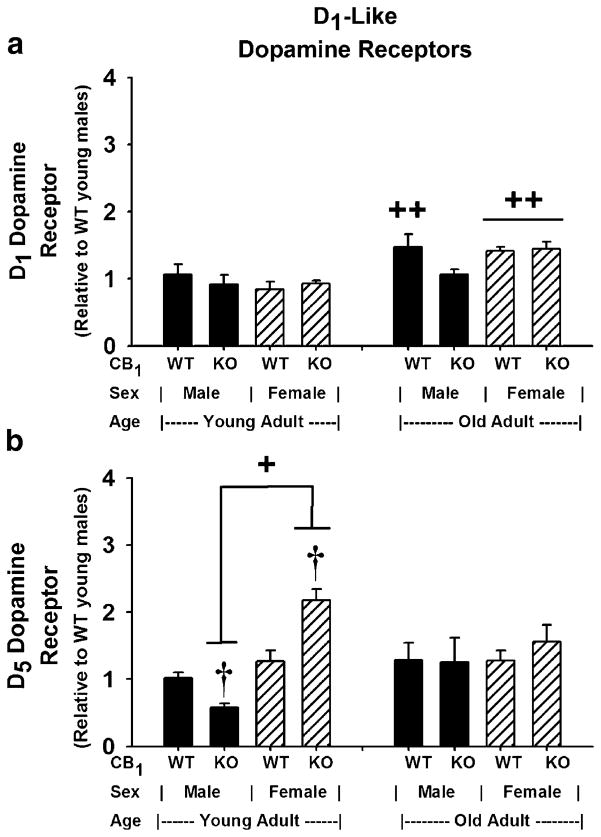
D1-like receptor (D1 and D5) gene expression in striata isolated from CB1(+/+) and CB1(−/−) mice were examined in Fig. 4. Evaluation of the data from Fig. 4a revealed significant differences in D1 dopamine receptor gene expression that were due mainly to the effect of age [F(1,38) =12.79, p=0.001]. Subsequent pairwise comparisons of all means revealed significantly higher D1 dopamine receptor gene expression in old over young CB1(+/+) mice regardless of sex (p<0.03) and higher gene expression in old over young female CB1(−/−) mice (p<0.03). There were no significant main effects due to genotype [F(1,38)= 0.95, p=0.336] or sex [F(1,38) =0.04, p=0.834] nor were there significant interactions between the groups: genotype and sex [F(1,38)=2.15, p=0.151]; genotype and age [F(1,38)= 0.49, p=0.487]; sex and age [F(1,38)=1.39, p=0.246]; genotype, sex, and age [F(1,38) =0.23, p=0.636].
Fig. 4.
Striatal D1-like dopamine receptor subtype gene expression: Influence of CB1 ablation, sex, and age. a D1 dopamine receptor and b D5 dopamine receptor. Gene expression levels were determined in total RNA isolated from brain striata of young and old adult CB1(+/+) and CB1(−/−) mice (n=3–10) and subjected to analysis as described in Fig. 2. a (D1 dopamine receptor gene expression): ++differs due to age, p<0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. b (D5 dopamine receptor gene expression): +differs due to sex, p<0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. †WT vs. KO mice of the same age and sex differ with a two-sample t test comparison; p≤0.012
Evaluation of the data from Fig. 4b using a GLM ANOVA revealed significant differences in D5 dopamine receptor gene expression due to the primary interaction between genotype and sex [F(1,40)=5.18, p=0.028] and the main effect of sex [F(1,40) =11.79, p=0.001]. There were no main effects due to genotype [F(1,40) =0.00, p=0.971] or age [F(1,40) =0.10, p=0.757], and there were no primary or second-order interactions between genotype and age [F(1,40)= 0.01, p=0.905]; sex and age [F(1,40)=1.71, p=0.199]; or genotype, sex, and age [F(1,40)=1.54, p=0.221]. Therefore, the primary interaction between genotype and sex was attributed to the main effect of sex. Subsequent pairwise comparisons revealed significantly higher D5 dopamine receptor gene expression in female over male young CB1(−/−) mice (p<0.03). A two-sample t test was used to compare the differences between CB1(+/+) and CB1(−/−) mice of the same sex and age. The results showed a reduction in receptor gene expression in young male mice (t=4.32, df=10, p=0.002) and an increase in receptor gene expression in young female mice (t=−3.53, df=6, p=0.012) due to CB1 ablation.
Dopamine D2-like receptor subtype gene expression: influence of CB1 ablation, sex and age
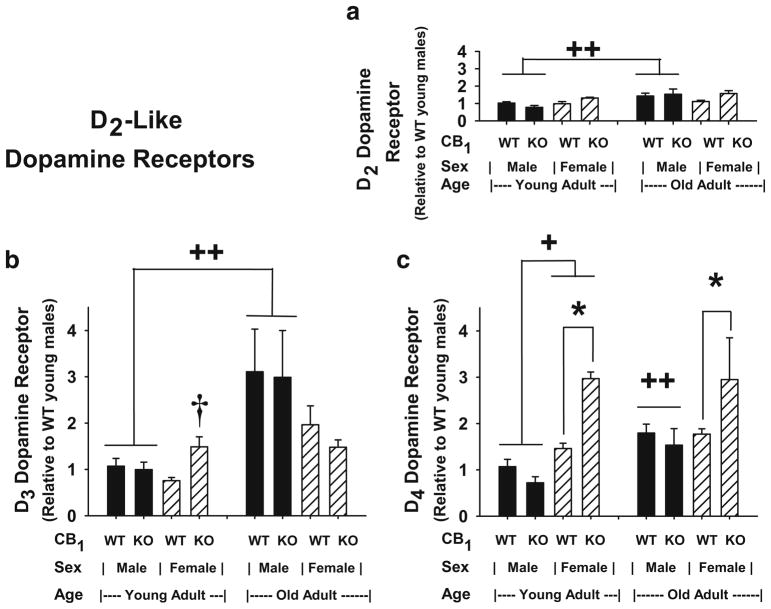
Evaluation of the data from Fig. 5a indicate that the combined effects of genotype and sex [F(1,41) =5.01, p= 0.031] appeared to influence the main effect of age [F(1,41)= 7.56, p=0.009] to significantly increase D2 dopamine receptor gene expression in old over young male mice (p<0.006). There were no main effects due to genotype [F(1,41) =0.54, p=0.467] or sex [F(1,41) =1.37, p=0.248], and there were no primary or second-order interactions between genotype and age [F(1,41) =0.57, p=0.455], sex and age [F(1,41) =1.69, p=0.200], and genotype, sex, and age [F(1,41) =0.41, p=0.527].
Fig. 5.
Striatal D2-like dopamine receptor subtype gene expression: Influence of CB1 ablation, sex, and age. a D2 dopamine receptor, b D3 dopamine receptor, and c D4 dopamine receptor. Gene expression levels were determined in total RNA isolated from brain striata of young and old adult CB1(+/+) and CB1(−/−) mice (n=3–10) and subjected to analysis as described in Fig. 2. a (D2 dopamine receptor gene expression): ++differs due to age, p<0.006; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. b (D3 dopamine receptor gene expression): ++differs due to age, p<0.01; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. †WT vs. KO mice of the same age and sex differ with a two-sample t test comparison; p=0.011. c (D4 dopamine receptor gene expression): *differs due to genotype (WT vs. KO), p<0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. +differs due to sex, p<0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests. ++differs due to age, p< 0.03; GLM ANOVA with Tukey–Kramer and Bonferroni multiple comparison tests
In Fig. 5b, significant differences in D3 dopamine receptor gene expression were due to the main effect of age [F(1,41)=9.85, p=0.003], with significantly higher gene expression in old over young male mice (p<0.01). There were no main effects due to genotype [F(1,41) =0.09, p= 0.763] or sex [F(1,41) =0.21, p=0.651] and no significant interactions between genotype and sex [F(1,41) =0.73, p= 0.398]; genotype and age [F(1,41) =1.21, p=0.278]; sex and age [F(1,41) =0.71, p=0.404]; or genotype, sex, and age [F(1,41) =1.11, p=0.299]. A t test analysis comparing CB1(+/+) to CB1(−/−) mice of the same sex and age showed higher receptor gene expression in CB1(−/−) over CB1(+/+) in young female mice (t=−3.60, df=6, p=0.011).
Differences in D4 dopamine receptor gene expression, in Fig. 5c, were due to interactions between genotype and sex [F(1,39) =16.51, p<0.0005] independent from the effect of age and to the main effects of sex [F(1,39) =24.66, p<0.0005], genotype [F(1,39) =6.56, p=0.014], and age [F(1,39) =5.12, p=0.029]. Comparisons of all means indicated D4 dopamine receptor gene expression was significantly higher in CB1(−/−) over CB1(+/+) in both young and old female mice (p<0.03). Furthermore, gene expression was higher in female over male young mice (p<0.03) and higher in old over young male mice (p<0.03) regardless of genotype. There were no primary interaction between genotype and age [F(1,39)=0.09, p=0.768] or sex and age [F(1,39) =2.33, p=0.135] and no second-order interactions among genotype, sex, and age [F(1,39) =0.26, p=0.614].
Discussion
CB1 receptor ablation in mice produces early mortality and deterioration in cognitive, memory, and motor function which has been characterized as “accelerated aging” (Bilkei-Gorzo et al. 2005; Steiner et al. 1999; Zimmer et al. 1999). Some of these phenotypic deficits involve neostriatal regulation, including increased immobility or catalepsy, hypoactivity, decline in skills learning, deficit social memory, and reduced operant responding to reinforcing stimuli (Bilkei-Gorzo et al. 2005; Holter et al. 2005). The reduced operant response reflects reduced incentive in that CB1(−/−) mice are characterized by a genotypic reduction in the consumption of sweet foods and alcohol and self-administration of reinforcing substances such as morphine (Cossu et al. 2001; Houchi et al. 2005; Hungund et al. 2003; Ledent et al. 1999; Martin et al. 2000; Sanchis-Segura et al. 2004). This behavioral phenotype has been linked with neurochemical differences in CB1(−/−) striatal tissue, including differences in the expression of substance P, opioid peptides, and enzymes that synthesize neurotransmitters (Steiner et al. 1999). Furthermore, reinforcing substances, which characteristically are accompanied by increased dopamine in the nucleus accumbens, fail to evoke dopamine accumulation in CB1(−/−) mice (Hungund et al. 2003; Mascia et al. 1999).
The goal of the present study was to extend our knowledge of the effects of CB1 receptor ablation on gene expression for members of the opioid (δ-OR, μ-OR, PPENK, and PPDYN) and dopamine (D1 through D5 receptor) systems in the striatum. This was accomplished by including in our evaluation the influence of the primary effects and interactions of sex and age on differences produced in CB1 transgenic mice. The results, presented in Figs. 2, 3, 4, and 5 and summarized in Table 1, showed genotypically greater expression of striatal opioid peptides and D3, D4 and D5 dopamine receptors in CB1(−/−) compared with CB1(+/+) mice. It was striking that these genotypic differences were restricted to females, particularly young adult females.
Table 1.
Relative differences in the expression of selected genes in mouse striatal tissue presented as a function of the following factors—genotype: CB1(−/−) relative to CB1(+/+) mice; sex: female relative to male mice; age: old relative to young adult mice
| Genes | Genotype CB1(−/−)/CB1(+/+) |
Sex Female/Male |
Age Old/Young |
|
|---|---|---|---|---|
| Opioid peptides | PPENK | ↑ Femalea | ↑ Youngb | –c |
| ↓ Male [young] | ↑ Oldb | – | ||
| PPDYN | ↑ Femalea | – | – | |
| ↓ Male [young] | – | ↑ Maleb | ||
| Opioid receptors | δ-OR | – | – | ↑ Femaleb |
| – | – | ↑ Maleb | ||
| μ-OR | – | ↑ Youngb | – | |
| ↑ Male [old] | ↑ Oldb | – | ||
| κ-OR | – | – | – | |
| – | – | ↑ Maleb | ||
| D1-like dopamine receptors | D1 dopamine receptor | – | – | ↑ Femaleb |
| – | – | ↑ Male [(+/+)] | ||
| D5 dopamine receptor | ↑ Female [young] | ↑ Young [(−/−)] | – | |
| ↓ Male [young] | – | – | ||
| D2-like dopamine receptors | D2 dopamine receptor | – | – | – |
| – | – | ↑ Maleb | ||
| D3 dopamine receptor | ↑ Female [young] | – | – | |
| – | – | ↑ Maleb | ||
| D4 dopamine receptor | ↑ Femalea | ↑ Youngb | – | |
| – | – | ↑ Maleb |
(+/+), CB1(+/+): mice with wild-type CB1 cannabinoid receptors; (−/−), CB1(−/−): transgenic mice deficient in CB1 cannabinoid receptors (Zimmer et al. 1999); young: adult mice 60–90 days old; old: adult mice 140–300 days old
PPENK preproenkephalin, PPDYN preprodynorphin, δ-OR delta opioid receptor, μ-OR mu opioid receptor, κ-OR kappa opioid receptor
Independent of age
Independent of genotype
No significant difference
Age-related differences in gene expression
Age-related differences were observed, particularly in males, in the expression of PPDYN and δ-OR in the opioid receptor system and in D1, D2, D3, and D4 dopamine receptor expression. Genotypic differences in gene expression were age-related in males for opioid peptide and μ-OR expression and in females for D3 and D5 dopamine receptors expression. The effect of age and CB1 receptor ablation on behavior has been extensively investigated in mice with C57BL/6 and CD1 backgrounds (Bilkei-Gorzo et al. 2005). In young mice (6–7 weeks), CB1(−/−) and CB1(+/+) mice perform the same in learning and memory paradigms including skill learning, partner recognition, and operant conditioning. In contrast, mature adult (3–5 months) CB1(−/−) perform poorly, but comparable to older (14–17 months) CB1(+/+) mice. These studies demonstrate an acceleration of the age-related decrement in cognitive and motor function in CB1(−/−) mice. One explanation for these age-related differences may be the sensitivity of neurons to neurotoxicity and the protective nature of endocannabinoids exerted through CB1 receptors (Veldhuis et al. 2003). However, it is not clear whether striatal neurons are as vulnerable to neurotoxicity as some areas of the hippocampus.
Significant age-related differences have been observed in the ability of the endocannabinoid system to modulate the intake of reinforcing substances such as ethanol and palatable food. In C57BL/6 mice that are in the same age range as the young adults used in the present study (6–10 weeks), CB1(+/+) mice prefer ethanol to a greater extent than do CB1(−/−) mice or CB1(+/+) mice treated with the CB1 antagonist SR141716 (Wang et al. 2003). Greater food intake is also observed in food-restricted juvenile CB1(+/+) mice compared with CB1(−/−) mice (Di Marzo et al. 2001) or CB1(+/+) mice treated with SR141716 (Wang et al. 2003). Juvenile mice have greater ethanol and food intake than old mice (26–48 weeks), and the ethanol and food intake in older adult mice cannot be reduced by SR141716 (Wang et al. 2003). Therefore, age has significant behavioral consequence when CB1 receptor activity is modified.
Our studies demonstrated several potential neurochemical substrates, in the opioid and dopamine receptor system, which may account for the genotype and age-related differences in motor behaviors and response to reinforcing substances. In previous studies, no differences in anandamide or 2-arachidonoylglycerol content were found in the limbic forebrain of young compared with old mice (Wang et al. 2003). Similarly, no differences were detected in CB1 receptor density in young compared with old mice. However, these studies do find twofold greater HU210-stimulated GTPγS binding in limbic forebrain in young versus old mice (Wang et al. 2003).
Sexual dimorphic effects of cannabinoids
In our study, PPENK and the μ-OR gene expression was greater in young and old adult females compared with males, with no genotypic increase in PPENK occurring in males but a genotypic increase in μ-OR in old adult males. The D3 and D5 receptor expression was significantly greater in young adult female CB1(−/−) compared with CB1(+/+), whereas no such increase was observed with males. In male mice, older adults expressed greater levels of D2 and D3 receptors than younger males. One explanation for phenotypic differences in the CB1(−/−) might be the developmental perturbations of the lack of cannabinoid receptors during critical stages of neurogenesis. Developmental studies (Rodriguez de Fonseca et al. 1993) have previously demonstrated a sexual dimorphic pattern of CB1 receptors arising in prenatal striata. Prenatal exposure to cannabinoid agonists modifies μ-OR density, and it differs with the sex of the animal (Perez-Rosado et al. 2000; Vela et al. 1998), leading to the possibility of differences established before birth in the CB1(−/−) genotype that might lead to a sexually dimorphic character when behaviors are tested as adults.
Behaviorally, Tseng and Craft (2001) found that Δ9-THC produces greater hypoactivity measures in female compared with male rats. The relatively greater hypoloco-motion and catalepsy responses to cannabinoid agonists in females are also observed with 11-OH-THC and CP55940, indicating that the sexual dimorphism is not attributed to pharmacokinetic disposition of Δ9-THC (Tseng and Craft 2001). The catalepsy response is blocked by SR141716, indicating that CB1 receptors are responsible (Tseng and Craft 2004). Sexually dimorphic responses in reinforcement behaviors have also been observed. Hungund et al. (2003) report that in a population of young adult C57BL/6 mice (13–15 weeks of age), the female mice consume significantly more alcohol (two-bottle choice test) than males. The ablation of the CB1 receptor significantly reduces the response in male mice. However, the magnitude of the reduction in females is much greater than males, as consumption of alcohol in both male and female mice does not differ.
Regulation of the dopaminergic and opioid mechanisms in the striatum and nucleus accumbens
The influence of CB1 receptors on enkephalin-containing neurons in striata has previously been reported. Cannabinoid treatment of rats with the potent agonist CP55940 for 18 days resulted in a robust increment in PPENK mRNA in both the striatum and nucleus accumbens (Manzanares et al. 1998). This may represent a means by which cannabinoid receptors mediate the activation of opioid receptors (Vigano et al. 2005). If our observed increases in PPENK in CB1(−/−) mice are also considered, these studies would suggest that the enkephalin-containing neurons are under tonic regulatory control by endocannabinoids. The converse interaction, the influence of enkephalin on the cannabinoid responses, is not as striking, inasmuch as no differences were observed between PPENK (−/−) and WT C57BL/6J mice in the ability of Δ9-THC to decrease locomotion or invoke catalepsy (Valverde et al. 2000). This would indicate that enkephalin-containing neurons are not intrinsic to a pathway that mediates these responses to cannabinoid drugs. Nevertheless, some of the withdrawal effects after chronic Δ9-THC treatment might involve enkephalin-containing neurons in the striatum (Valverde et al. 2000).
The influence of CB1 receptors on dynorphin-containing neurons in the striatum are beginning to be established. PPDYN(−/−) mice are no different from WT C57BL/6 in the Δ9-THC-decreased locomotion response attributed to striatal neurons. However, PPDYN(−/−) mice are characterized by the absence of Δ9-THC-induced conditioned place aversion (Zimmer et al. 2001). This suggests that dynorphin-containing neurons are in the pathway for the Δ9-THC dysphoric response. Nor-BNI also blocked Δ9-THC conditioned place aversion, indicating that dynorphin is working through κ-OR (Zimmer et al. 2001). It is not clear whether the striatum is involved in the anxiety and dysphoria due to Δ9-THC. The aversive response to kappa opioids was reduced in the CB1(−/−) phenotype, indicating that the aversive responses are a coordinated mechanism rather than one that is linearly downstream from the other (Zimmer et al. 2001). However, limited observations in our present study showed no difference in κ-OR expression due to genotype.
An extensive literature has developed surrounding the interactions between the endocannabinoid and dopaminergic systems in the striatum (Gerdeman et al. 2003; van der Stelt and Di Marzo 2003). CB1 receptors are expressed in D1-and D2-like receptor expressing neurons in the striatum (Hermann et al. 2002), suggesting a degree of cross-talk between these receptor systems. Indeed, D2-like receptors (D2, D3, and D4) have been shown to be functionally coupled to the endocannabinoid system, as dopamine increased anandamide synthesis and release in dorsal striata, and postsynaptic D2 receptor stimulation parallels the increase in endocannabinoid in striata (Giuffrida et al. 1999). In situ hybridization showed that chronic treatment with D2 antagonists up-regulated CB1 mRNA in rat caudate-putamen (Mailleux and Vanderhaeghen 1993). However, D1-like (D1, D5) receptor activation using the agonist SKF38393 did not exhibit similar effects on the endocannabinoid system (Giuffrida et al. 1999).
Influence of D4 dopamine receptor activity on behavior
Genetic modification of members of the endocannabinoid, opioid, and dopamine receptor systems have been linked to various neuropathologies with behavioral consequences, including those related to drug addiction, attention deficit hyperactivity disorder (ADHD), and bipolar disease (Saxon et al. 2005; Schmidt et al. 2002; Wong et al. 2000). However, many of these links or associations have been questioned due to conflicts among the published data (Chakrabarti et al. 2006; Helmeste and Tang 2000; Hoenicka et al. 2007; Hopfer et al. 2006; Saxon et al. 2005; van den Wildenberg et al. 2007; Wong et al. 2000). In the present study, large differences in D4 dopamine receptor gene expression were detected in CB1 ablated female mice, but gene expression independent of genotype was a function of age or sex. D4 receptor expression has specifically been linked to neuropathologies, particularly ADHD, whose relationship to genetic modification of the receptor is currently being considered (Helmeste and Tang 2000; Wong et al. 2000). Yet, D4-receptor-ablated mice exhibited hyperactivity in open field test, supersensitivity to the locomotor-stimulating effects of ethanol, cocaine and methamphetamine, and elevated synthesis of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid in the caudate-putamen, but not in the nucleus accumbens. These D4-receptor-deficient mice were more adept than wild-type mice at complex motor functions as determined by rotarod performance tests. This has been attributed to the elevated dopamine levels (Rubinstein et al. 1997). Spontaneously hypertensive rats (SHR), considered a suitable rat model for ADHD, are hyperactive in the open field test. In the SHR strain, D4 dopamine receptor gene expression was reduced in the prefrontal cortex, but not in the striatum (Li et al. 2007). In an ADHD model induced by lateral ventricle 6-hydroxydopamine injection, wild-type mice developed hyperactivity, but D4-receptor-ablated mice did not (Avale et al. 2004). D4 receptor antagonists (i.e., PNU-101387G, L-745,870, and U-101,958 but not S-18126) blocked the hyperactivity in this ADHD mouse model (Avale et al. 2004; Zhang et al. 2001, 2002). Despite these observations, the underlying mechanism(s) are still open to speculation.
A possible resolution to this question may reside in further studies involving CB1 transgenic mice. CB1-deficient mice lack normal dopamine-mediated suppression of PKA activity, mediated by postsynaptic D4 receptors, which blocks GABAA currents in the globus pallidus (Engler et al. 2006; Shin et al. 2003). Selective genes in striata, including those for opioid peptides, are influenced by increased cAMP and PKA activation via a cAMP response element binding protein (CREB)-mediated mechanism. Therefore, modulation of CB1 receptor activity could influence, either directly or indirectly, CREB-mediated mechanisms that affect gene expression that impacts memory, addiction, depression, and anxiety (Carlezon et al. 2005).
In conclusion, differences in opioid peptide and in D3, D4, and D5 dopamine receptor gene expression, which were greater in females, were observed in striata from CB1-ablated mice. Meanwhile, greater differences independent of genotype were observed in the gene expression for PPENK, μ-OR, and D4 dopamine receptors in females and were also observed in the gene expression for PPDYN, δ-OR, and dopamine receptor subtypes D2, D3, and D4 in old males. These significant genotypic, age-dependent, and sexually dimorphic differences in gene expression may contribute to the phenotypic deficit behaviors associated with these genes. They may also contribute to the various behavioral differences that have been observed with these featured groups.
Acknowledgments
The funding to support these studies has come from the National Institute on Drug Abuse (NIDA) cooperative agreement U24-DA12385 to build a drug abuse research program at a historically Black college or university (HBCU), and a National Center for Minority Health and Health Disparities EXPORT grant P20-MD00175. SOF was supported by NIGMS S06-GM08049. ACH was supported by NIDA Senior Research Scientist Award K05-DA00182 and R01-DA03690. CH was a participant in the Wake Forest University Summer Research Opportunities Program.
Contributor Information
Tonya M. Gerald, Chemistry Department, North Carolina Central University, Durham, NC 27707, USA. Neuroscience/Drug Abuse Research Program JLC-BBRI, North Carolina Central University, Durham, NC 27707, USA
Allyn C. Howlett, Neuroscience/Drug Abuse Research Program JLC-BBRI, North Carolina Central University, Durham, NC 27707, USA. Department of Physiology and Pharmacology, Wake Forest University Health Sciences, Winston-Salem, NC 27157, USA
Gregg R. Ward, Neuroscience/Drug Abuse Research Program JLC-BBRI, North Carolina Central University, Durham, NC 27707, USA. Department of Life Sciences, Winston-Salem State University, Winston-Salem, NC 27110, USA
Cheryl Ho, Department of Physiology and Pharmacology, Wake Forest University Health Sciences, Winston-Salem, NC 27157, USA.
Steven O. Franklin, Email: sfranklin1@nc.rr.com, Chemistry Department, North Carolina Central University, Durham, NC 27707, USA. Neuroscience/Drug Abuse Research Program JLC-BBRI, North Carolina Central University, Durham, NC 27707, USA. Department of Physiology and Pharmacology, Wake Forest University Health Sciences, One Medical Center Blvd., Winston-Salem, NC 27156, USA
References
- Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/ hyperactivity disorder. Mol Psychiatry. 2004;9:718–726. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol. 2005;40:15–24. doi: 10.1093/alcalc/agh111. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci USA. 2005;102:15670–15675. doi: 10.1073/pnas.0504640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur J Neurosci. 2006;23:1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC. Epidemiological patterns of extra-medical drug use in the United States: evidence from the National Comorbidity Survey Replication, 2001–2003. Drug Alcohol Depend. 2007;90:210–223. doi: 10.1016/j.drugalcdep.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Engler B, Freiman I, Urbanski M, Szabo B. Effects of exogenous and endogenous cannabinoids on GABAergic neuro-transmission between the caudate-putamen and the globus pallidus in the mouse. J Pharmacol Exp Ther. 2006;316:608–617. doi: 10.1124/jpet.105.092718. [DOI] [PubMed] [Google Scholar]
- Gerald TM, Ward GR, Howlett AC, Franklin SO. CB(1) knockout mice display significant changes in striatal opioid peptide and D(4) dopamine receptor gene expression. Brain Res. 2006;1093:20–24. doi: 10.1016/j.brainres.2006.03.088. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez dF, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Manwani SG, Nargiso JE. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:413–446. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Helmeste DM, Tang SW. Dopamine D4 receptors. Jpn J Pharmacol. 2000;82:1–14. doi: 10.1254/jjp.82.1. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hoenicka J, Ponce G, Jimenez-Arriero MA, Ampuero I, Rodriguez-Jimenez R, Rubio G, Aragues M, Ramos JA, Palomo T. Association in alcoholic patients between psychopathic traits and the additive effect of allelic forms of the CNR1 and FAAH endocannabinoid genes, and the 3′ region of the DRD2 gene. Neurotox Res. 2007;11:51–60. doi: 10.1007/BF03033482. [DOI] [PubMed] [Google Scholar]
- Holter SM, Kallnik M, Wurst W, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol. 2005;510:69–74. doi: 10.1016/j.ejphar.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP, Rhee SH, Smolen A, Krauter K, Hewitt JK, Ehringer MA. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet B Neuropsychiatr Genet. 2006;141:895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, Yew DT. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int. 2007;50:848–857. doi: 10.1016/j.neuint.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study. J Neurochem. 1993;61:1705–1712. doi: 10.1111/j.1471-4159.1993.tb09807.x. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res Mol Brain Res. 1998;55:126–132. doi: 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Mascia MS, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A, Fratta W. Lack of morphine-induced dopamine release in the nucleus accumbens of cannabinoid CB (1) receptor knockout mice. Eur J Pharmacol. 1999;383:R1–R2. doi: 10.1016/s0014-2999(99)00656-1. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Vigano D, Rubino T. Endocannabinoids and drug dependence. Curr Drug Targets CNS Neurol Disord. 2005;4:643–655. doi: 10.2174/156800705774933014. [DOI] [PubMed] [Google Scholar]
- Perez-Rosado A, Manzanares J, Fernandez-Ruiz J, Ramos JA. Prenatal Delta(9)-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Brain Res Dev Brain Res. 2000;120:77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline BH, Marsicano G, Lutz B, Spanagel R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology (Berl) 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Oreskovich MR, Brkanac Z. Genetic determinants of addiction to opioids and cocaine. Harv Rev Psychiatry. 2005;13:218–232. doi: 10.1080/10673220500243364. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Shin RM, Masuda M, Miura M, Sano H, Shirasawa T, Song WJ, Kobayashi K, Aosaki T. Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons. J Neurosci. 2003;23:11662–11672. doi: 10.1523/JNEUROSCI.23-37-11662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Bonner TI, Zimmer AM, Kitai ST, Zimmer A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. CB(1) receptor mediation of cannabinoid behavioral effects in male and female rats. Psychopharmacology (Berl) 2004;172:25–30. doi: 10.1007/s00213-003-1620-x. [DOI] [PubMed] [Google Scholar]
- Valverde O, Maldonado R, Valjent E, Zimmer AM, Zimmer A. Cannabinoid withdrawal syndrome is reduced in pre-proenkephalin knock-out mice. J Neurosci. 2000;20:9284–9289. doi: 10.1523/JNEUROSCI.20-24-09284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg E, Janssen RG, Hutchison KE, van Breukelen GJ, Wiers RW. Polymorphisms of the dopamine D4 receptor gene (DRD4 VNTR) and cannabinoid CB1 receptor gene (CNR1) are not strongly related to cue-reactivity after alcohol exposure. Addict Biol. 2007;12:210–220. doi: 10.1111/j.1369-1600.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Vela G, Martin S, Garcia-Gil L, Crespo JA, Ruiz-Gayo M, Javier Fernandez-Ruiz J, Garcia-Lecumberri C, Pelaprat D, Fuentes JA, Ramos JA, Ambrosio E. Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998;807:101–109. doi: 10.1016/s0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, Veldink GA, Vliegenthart JF, Bar PR, Nicolay K, Di Marzo V. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci. 2003;23:4127–4133. doi: 10.1523/JNEUROSCI.23-10-04127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Hofler M, Sonntag H, Wittchen HU. The natural course of cannabis use, abuse and dependence over four years: a longitudinal community study of adolescents and young adults. Drug Alcohol Depend. 2001;64:347–361. doi: 10.1016/s0376-8716(01)00137-5. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? Eur J Pharmacol. 2000;410:183–203. doi: 10.1016/s0014-2999(00)00815-3. [DOI] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Baldessarini RJ. Role of dopamine D(4) receptors in motor hyperactivity induced by neonatal 6-hydroxydopamine lesions in rats. Neuropsychopharmacology. 2001;25:624–632. doi: 10.1016/S0893-133X(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Zhang K, Davids E, Tarazi FI, Baldessarini RJ. Effects of dopamine D4 receptor-selective antagonists on motor hyperactivity in rats with neonatal 6-hydroxydopamine lesions. Psycho-pharmacology (Berl) 2002;161:100–106. doi: 10.1007/s00213-002-1018-1. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Valjent E, Konig M, Zimmer AM, Robledo P, Hahn H, Valverde O, Maldonado R. Absence of delta -9-tetrahy-drocannabinol dysphoric effects in dynorphin-deficient mice. J Neurosci. 2001;21:9499–9505. doi: 10.1523/JNEUROSCI.21-23-09499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]