Abstract
Due to the increasing demand to generate thick and vascularized tissue engineered constructs, novel strategies are currently being developed. An emerging example is the generation of oxygen-releasing biomaterials to tackle mass transport and diffusion limitations within engineered tissue-like constructs. Biomaterials containing oxygen releasing molecules can be fabricated in various forms such as, hybrid thin films, microparticles, or three dimensional (3D) scaffolds. In this perspective, we will summarize various oxygen-releasing reagents and their potential applications in regenerative engineering. Moreover, we will review the main approaches to fabricate oxygen-releasing biomaterials for a range of tissue engineering applications.
Keywords: oxygen-releasing biomaterials, peroxides, cell survival, tissue engineering
1. Introduction
Every year millions of people suffer from loss of life or other health related issues associated with organ failure (1). Due to the limitations with the number of available organ donors, there is an increasing demand for artificial tissue replacements. Tissue engineering aims to repair diseased or damaged tissues to improve human health (2). A crucial requirement to fabricate vascularized thick tissue constructs is to provide sufficient oxygen for the metabolically active cells encapsulated within the 3D structure of the engineered scaffolds. The presence of oxygen facilitates graft maturation especially during the early stages of tissue formation.
The dimensions of tissue engineered constructs should comply with the mass transport and diffusion limits to yield viable tissue constructs. For example, when the tissue thickness exceeds 1 mm, due to the limited oxygen diffusion into the 3D matrix, hypoxia conditions establish within the microenvironment as a function of time. This results in reduced cell viability within the 3D constructs. To tackle this hurdle, there have been recent efforts focusing on generation of oxygen releasing biomaterials as transplantable tissue-like constructs (3, 4).
Inadequate levels of oxygen have been shown to induce apoptosis and necrosis within 3D tissues both in vitro and in vivo (5–8). The use of oxygen-releasing constructs could decrease apoptosis and necrosis by providing sufficient oxygen for an extended period of time (3, 4). It may be of benefit to utilize these materials during maturation of newly formed functional tissues. Another potential application for oxygen-releasing biomaterials is the treatment of ischemic tissues, such as the cardiac muscle after myocardial infarction. Moreover, these biomaterials have great potential to promote healing for large and chronic wounds. However, one common problem with oxygen-delivering materials is the sudden release of oxygen, which typically damages the cells. Therefore, it is essential to design biomaterials with sustained oxygen-release capabilities for tissue engineering applications. The release kinetics of an ideal oxygen-releasing biopolymer should be tunable and extended from days up to weeks to allow sufficient time for revascularization and maturation of the engineered graft within the host system.(9).
The most common oxygen-releasing materials include sodium percarbonate (4), calcium peroxide (3, 9), magnesium peroxide (4), hydrogen peroxide (10, 11), and fluorinated compounds (12–14). Solid peroxides decompose upon exposure to water to release oxygen. However, if this process takes place too quickly, it may significantly damage the cells due to free radical formation (15). The rate of oxygen release via peroxide compounds is influenced by a number of factors such as, temperature, pH, and presence of a buffer or catalyst (16, 17). For example, when solid peroxide compounds react with water they form metal hydroxides, which induce an increase in the pH and the amount of released oxygen (18). Alternatively, the use of buffers provide adjustment of pH and therefore oxygen generation. Moreover, the purity and solubility of peroxides significantly affect the kinetics of oxygen release.
Oxygen-releasing biomaterials can be produced in various forms including thin films, microparticles, 3D scaffolds, or cell-laden hydrogels. The use of these biomaterials improves cell survival under hypoxic conditions where oxygen supply is limited. In addition, oxygen-producing biomaterials can potentially enhance vascularization and angiogenesis events. This is crucial for a wide range of applications in regenerative engineering especially for cardiac and pancreatic tissue formation and wound healing processes.
2. Oxygen-Generating Materials
Solid inorganic peroxides, such as calcium peroxide (CaO2), sodium percarbonate ((Na2CO3)2.1.5H2O2), and magnesium peroxide (MgO2) have been proposed to generate oxygen within liquid environments. As described in the chemical reactions below, formation of hydrogen peroxide (H2O2) takes place in the first step upon exposure of solid inorganic peroxides to water (Equations 1–3). This is subsequently followed by decomposition of H2O2 into oxygen in the second step (Equation 4) (17, 18).
| (1) |
| (2) |
| (3) |
| (4) |
MgO2 allows for the slowest oxygen formation compared to the rest of the solid reagents given above due to its lower solubility. For example, it was found that the equilibrium coefficients for MgO2 and CaO2 are 1.8×10−11 and 9.8×10−9, respectively (18) indicating the lower solubility and consequently slower reaction rate of MgO2 compared to CaO2 with water (19). Moreover, the availability of higher purity commercial formulations for CaO2 enables more efficient delivery of oxygen compared to MgO2 (20). For instance, CaO2 can be found with 60–80% purity, whereas MgO2 can only be obtained around 15–25% purity by weight (21). Therefore, CaO2 is commonly preferred reagent among the other solid peroxides. In addition, CaO2 is an inexpensive reagent and has a long history of applications as an oxygen-releasing compound. All of the above solid inorganic peroxides have been used in tissue engineering as oxygen-releasing reagents due to their cellular compatibility (3, 4, 22).
The rate of oxygen release is of significant importance for tissue construct formation. For example, if oxygen release takes place too quickly, the O2 cannot be utilized due to supersaturation. On the other hand, too slow oxygen release rates do not provide sufficient source to maintain healthy cellular function. Therefore, the ability to provide oxygen in a controlled and sustained manner may have important implications for biological systems. The rate of oxygen formation from peroxide compounds depends on a number of factors, including temperature, pH, the ratio of solid peroxide to water, amount of catalyst, and type of catalyst (23, 24). In addition, hydrophilicity of the surrounding biopolymer also influences the release rate of oxygen from the source. For example, if a hydrophobic material is used to encapsulate solid peroxides, the rate of oxygen release reaction is slow due to the slow diffusion of water into the hydrophobic materials (9). In this case, solid peroxide particles do not immediately come into contact with water, which induce slow release of oxygen. On the other hand, in case of hydrophilic materials, water adsorption happens quickly and thus solid peroxide particles start decomposing and generate oxygen faster. Both hydrophilic and hydrophobic oxygen-releasing biomaterials have been used for various tissue engineering applications (3, 4, 22).
Catalase is often times used as a catalyst for facilitating the conversion of H2O2 into oxygen (25). Catalase is an enzyme, present in liver and blood of mammals, and used to decompose H2O2 into water and oxygen (16) with very high turnover efficiency (26). This enzyme is composed of four heme (iron containing organic ring) groups embedded within the structure to be utilized in the oxygen conversion processes. Although the exact mechanism that catalase function is unknown, it is believed that the mechanism of decomposition reaction of H2O2 is given in Equations (5) and (6). Without catalase, the formation of H2O2 may lead to unwanted side reactions and cellular damage. Therefore, using a catalyst is a common strategy for the conversion of H2O2.
| (5) |
| (6) |
In addition to the physical and chemical properties of biomaterials, the oxygen consumption rate is also influenced by the cellular density and the metabolism. For instance, hypoxia condition is accelerated throughout the construct in the case of high density of highly metabolically active cells (9).
Although solid peroxides are commonly utilized as oxygen source, liquid H2O2 can also be used for oxygen generation. In this case, only the Equation (4) takes place for the formation of oxygen (15). It has been previously shown that CaO2, (Na2CO3)2.1.5H2O2, or MgO2 are more effective sources for in situ oxygen formation as compared to liquid H2O2 (16, 19, 27, 28); this is because oxygen generation can be achieved in a more controlled and sustained manner by the use of solid peroxides, which is a highly desired feature for tissue engineering research.
In addition to solid and liquid peroxides, it is also possible to use perfluorocarbons (PFCs) as oxygen supply for tissue engineering applications. The PFCs have the ability to dissolve large amounts of physiologically important gases, such as oxygen and carbon dioxide. These fluorinated liquids could potentially be used for tissue engineering applications where there is a high demand for oxygen (i.e. hypoxia conditions) (13, 29, 30). PFCs can be used as aqueous emulsion systems (12) or can be embedded into a suitable biomaterial (14) to supply oxygen. In liquid emulsions, oxygen trapped in the core of particles can only be transferred by diffusion since PFC droplets have higher density and are immiscible with water (13, 14).
The amount of released oxygen is described as “dissolved oxygen (DO)” in aqueous solutions and measured by using different approaches such as with the use of oxygen sensors (4, 9) or optical set-ups that utilize dye complexes (31). To determine the kinetics of oxygen-release, one common approach is to use electrochemical sensors that can reduce oxygen on a noble metal electrode, such as platinum (32). In this method, dissolved oxygen is measured in the liquid medium in terms of ppm or mm-Hg. However, due to their shortcomings, such as inaccurate measurements in low-oxygen environments, alternative methods have been developed. For instance, O'Neal et al. have fabricated an optical system, where a ruthenium complex is used to detect the amount of oxygen in cell culture media (31). In this study, the release kinetics of oxygen was determined based on the change in intensity of color. Alternatively, oxygen concentration in liquid environments can be determined by Trinder reaction (22).
3. Applications of Oxygen-Releasing Biomaterials in Tissue Engineering
Numerous approaches have been developed to incorporate oxygen-releasing molecules into biomaterials for in situ generation of oxygen (Figure 1). The most widely used methods are adsorption of oxygen-releasing molecules into fibers (33) or scaffolds (3), encapsulating them within 3D polymer networks (15) and direct administration of oxygen carrying reagents into the liquid medium (10). Various assays have been performed to test cellular response to oxygen-releasing biomaterials such as cell viability, metabolic activity, proliferation and apoptosis assays (Live/Dead, MTT, MTS, caspase activity, lactate dehydogenease) (3, 4, 9, 15).
Figure 1.
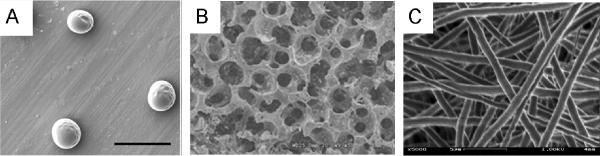
SEM images for solid peroxide incorporated biomaterials. (A) Core -shell H2O2-PVP microspheres (15) Copyright (2012) with permission from Elsevier, (B) 5% CaO2-PLGA scaffolds (3) Copyright (2009) with permission from Elsevier, (C) 10% CaO2-PCL electrospun nanofibers (33), Copyright (2011) American Chemical Society.
Solid peroxide compounds have been incorporated into electrospun nanofibers to form oxygen-releasing fibrous scaffolds for tissue engineering applications. For instance, in one report CaO2 particles were blended into polycaprolactone (PCL) at different concentrations (1, 5, 10% (w/w)) prior to electrospinning to fabricate hybrid nanofibers with or without the addition of a cytoprotective reagent, ascorbic acid (33). The incorporation of CaO2 into the PCL nanofibers was validated by Alizarin Red staining (Figure 2.A–D). The release of oxygen was determined by incubating the nanofibers in deionized water for different time points and testing the presence of calcium in the solution by a colorimetric assay (Figure 2.E). The burst release of CaO2 was found to occur in day 1. The addition of ascorbic acid was found to enhance the burst release due to enhanced pore sizes. The evaluation of antibacterial properties of the oxygen-releasing nanofibers was determined by incubating them with S. epidermidis and E. coli. The results indicated that there was significantly less bacterial activity when CaO2-PCL nanofibers were used. To test mammalian cell response against CaO2-PCL nanofibers, human osteoblast cells were seeded on the nanofibers and their proliferation was measured up to 4 days. CaO2 cytotoxicity was reported to be significantly higher on day 1 compared to day 4, which was due to the burst release of oxygen at day 1. The CaO2-PCL nanofibers could be used for preventing the colonization of bacteria on the surface of artificial prostheses and decreasing the risks of infection. Although this strategy was shown to be potentially useful, sustained delivery of oxygen is crucial and needs to be addressed for numerous applications in tissue engineering.
Figure 2.
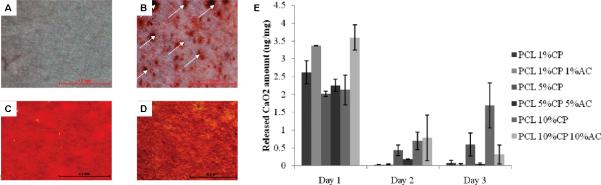
Characterization of CaO2 incorporated PCL nanofibers. Alizarin Red staining for calcium in (A) pure PCL nanofibers, (B) 1 % CaO2 blended PCL nanofibers, (C) 5 % CaO2 blended PCL nanofibers, (D) 10 % CaO2 blended PCL nanofibers, (E) Quantification of the amount of CaO2 released from the nanofibers with and without ascorbic acid (AC), which enhanced the burst release (33), Copyright (2011) American Chemical Society.
Towards clinical translation, Harrison et al. (2007) encapsulated peroxide compounds within polymeric scaffolds to study cellular response under hypoxic conditions (4). In this work, (Na2CO3)2.1.5H2O2-dispersed poly(lactic-co-glycolic acid) (PLGA) films were generated by a solvent casting technique. The release of oxygen from the film was confirmed by observation of gas bubbles over 24 h. The release of oxygen slowed down and was complete by 70 h. The (Na2CO3)2.1.5H2O2-PLGA films were then implanted subcutaneously in a skin flap nude mouse model to determine the amount of necrosis at different time points up to 7 days. It was observed that the oxygen-releasing films significantly decreased in vivo necrosis and lactate levels indicating the benefits of using oxygen supplementation in a wound healing model (Figure 3). It should be noted that the generation of oxygen for prolonged periods of time is preferred for a wide range of tissue engineering applications. Therefore, it is essential to develop approaches which control sustained release of oxygen over longer periods of time. For example, encapsulation of (Na2CO3)2.1.5H2O2 within a more hydrophobic polymer could potentially address this issue. The key finding of this work was the reduced tissue death by oxygen releasing films demonstrating the benefits of localized effect of oxygen delivery in ischemic tissues.
Figure 3.
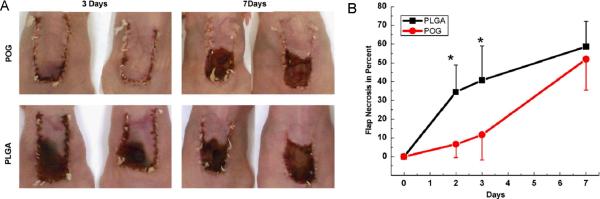
Polymeric oxygen generating (POG) PLGA films for tissue regeneration. (A) In vivo model demonstrating flap necrosis, (B) Quantification of percent flap necrosis (4) Copyright (2007) with permission from Elsevier.
In addition to solid peroxides, H2O2 in the liquid form has previously been used in oxygen delivery studies. For example, Li et al. (2012) have developed a method to fabricate core-shell oxygen-releasing microspheres for augmentation of cell survival under hypoxic conditions (15). They first generated microspheres of H2O2-bound poly(2-vinlypyrridione) (PVP) loaded PLGA by using a co-axial device (Figure 4). Then, the microspheres and cardiosphere-derived cells (CDCs) were encapsulated within a temperature sensitive hydrogel system consisted of acrylic acid (AAc), N-isopropylacrylamide (NIPAAm), and hydroxyethyl methacrylate-oligo(hydroxybutyrate) (HEMA-oHB) to test the survival and differentiation ability of CDCs. The rate of release of oxygen was tunable by varying the ratio of H2O2 to PVP. Cell viability was around 57.3% when CDCs were exposed the hypoxia conditions within the control hydrogels (without H2O2) at day 7. In addition, differentiation of CDCs has stopped upon exposure to hypoxia after 1 week. On the other hand, the viability of CDCs was found to be significantly enhanced within the oxygen-releasing hydrogel system. Similarly, the differentiation capacity of CDCs was significantly augmented in the oxygen-delivering hydrogel. It was reported that this strategy enabled release of oxygen up to 2 weeks. H2O2-releasing microspheres could be used for sustained delivery of oxygen to myocardial-infarcted tissues, which have been exposed to hypoxia during the early stages of the damage. This approach may also be of benefit to use in other cellular therapies where effective delivery of cells is required.
Figure 4.
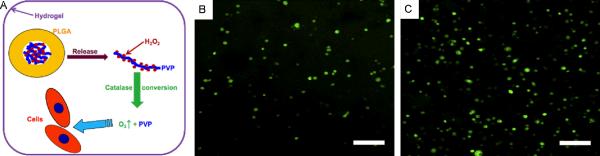
Oxygen-releasing H2O2-PVP-PLGA scaffolds to enhance CDC survival and differentiation upon exposure to 1% oxygen. (A) Schematic for the synthesis of H2O2-PVP-PLGA scaffolds, CDCs were encapsulated within a thermoresponsive hyd rogel (B) without and (C) with oxygen-releasing H2O2-PVP-PLGA blend. The viability of CDCs was determined by a Live/Dead assay after 14 days of culture under hypoxic conditions (15) Copyright (2012) with permission from Elsevier.
Oxygen releasing materials are beneficial for the formation of 3D scaffolds, which are more useful over 2D techniques for various tissue engineering applications, such as vascularization. The generation of pre-vascularization within the engineered scaffolds could address the issues relevant to adequate delivery of oxygen. In this context, incorporation of oxygen-releasing molecules within engineered tissues may provide additional oxygen to the construct and avoid the problems caused by the lack of oxygen, such as tissue necrosis. To test this hypothesis, Oh et al. (2009) have encapsulated solid CaO2 particles within 3D PLGA by using a porogen leaching procedure to enable the release of oxygen during 10 days (3) (Figure 1.B). The release of oxygen from the scaffold was determined by incubating the scaffolds in serum free media in a glovebox under hypoxia conditions (1% oxygen) and daily measurement of the dissolved oxygen in the medium by a gas analyzer during 10 days. The amount of oxygen in the media was found to be significantly higher when compared to the PLGA scaffold without CaO2 particles. To test the cellular response to oxygen-releasing material, 3T3 fibroblast cells were seeded on the PLGA scaffolds and incubated under hypoxia condition. Cellular viability and growth were tested using a standard MTS assay at days 1, 3, 5, 7, and 10. Cellular activity was observed to be significantly decreased after 3 days in the control scaffolds without CaO2 particles, whereas, the oxygen releasing scaffolds exhibited increasing trend for the metabolic activity until day 10. The oxygen-releasing scaffold maintained significantly higher levels of oxygen under hypoxic conditions compared to control samples of plain PLGA scaffolds. Similarly, cellular viability was found to be improved for the oxygen-releasing scaffolds. The fabricated oxygen-releasing scaffolds in this study could be useful as transplantable constructs in vivo and expected to maintain viable tissue constructs until vascular network formation.
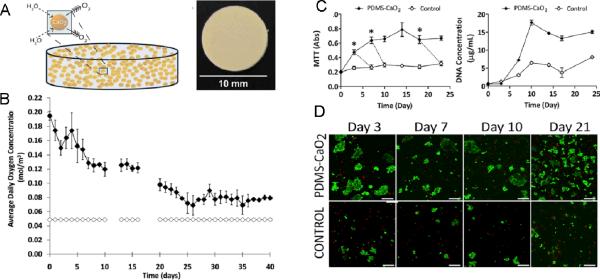
The viability and proper function of cells, and conservation of the cellular energy have important implications especially during the early stages of engineered graft maturation and angiogenesis events. Oxygen-releasing tissue constructs are not only crucial for fabrication of vascularized constructs, but also they are highly important for the cell types that demonstrate high metabolic activity and require high levels of oxygen for their survival and function. Therefore, consumption of oxygen within cell-loaded constructs significantly influences the cellular outcome. Thus, encapsulated cell type and cell density also affect the viability and growth processes. For example, beta-cells possess high metabolic activity and hence require elevated levels of oxygen for tissue formation. In one study, Pedraza et al. (2012) has generated CaO2-encapsulated PDMS disks and placed the oxygen-releasing cores inside beta-cell laden agarose gels (9).
In this study, PDMS was chosen as the CaO2 encapsulating polymer due to its hydrophobic properties that improves the efficiency of sustained release of oxygen by delaying the formation H2O2 upon contact of water. Steady release of oxygen from the CaO2-encapsulated PDMS disks was monitored over an extended time period, for 6 weeks. On the other hand, no change in oxygen levels was observed for the control PDMS disks (without CaO2) in the buffer solution (Figure 5.B). After confirmation of sustained release of oxygen, the cytocompatibility of the resulting biomaterial was tested by proliferation of MIN6 beta cells within oxygen-releasing hydrogel over 3 weeks, under both normoxic and hypoxic conditions. Cellular response was measured by total DNA content, metabolic activity (MTT), caspase activity, and lactate dehydrogenase release assays. They have demonstrated that CaO2-encapsulated PDMS augmented cell survival by preventing the formation of oxygen gradients throughout the hydrogel construct. As expected, scaffolds without CaO2 showed significantly lower cell viability and proliferation. This approach could have important implications in transplantation of pancreatic cells to treat diabetes.
Figure 5.
Oxygen-releasing agarose hydrogels. (A) CaO2-encapulated PDMS disk, (B) Quantification of released oxygen over 6 weeks (solid diamonds: CaO2-encapsulated PDMS disks, open diamonds: control PDMS disks without CaO2), (C) Results for metabolic activity and DNA content assays for MIN6 beta cells encapsulated within oxygen releasing agarose hydrogels over 3 week time period, (D) Live/Dead staining for MIN6 beta cells encapsulated within oxygen releasing 3D agarose hydrogels at day 21 (9). Copyright (2012) PNAS.
4. Conclusions and Future Perspectives
The inability to administer sufficient oxygen to thick artificial tissues and healing wounds brought about a growing interest for the design and development of novel functional biomaterials. In this perspective, we have reviewed the major technologies to incorporate oxygen-releasing molecules into biomaterials for different tissue engineering applications. The elimination of the onset of hypoxia within engineered constructs from the time of implantation to formation of functioning vasculatures is an exciting development towards translation into clinic. It is expected that these strategies will open up new research avenues for numerous applications in regenerative engineering. With the advances in oxygen releasing biopolymers, it is expected to significantly enhance cell viability as well as improving tissue function. These strategies are attractive for a wide range of areas in tissue engineering, such as wound healing, cardiac repair, and beta-cell transplantation. It is anticipated that the demand for oxygen-releasing polymers will exponentially increase in the next few years due to the enormous need to fabricate offthe-shelf engineered products to regenerate/repair different organs and tissues.
Acknowledgements
This paper was supported by the Office of Naval Research Young National Investigator Award, the Presidential Early Career Award for Scientists and Engineers (PECASE), the National Science Foundation CAREER Award (DMR 0847287), and the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392).
References
- 1.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Proc Natl Acad Sci USA. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 4.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Biomaterials. 2007;28(31):4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Lewis MC, MacArthur BD, Malda J, Pettet G, Please CP. Biotechnol Bioeng. 2005;91(5):607–615. doi: 10.1002/bit.20508. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Hochberg M. J of Exp Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radisic M, Yang LM, Boublik J, Cohen RJ, Langer R, Freed LE, et al. Am J Physiol-Heart C. 2004;286(2):H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 8.Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G. Biotechnol bioeng. 1999;64(5):580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Proc Natl Acad Sci USA. 2012;109(11):4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng SM, Choi JY, Han HS, Huh JS, Lim JO. Int J Pharm. 2010;384(1–2):120–127. doi: 10.1016/j.ijpharm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bae SE, Son JS, Park K, Han DK. J Control Release. 2009;133(1):37–43. doi: 10.1016/j.jconrel.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Chin K, Khattak SF, Bhatia SR, Roberts SC. Biotechnol Progr. 2008;24(2):358–366. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- 13.White J, Stoppel W, Roberts S, Bhatia S. J Biomed Mater Research Part A. 2013;101A(2):438–446. doi: 10.1002/jbm.a.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifu DG, Isimjan TT, Mequanint K. Acta Biomater. 2011;7(10):3670–8. doi: 10.1016/j.actbio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Guo X, Guan J. Biomaterials. 2012;33(25):5914–5923. doi: 10.1016/j.biomaterials.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Schmidtke T, White D, Woolard C. J Hazard Mater. 1999;64(2):157–65. doi: 10.1016/s0304-3894(98)00243-x. [DOI] [PubMed] [Google Scholar]
- 17.Northup A, Cassidy D. J Hazard Mater. 2008;152(3):1164–1170. doi: 10.1016/j.jhazmat.2007.07.096. [DOI] [PubMed] [Google Scholar]
- 18.Waite AJ, Bonner JS, Autenrieth R. Environ Eng Sci. 1999;16(3):187–199. [Google Scholar]
- 19.Borden RC, Goin RT, Kao CM. Ground Water Monit R. 1997;17(1):70–80. [Google Scholar]
- 20.Cassidy DP, Irvine RL. J Hazard Mater. 1999;69(1):25–39. doi: 10.1016/s0304-3894(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 21.White DM, Irvine RL, Woolard CR. J Hazard Mater. 1998;57(1–3):71–78. [Google Scholar]
- 22.Fraker CA, Mendez AJ, Stabler CL. J Phys Chem B. 2011;115(35):10547–10552. doi: 10.1021/jp204146n. [DOI] [PubMed] [Google Scholar]
- 23.Soleymani M, Moheb A, Babakhani D. Chem Eng & Technol. 2011;34(1):49–55. [Google Scholar]
- 24.Roy CB. J Catal. 1968;12(2):129–133. [Google Scholar]
- 25.Raducan A, Cantemir AR, Puiu M, Oancea D. Bioproc Biosyst Eng. 2012;35(9):1523–1530. doi: 10.1007/s00449-012-0742-0. [DOI] [PubMed] [Google Scholar]
- 26.Chelikani P, Fita I, Loewen PC. Cell Mol Life S. 2004;61(2):192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardieck DL, Bouwer EJ, Stone AT. J Contam Hydrol. 1992;9(3):221–242. [Google Scholar]
- 28.Spain JC, Milligan JD, Downey DC, Slaughter JK. Ground Water. 1989;27(2):163–167. [Google Scholar]
- 29.Khattak SF, Chin KS, Bhatia SR, Roberts SC. Biotechnol Bioeng. 2007;96(1):156–166. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 30.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Am J Physiol-Heart C. 2005;288(3):H1278–1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 31.O'Neil P, Meledeo MA, Davis JR, Ibey BL, Pishko M, Cote G. IEEE Sens J. 2004;4(6):728–734. [Google Scholar]
- 32.Schneider N, Lejeune JP, Deby C, Deby-Dupont GP, Serteyn D. Vet J. 2004;168(2):167–173. doi: 10.1016/j.tvjl.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Zhu Y, Bawa HK, Ng G, Wu Y, Libera M, et al. Acs Appl Mater & Inter. 2011;3(1):67–73. doi: 10.1021/am100862h. [DOI] [PubMed] [Google Scholar]