Abstract
Rationale
A differential-reinforcement-of-low-rate schedule (DRL) delivers reinforcement only when the interresponse time (IRT) exceeds a fixed time interval, thereby shaping rats to discriminate the timing of their responses. However, little is known about the motor behavior and location of the rats in the chamber during the IRTs that lead to reinforcement. Although amphetamine is known to disrupt DRL timing behavior, the effects of this drug on non-operant motor behavior during DRL performance has not yet been quantified.
Objective
The purpose of this research was to measure the motor behavior (movement trajectories in the horizontal plane and spatial location in the plane) during longer IRT’s after either vehicle or amphetamine treatment.
Method
Experimental chambers were constructed with a force-plate actometer as the floor, and while performing the operant task, the rats’ motor behaviors were measured continuously with high temporal and spatial resolution. Separate groups of 8 male Sprague Dawley rats were maintained on either DRL 24-s or DRL 72-s schedules of water reinforcement in 4-hr recording sessions.
Results
Analyses of IRT distributions showed that the rats’ timing behavior conformed to their respective DRL requirements. In the absence of drug, analysis of motor behavior in pre-reinforcement intervals showed that rats located themselves away from the operandum, and exhibited very low levels of movement. Rats exhibited a significant temporal diminution of horizontal movement that reached a minimum 4–8 s before the rats moved to the operandum to execute operant responses. Amphetamine treatment increased locomotion, abolished the temporal movement gradient, and brought the rats closer to the operandum compared to vehicle treatment. Movement changes induced by amphetamine were accompanied by degraded timing behavior.
Conclusions
Taken together, the data show that DRL training induced rats to locate themselves away from the operandum and to remain nearly motionless during longer IRTs, and that amphetamine treatment interfered with this complex of behavioral features
Keywords: Differential reinforcement of low rate, DRL 72 s, d-amphetamine, temporal discrimination, focused stereotypy, sensitization, force-plate actometer, rat
Introduction
In the differential-reinforcement-of-low-rate (DRL) schedule of reinforcement, a response produces a reinforcer only if the time interval since the previous response exceeds a fixed time interval set by the experimenter (e.g., Richards et al. 1993). Over the course of extensive training on this procedure, rats learn to control many of their responses temporally and eventually produce a frequency distribution of interresponse times that has a peak near the required time interval. Conceptual analyses of DRL performance have led to a consensus that both timing behavior (estimating and/or remembering how long it has been since the last response) and response inhibition (refraining from making the response before the interval times out) are key biobehavioral process that govern DRL-engendered behavior (e.g., Sanabria and Killeen 2008). Interest in these biobehavioral processes has led to the use of the DRL procedure in a wide variety of research contexts, including 1) behavioral theories of timing (e.g., Killeen and Fetterman 1988), 2) behavioral pharmacology of DRL performance (Richards et al. 1993), 3) hippocampal involvement in timing behavior (e.g., Meck et al, 1984; Bannerman et al. 1999; Young & McNaughton 2000; Costa et al. 2005), 4) screening drugs for antidepressant activity (e.g., O’Donnell et al. 2005), 5) validating animal models of ADHD (e.g., Bull et al. 2000; van den Berg et al. 2006; Sanabria & Killeen 2008), 6) evaluating animal models of psychosis (e.g., Featherstone et al. 2007), and 7) investigating animal models of impulsive behavior (e.g., Monterosso & Ainslie 1999). Despite such interest in the DRL schedule, little is known about collateral behavior (behavior occurring between separate operant responses) of rats during DRL performance, and the literature to date contains mixed and conflicting interpretations of the role of behavior during the IRT (cf. Laties et al. 1965; McIntire et al. 1983; McMillan 1969: Zuriff 1969).
Accordingly, one purpose of the experiment reported here was to obtain high resolution measurements of the rats’ locomotor activity, spatial location, and forcefulness of bodily movement in an operant conditioning chamber while their responding was maintained by a DRL-24-s or a DRL 72-s schedule of water reinforcement. A second purpose was to perturb the behavior with amphetamine, a drug having behavioral effects that are related to each of the research contexts listed above. More specifically, amphetamine has been reported to impair DRL-maintained performance by substantially increasing response rate, decreasing reinforcers per hour and shifting the peak of the IRT distribution to the left (e.g., Balcells-Olivero et al. 1997).
The two DRL parameters, 24-s and 72-s, were chosen for specific reasons. As judged by rewards earned per hour and by how closely the peak in the IRT distribution coincides with the DRL requirement, the 24-s parameter poses a less difficult task than the 72-s DRL requirement (see Doughty & Richards 2002, who compared DRL 18-s and DRL 72-s). Thus, the two DRL parameters represent levels of task difficulty. The 72-s DRL parameter is at the high end of the difficulty dimensions in experiments with rats, and there is an established literature with this parameter value in the context of a screen for antidepressant activity of drugs (e.g., O’Donnell et al. 2005). The 24-s requirement is near the high end of the range of parameters used to evaluate the effects of hippocampal lesions on timing behavior (e.g., Costa et al. 2005; Bannerman et al.1999) or to examine hippocampal neuronal firing rates during DRL performance (Young & McNaughton 2000).
Assessing the effects of amphetamine on DRL-maintained behavior is complicated by the fact that the behavioral response to this drug may change as repeated dosing ensues (e.g., Blackman 1989). For example, both tolerance (e.g., Schuster and Zimmerman 1962) and sensitization (e.g., Balcells-Olivero et al. 1997) to amphetamine’s effects on DRL-maintained behavior have been reported following repeated administrations of the drug. Moreover, evidence for amphetamine-induced behavioral sensitization has been observed after a single dose that induced focused stereotypy (Kuczenski & Segal 1999). These results and many others (summarized by Blackman 1989) suggest the difficulty of interpreting within-subject amphetamine dose-effect experimental designs where dose is confounded with repeated treatment. As a consequence of these considerations, we attempted to achieve stability of behavioral response to amphetamine by exposing DRL-trained rats to a sensitizing series of focused stereotypy-inducing amphetamine treatments (5.0 mg/kg). In addition, we used 4-hr periods of behavioral measurement so that behavior could be expressed across a relatively broad range of brain concentrations of amphetamine (Osterhaus et al. 2007; Kuczenski and Seigel 1999). Thus, in the current study, effects of amphetamine on DRL-maintained behavior were examined under conditions in which both between-session and within-session sensitization (within-session sensitization to amphetamine has been described by Kuczenski and Seigel, 1999) were thought to have reached near steady state levels.
Methods and Materials
Subjects
Sixteen experimentally naïve male, Sprague Dawley rats (Harlan, Indianapolis) approximately 70 days of age when training procedures began and 145 days old and mean body weight of 370.0 g (SEM 6.9) at the time of the first injection were used. The rats were maintained on a restricted watering regimen of 15 ml per day that permitted a slow weight gain of a few grams per week throughout the study. Water obtained in the operant conditioning session was included in the 15 ml total. If water intake in the experimental chambers exceeded 15 ml, no additional water was given. Food was freely available in the individual home cages. Lights in the colony room were programmed on a 12:12 hr light: dark cycle (lights on at 6:00 AM). Behavioral measurements were recorded during the light portion of the lighting cycle. The rats were housed individually in plastic tub cages with Aspen wood (non aromatic) shavings as bedding. Use of the animals was approved by the University of Kansas Institutional Animal Care and Use Committee, and procedures adhered to the National Institutes of Health 1996 edition of the Guide for the Care and Use of Laboratory Animals.
Apparatus
The behavior of the rats was recorded in 4 concurrently operating “hybrid” chambers, each consisting of a force-plate actometer (Fowler et al. 2001) as the floor and operant conditioning fixtures (an operandum and a water port served by a peristaltic pump on one wall). Each chamber was enclosed in a sound-attenuating cabinet (Med Associates, ENV-018MD). The sensing area of the load plate of the actometer was 28 cm × 28 cm. A transparent Plexiglas™ chamber, 28 cm square and 23 cm high, rested on four vertical supports that allowed the cage to be positioned 2 mm above the load plate. Two apertures were cut into one wall of the chamber. One aperture provided access to the operandum and was 2.5 cm high and 3.0 cm wide with the left edge located 3.0 cm from the adjacent wall and the lower edge located 5.0 cm above the floor. The 1.8 cm diameter disk-shaped operandum was located outside the cage such that the perimeter of the disk closest to the wall was 1.6 cm from the inside surface of the chamber. When extended fully, the center of the rat’s forepaw could reach the center of the sensing disk. The disk was mounted on an isometric force-transducer (the same type of transducer used in the force-plate; see Fowler et al. 2001, for details). Each press that exceeded 12 gram equivalent weights in peak force counted as an operant response. Placing the operandum outside of the chamber ensured strict control over the topography of the response; that is, the rat was unable to bite or nose the operandum or to strike it in the course of ambulating along the inside perimeter of the chamber.
To the right of the operandum, a second aperture, 7 cm × 7 cm, was cut into the wall, such that its lower edge was 1.3 cm above the floor and its right edge was 2.5 cm from the adjacent wall. A 4-cm-deep transparent polycarbonate enclosure covered the opening and provided access to a 1.8 cm diameter aluminum disk via a 1.5 cm hole. The aluminum disk served as a water port. Water was pumped into the center of the disk by a Manostat peristaltic pump (Model 72-410-014) located outside the sound attenuating cabinet. The lick disk had a 1.2 cm diameter central depression 1 mm deep that contained the dispensed water and kept it from flowing off the horizontal disk surface. The horizontal distance from the center of the operandum to the center of the lick disk where water was dispensed was 18 cm. The distances and geometry were such that the rat had to move all 4 limbs in covering the distance from executing an operant response to licking from the water port. The onset of water delivery was accompanied by the click of a relay (Potter & Brumfield, model T9AS1D22-12) located 5 cm outside the chamber and half way between the operandum and water port. Additionally, an indicator light in the top of the water port aperture was illuminated during the pump cycle. A 14-V DC bulb located over the top of the chamber provided ambient illumination during sessions. Each hybrid chamber was serviced by a separate PC computer equipped with a LabMaster™ interface (Scientific Solutions, Mentor, Ohio). Data recording and experimental control were accomplished with a temporal resolution of 0.01 s through the use of custom software written in-house in Free Pascal (http://www.freepascal.org/).
Behavioral Training
Rats were shaped by the method of successive approximations to reach through the operandum aperture and strike the operandum with either forepaw. Manual shaping continued for 15 to 20 min per day until all rats had learned to press the operandum with peak forces that exceeded 12 g. Exteroceptive feedback was provided only for reinforced (RFD) responses and was limited to stimuli (relay click and light-on in the water port) that accompanied water delivery. Once the operant response occurred, all rats were given a 30-min session on Fixed Ratio 1 with a reinforcer volume of 0.06 ml. Over the next 25 days rats were exposed to progressively increasing DRL requirements starting with a 2-s DRL requirement in 30-min sessions and ending with a 24-s DRL requirement in a single 4-hr session (a total of 11.5 hr of experience). At this point, 8 rats were switched (randomly) to the DRL 72-s requirement and the 8 other rats remained at the 24-s requirement. When the 4-hr sessions were instituted, rats were run two days per week, either Wednesday and Saturday (DRL 24) or Tuesday and Friday (DRL 72); other experiments with different rats were conducted in the chambers on intervening days. Because of the 4-hr sessions, the 4 operant conditioning chambers were occupied for a minimum of 8 hr/day (4 rats in the morning and 4 rats in the afternoon) so that all 16 rats could not be run in the same day; running more than 8 rats per day would have made it impossible to ensure that rats were all run in the same light phase of the light-dark cycle. Rats then experienced 12 4-hr sessions with the reinforcer volumes of 0.09 ml and 0.18 ml for the DRL 24 and DRL 72 groups, respectively. Different water volumes were used for the two groups in an attempt to equate amount of water consumed across the 4-hr sessions while curtailing satiation effects so that all rats under non drug conditions would continue responding well into the 4th hr of the session. By the end of the 48-hr of training (12 sessions) under the final behavioral contingencies, the interresponse distributions for each rat reflected a distinct mode in the region of its respective DRL requirement.
Drug treatment
Immediately before the 13th session, each rat received an ip. saline injection in a volume of 1 ml/kg. d-amphetamine sulfate (Sigma-Aldrich, St. Louis, MO) in a dose of 5.0 mg/kg was then given before each of the next 5 4-hr sessions. Recall that sessions were separated by 3 days. The dosing schedule was designed to produce marked behavioral sensitization so the obtained data would be most relevant to procedures that use multiple amphetamine dosing. The 5.0 mg/kg dose was chosen because it produces an initial robust focused stereotypy syndrome followed by pronounced hyperlocomotion within a 4-hr time frame (e.g. Kuczenski and Segal 1999). d-amphetamine sulfate was dissolved in physiological saline in a concentration of 5.0 mg/ml. The dose was expressed in terms of the weight of the salt form of the compound.
Data analysis
Extensive quantitative analyses were performed to compare the effects of amphetamine with saline treatment on 1) operant conditioning measures: interresponse time distributions, number of responses, and reinforcers obtained per hr; 2) force plate actometer measures: distance traveled, focused stereotypy, and spatial location in the chamber; and 3) relationships between actometer-derived measures and measures of operant responses: spatial location and distance traveled in the intervals before RFD and UNRFD operant responses. Programs written in-house in Free Pascal were required for examining the relationships between the rats’ collateral movements in the actometer and selected features of the operant behavior. Statistical tests of hypotheses were generally carried out with multivariate repeated measures analyses of variance (ANOVA) as implemented in SYSTAT (Systat Software Inc., San Jose, CA), followed by post hoc ANOVA’s or t-tests. The α-level for statistical significance was set at p=.05. When assumptions of homoscedasticity were not met, ANOVA’s were based on Log10 transformations of the data, and the transformations were observed to homogenize the variances as expected.
Results
Given some of the unusual specifics of our procedures (e.g., operandum outside the chamber wall, isometric operandum, non-grid floor, sessions conducted on nonconsecutive days, assessing the effect of amphetamine in sensitized rats), the analyses of data first aimed to establish that the results were consistent with previous reports in four domains: 1) confirmation that behavioral sensitization had occurred, 2) stereotypy and locomotor activity effects of amphetamine at 5.0 mg/kg, 3) DRL performance by rats, 4) effects of amphetamine on DRL performance. The second analysis aimed to describe how the rats’ locomotor movements and use of space were affected by DRL parameter and by amphetamine treatment.
Effects of amphetamine: stereotypy, locomotor activity, evidence for sensitization
Actometer-derived data from the 5th amphetamine treatment were subjected to computer algorithms that calculated spatial confinement measures and power spectra of the vertical force of the rats’ movements on the force plate. These data (not shown) indicated that all rats began to express the focused stereotypy syndrome (absence of locomotion accompanied by a near 10-Hz rhythm of the power spectra of vertical force variation) within a few minutes of the amphetamine injection. These data were in close quantitative agreement with previously reported results for the same amphetamine-dosing and behavioral measurement conditions (Fowler et al. 2007), but without the presence of an operant response contingency. Also consistent with the cited report and with results reported by Segal and Kuczinski (1999), hyperlocomotion occurred upon cessation of the focused stereotypy at about the 2-hr point of the 4-hr recording session. Operandum pressing behavior was almost completely absent during the period when focused stereotypy was expressed.
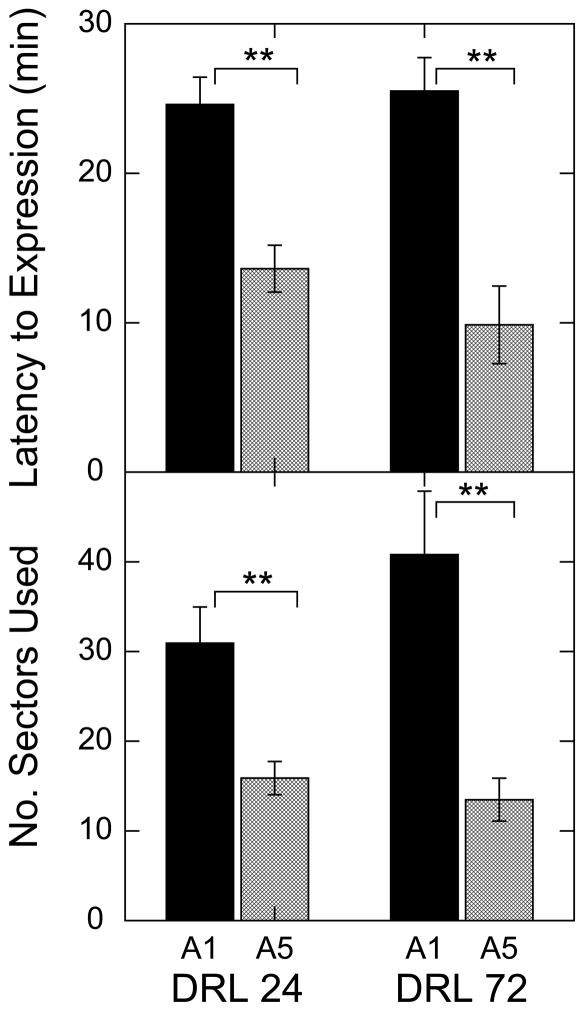
Evidence for behavioral sensitization of stereotypy was sought in terms of intensification of spatial confinement and reduced latency to express spatial confinement after amphetamine injection. Spatial confinement was scored by tallying the number of separate 1.75 cm × 1.75 cm squares on the floor of the chamber occupied by the rat in the first hour after drug injection. Latency to full expression of focused stereotypy was determined separately for each rat by calculating the time point (in 3 min time frames) when number of sectors fell to 20% of the maximum value expressed in the first hr of the session. As shown in Fig. 1, for both behavioral measures the response to the fifth injection of amphetamine (A5) was significantly more pronounced than the response to the first injection (A1).
Figure 1.
Spatial confinement (upper axes) and intensification of spatial confinement for the 5th amphetamine (5.0 mg/kg) injection compared to the 1st. These data are for the 1st hr of the sessions. Vertical brackets show +/− 1 SEM, while horizontal brackets and double asterisks indicate significant (p<.01, paired t-tests) differences between 1st and 5th injections.
DRL performance after saline or amphetamine
IRT distributions
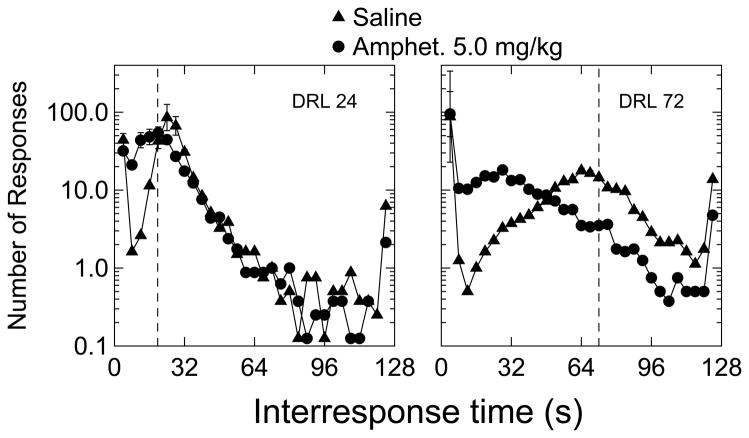
As shown in Fig. 2, under saline treatment conditions, IRT distributions peaked near the DRL requirement for each group. However, the distribution for the DRL 72-s group peaked 8 s below the 72-s requirement, whereas the distribution for the DRL24-s group peaked in the 4-s bin above the temporal requirement. Amphetamine treatment resulted in the expected leftward shift in the peak of the IRT distribution for both DRL parameters. As a proportion of the requirement, the left-shift of the peak in the IRT distribution was much greater for the 72-s group.
Figure 2.
Group mean of the interresponse time distributions for both DRL groups after saline or amphetamine treatment. Data are for IRT’s greater than 2.0 s (i.e., the majority of “burst responses” were eliminated). The bin size for these plots was 4 s. In each panel, the dashed vertical line marks the DRL requirement. Note that the ordinate is expressed as a log10 scale. The right-most data point in each frequency distribution depicts IRTs greater than 124 s. Brackets represent +/− 1 SEM. These data are for the entire 4 hr of both saline and amphetamine conditions.
Median IRT’s>2.0 s
Effects of DRL parameter and drug treatment were statistically confirmed by performing a 2-way ANOVA (after Log10 transformations) on the median IRT’s > 2.0 s (see Table 1). Significant DRL training parameter [F(1,14)=155.769, p<.001], drug treatment [F(1,14)=80.750, p<.001], and interaction [F(1,14)=22.459, p<.001] effects were obtained. Post hoc comparisons in Table 1 suggest that the interaction effect was primarily the result of a much greater effect of amphetamine on performance in the DRL 72-s group than in the DRL 24-s group.
Table 1.
Group mean of individual rat medians of IRT’s > 2.0 s for the indicated saline and drug conditions. Data are in seconds.
| Saline | 5.0 mg/kg d-amphetamine | |||
|---|---|---|---|---|
| DRL parameter (s) | Mean | SEM | Mean | SEM |
| 24 | 25.05a | 0.66 | 20.05c | 1.68 |
| 72 | 67.19b | 2.249 | 31.05d | 2.20 |
Notes:
- Comparison a–b: F(1,14)=543.002, p<.001.
- Comparison c–d: F(1,14)=17.344, p=.001.
- Comparison a–c: F(1,7)=11.192, p=.012.
- Comparison b–d: F(1,7)=78.873, p<.001.
Average response rates and reinforcement rates
Comparing response rates between saline and amphetamine administrations was not straightforward because early in the 4-hr session amphetamine almost completely suppressed operant responding, but substantially increased response rate once operant responding resumed. Therefore, averaging across the entire 4-hr session to obtain response rates would obscure important drug-treatment-related changes in behavior. Accordingly, we calculated response rate for the amphetamine condition by including only those responses made after operant responding resumed and divided by the duration of the opportunity to respond (i.e, the interval between resumption of operant responding and the end of the session). This duration of opportunity to respond varied for each rat ranging from 48 min to 153 min (mean=111.5 min, SEM=7.2 min). Response rates for the saline condition for each rat were then calculated for the same duration of opportunity but starting from the beginning of the session. Response rates for saline were not taken from a time period exactly the same as for the amphetamine session because when the amphetamine rats began to respond 111.5 min into the session, they had essentially earned no water, whereas at this time, the saline treated rats had been successfully obtaining reinforcers for almost 2 hr. Therefore, the motivational/satiation conditions would have been quite different for saline and amphetamine conditions if the fixed-time method had been used. The decision was made to try to equate the motivation/satiation conditions to avoid confounding motivational effects with the effects of amphetamine. Table 2 shows the group mean of average operant response rates of responding as a function of DRL parameter and drug condition. A 2-way ANOVA applied to these data yielded a significant DRL-parameter effect [F(1,14)=11.978, p=.004], a significant drug effect [F(1,14)=15.665, p=.001], and no interaction. A 2-way ANOVA on the reinforcement rates indicated a significant DRL parameter effect [F(1,14)=188.708, p<.001], a significant drug effect [F(1,14)=8.306, p=.012], and no interaction.
Table 2.
Group mean and standard errors of the mean (in parentheses) for response rates and reinforcement rates for the saline and amphetamine conditions for the indicated DRL parameters. The time intervals for calculations of response rate and reinforcement rate were based on the time interval marked by the resumption of responding after amphetamine treatment to the end of the session. Response rates for saline were based on the same length of time but starting at the beginning of the session. See text for rationale.
| Saline | 5.0 mg/kg d-Amphetamine | |||
|---|---|---|---|---|
| DRL parameter (s) | Response rate (r/hr) | Reinforcement rate (rft/hr) | Response rate (r/hr) | Reinforcement rate (rft/hr) |
| 24 | 144.0 (12.0) | 61.2 (6.0) | 174.0 (13.2) | 49.2 (4.2) |
| 72 | 78.0 (15.0) | 16.2 (2.4) | 138.6 (13.2) | 9.6 (0.6) |
Effects of DRL parameter and amphetamine on actometer behavioral measures
Distance moved
The purpose of this analysis was to determine if the DRL training influenced the amount of locomotor movement made by the rats during both reinforced (RFD) and unreinforced (UNRFD) IRTs. For RFD responses, this question was addressed by limiting the movement analyses to periods of time that preceded RFD responses (i.e., 24 s for DRL 24 s and 72 s for DRL 72 s). When an operant response produced a reinforcer, then no other operant response could have occurred to “contaminate” the movement measures within the prior DRL criterion interval as demanded by the DRL reinforcement contingency. To reduce the probability that the first seconds of a given RFD IRT contained the movements from operandum to the water port and the reward ingestion of an immediately preceding RFD IRT, this analysis did not contain the first 8 s of the pre-reinforcement interval. Calculating the locomotor movements for UNRFD IRTs is not as straightforward as for RFD ones because, whereas RFD IRTs represent those IRTs of 72 s (or 24 s) or longer, UNRFD IRTs can range from as little as a fraction of a second to 71.99 s. (or 23.99 s). Therefore, one needs to define what is of most interest in the class of UNRFD IRTs. Somewhat arbitrarily, the decision was made to define a “near-miss” subclass of IRTs as of greatest interest. Accordingly, UNRFD IRTs were taken as those in the range of 48.00 to 71.99s for the DRL 72 rats and 8.0 to 15.99 s for the DRL 24 group. In this analysis of UNRFD responses, the first 8 s of individual IRTs were omitted for the same reason that these IRTs were omitted for RFD responses. The distance traveled in pre-reinforcement intervals was normalized for comparing movements of the two DRL groups by dividing the cumulative distance traveled prior to RFD responses by the product of the number of RFD responses and the DRL parameter. For example, in the case of one rat that made 82 RFD responses in the DRL 72-s condition and traveled a total of 53.144 m across the 82 intervals, the computed normalized distance was 53.144 m/((64 s × 82 RFD responses)/60 s/min) = 0.608 m/min. The units reflect the normalization process and not speed of specific locomotor acts as ordinarily conceived (i.e. this measure is an aggregation of many classes of movement including movements too small to be described as locomotion).
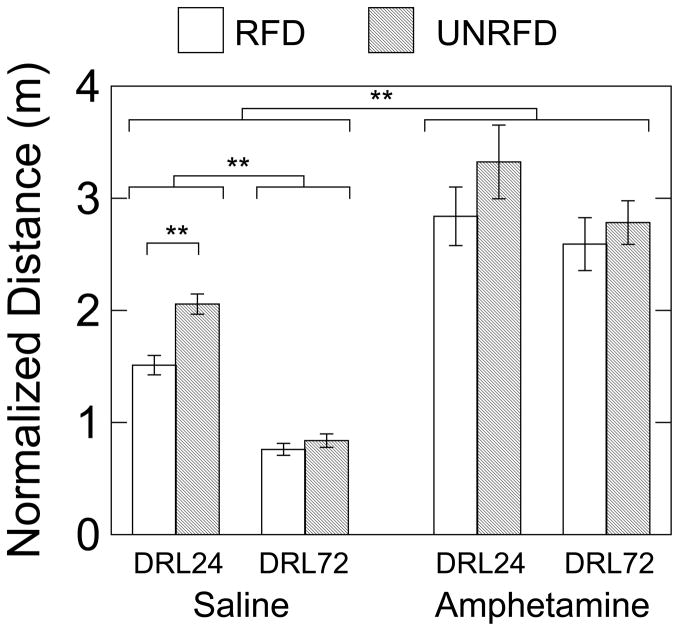
Fig. 3 shows normalized distance for RFD and UNRFD response classes as a function of DRL parameter and drug treatment. A three way ANOVA (DRL parameter, RFD vs UNRFD, saline vs amphetamine) indicated that the three main effects were significant (p<.001), without a three-way interaction. DRL parameter significantly interacted with reinforcement condition, F(1,14)=21.756, p<.001, and with drug condition, F(1,14)=35.023, p,.001). Thus, as shown in Fig. 3, when no drug was given the smaller DRL 24-s parameter was accompanied by greater amounts of movement than the DRL 72 s parameter. In addition, UNRFD responses in the DRL 24-s group were associated with increased movement compared to RFD IRTs, but this difference was not observed in the DRL 72-s group. Amphetamine increased movement to about equal levels regardless of DRL parameter as shown by a post hoc DRL-by-reinforcement-condition ANOVA: no DRL effect and no interaction effect, but a significant effect of reinforcement condition, F(1,14)=20.703, p<.001.
Figure 3.
Group mean normalized distance traveled during reinforced IRTs and longer unreinforced IRTs after saline or amphetamine injection. Double asterisks indicate significant differences (p<.01) as determined by analysis of variance. Horizontal brackets show the groupings of data to which the double asterisks pertain (e.g., the main effect of saline versus amphetamine was significant). Vertical brackets on each bar show +/− 1 SEM..
Temporal gradient of locomotion during pre-reinforcement intervals
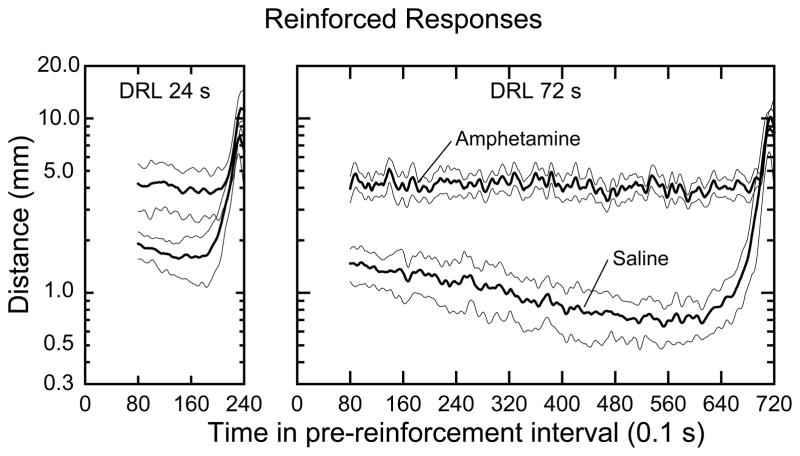
Although the immediately preceding data showed that DRL parameter, amphetamine treatment, and UNRFD- versus RFD-response class had significant influences on amount of locomotor activity in the operant conditioning chamber, these data did not show how activity was temporally distributed across the pre-response intervals. This question was addressed by data presented in Fig. 4. When saline was tested, locomotor activity was relatively low during most of the pre-reinforcement/response interval, and on the average locomotion began to increase about 4 s before the operant response was made. If the rat was not in the immediate vicinity of the operandum aperture (which was often the case, see below for location information), it had to travel from wherever it was in the chamber to the operandum aperture. Travel toward the operandum is reflected by the sharp increase in distance as the time for reinforcement availability approached. Interestingly, the data for the saline condition display a gradual decline in movement that reached minima around 8 s before the RFD response. In both DRL groups the gradual decline was significant by linear regression, where the time interval for regression was 8 s past the beginning of the interval (left-most saline point for each group in Fig. 4) to the minimum point reached for each DRL group (statistics not shown, but note the 95% confidence intervals in Fig. 4). Thus, the present data show two movement features during the pre-reinforcement interval: a long “waiting” phase, wherein the rat moved progressively less with the passage of time, and a “travel” phase wherein the rat traveled quickly to the operandum and pressed it. Amphetamine treatment substantially elevated the amount of locomotion, and this increase was approximately evenly distributed across the pre-reinforcement interval. The lower variance in the amphetamine function for the DRL 24-s group compared to the DRL 72-s group was the result of more averaging for the DRL 24 group which had about 4 times as many RFD responses as the DRL 72 group after amphetamine treatment.
Figure 4.
Group mean amount of locomotion (thick lines) as a function of time in the pre-reinforcer interval for the indicated DRL and drug conditions. Thin lines paralleling the thick lines are the 95% confidence intervals. Note that the ordinates are expressed on a log10 scale. An explanation for why the plotted data begin at 8 s instead of at 0 s is given in the text. Upon plotting, the data were subjected to a lowess smooth in order improve the visibility of the confidence intervals around the mean curves. The amount of smoothing used did not affect the breadth of the confidence intervals.
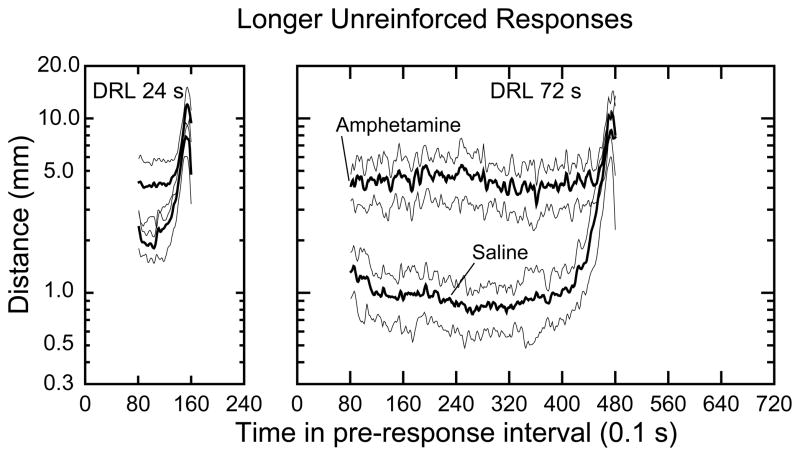
In Fig. 5 the DRL 24-s and DRL 72-s group mean distances for longer UNRFD responses are presented. Under saline treatment conditions the temporal gradients of the UNRFD pre-response intervals were not as pronounced as they were for the RFD responses shown in Fig. 4. However, this difference may reflect averaging together of 40-s intervals that varied by as much as 20 s in the IRT from which the 40 s was drawn. Nevertheless, the data show that amounts of movement during relatively long UNRFD IRTs were approximately a low as for RFD responses after saline treatment. In addition, and also similar to the data in Fig 4, amphetamine elevated movement comparably for the DRL 24-s and DRL 72-s groups and resulted in no temporal gradient.
Figure 5.
Group mean amount of locomotion as a function of time in relatively long unreinforced IRTs. Graphics conventions are the same as in Fig. 4.
Spatial location of rats in the intervals before RFD and UNRFD responses
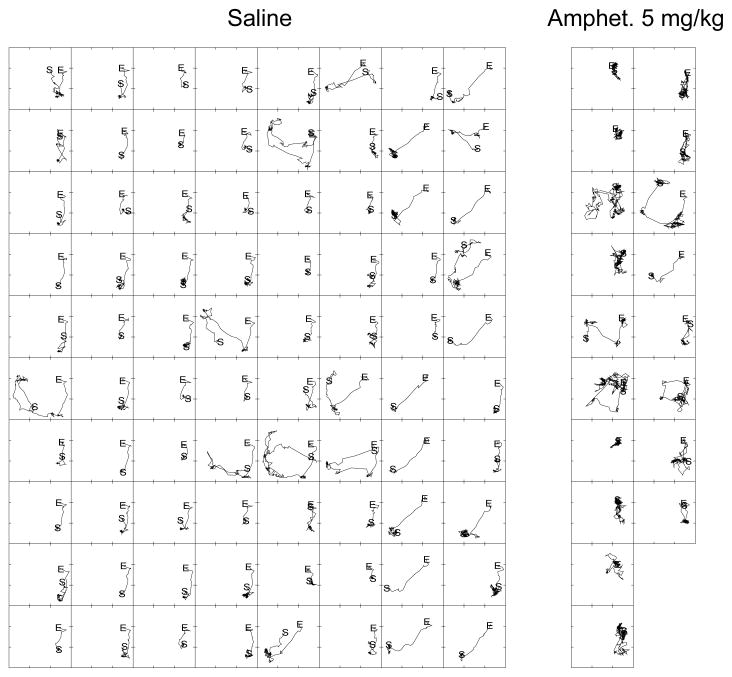
In Fig. 6, movement trajectories for one rat illustrate how a rat’s position changed during the 80 RFD intervals of the saline session and the 18 RFD intervals of the fifth amphetamine session. Under saline conditions the rat’s locomotion was confined to the vicinity of the operandum and water port, and the rat’s starting location (i.e., its location when the 72-s interval before reinforcement began) was predominantly near the water port (i.e., note the locations of the “S” across the array of 80 axes in Fig. 6). Amphetamine treatment disrupted the space-usage pattern that was observed after saline injection. Under amphetamine, with few exceptions, the location of the rat at the start of an interval tended to be near the operandum aperture and not near the water port. The increased locomotor activity induced by amphetamine relative to saline is clearly visible in Fig. 6. Spatial trajectories like those shown in Fig. 6 were plotted for each saline and amphetamine session and inspected (up to 80 plots per rat and treatment condition for a total of 1928 plots). These plots were generally consistent with the data shown in Fig. 6.
Figure 6.
Illustrative movement trajectories for one DRL-72-s-trained rat with the starting locations (“S”) and the ending locations (“E”) of a rat’s movements in the 72-s before reinforced responses. Data are for an entire session after saline or amphetamine treatment for one rat. In each grouping of plots, the 1st reinforced response is at the top left. Time advances down the ten columns of axes, jumps to the top of the next column to the right and advances down the column, and so on. The axes enclosing the tracings represent the actometer sensing area, and the upper and lower tick marks along the left border designate the approximate location of the operandum aperture and water port, respectively.
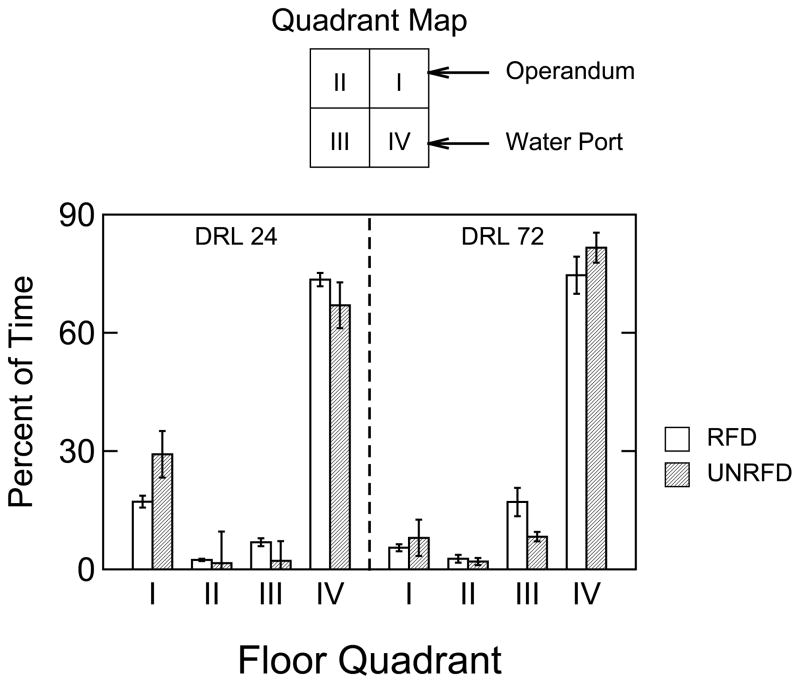
The impracticality of presenting thousands of plots led to the summary analysis of the rats’ locations shown in Fig. 7, which gives a comparison between locations for RFD responses and UNRFD responses by spatial quadrant of the chamber’s floor. Each bar in Fig. 7 represents a group mean with individual subjects weighted equally regardless of the number of qualifying IRTs made by each subject. A three-way ANOVA (DRL X RFD-UNRFD X quadrant) was applied to the data shown in Fig. 7. Significant main effects were obtained for the quadrant and RFD-UNRFD factors but not for DRL. The three-way interaction was not significant, but all the two way interactions were significant: RFD-UNRFD X DRL: F(1,14)=5.115, p=.04; RFD-UNRFD X quadrant: F(3,12)=24.521, p<.001; and quadrant X DRL: F(3,12)=25.265, p<.001. Post hoc 2-way ANOVAs (RFD-UNRFD X DRL) were performed separately for each quadrant in order to investigate these interactions. The DRL 24-s group spent more time in Quadrant I than the DRL 72-s group, F(1,14)=66.604, p<.001, and the UNRFD- relative to the RFD- condition increased time in this quadrant in both groups, F(1,14)=25.939, p<.001 (the interaction was not significant). There were no significant effects in Quadrant II. In Quadrant III, the DRL factor was significant, F(1,14)=11.985, p<.01, with the DRL 72-s rats showing a larger amount of time spent than the DRL 24-s rats. In addition, the UNRFD condition reduced time in Quadrant III compared to the RFD condition, F(1,14)=41.769, p<.001 (the interaction was not significant). In Quadrant IV, main effect were not significant, but the interaction was significant, F(14)=7.122, p<.02, suggesting that the UNRFD condition decreased Quadrant IV occupancy in the DRL 24-s group while increasing it in the DRL 72-s group (see Fig. 7).
Figure 7.
Group mean percent of time spent in each force-plate quadrant during intervals preceding reinforcement delivery (open bars) or preceding relatively long IRTs that were unreinforced (cross-hatched bars) for DRL 24-s and DRL 72-s trained rats. On the abscissa the Roman numerals denote the floor spatial quadrant. Brackets represent +/− 1 SEM.
Effects of amphetamine on quadrant occupancy
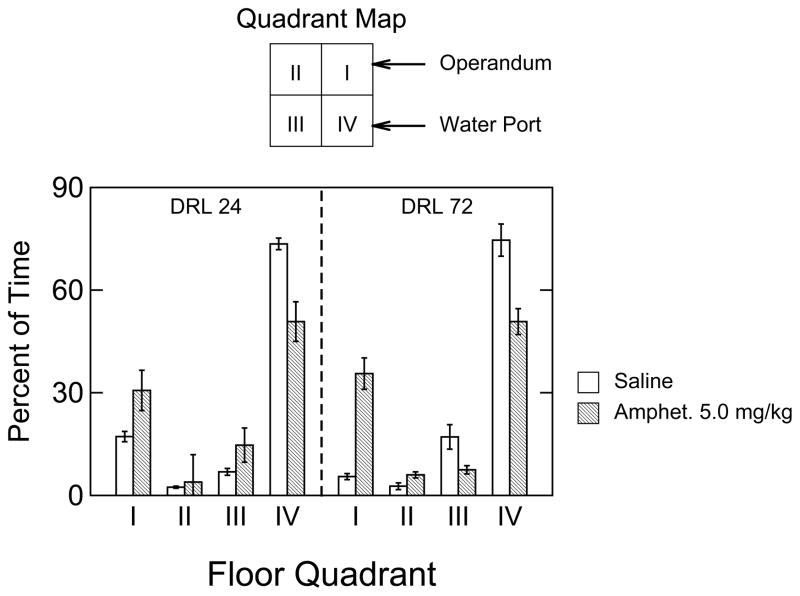
The first step in this analysis was to determine if there was any difference between RFD and UNRFD responses after amphetamine treatment. A 3-way ANOVA (DRL X RFD-UNRFD X Quadrant) detected a significant main effect of Quadrant, F65.190, p<.001, but all other main effects and interaction effects were not significant. Therefore, attention was focused on a comparison between saline and amphetamine treatments for the percent occupancy data based on RFD responses (see Fig. 8). A three-way ANOVA, applied to these data indicated four significant effects: saline versus amphetamine, F(1,14)=12.939, p=.003; quadrant used, F(3,42)=154.184, p<.001; drug treatment X quadrant interaction, F(3,42)=14.771, p<.001; and drug treatment X quadrant X DRL interaction, F(3,42)=7.395, p<.001. To explore the two- and three-way interaction effects, two-way ANOVAs (DRL X drug treatment) were performed separately for each quadrant. For Quadrant I, the DRL, amphetamine, and interaction effects were significant effect: F(1,14)=10.366, p<.01; F(14)=39.338, p<.001; and F(1,14)=14.135, p<.01; respectively. These statistics support what one sees upon inspection of Fig. 8 at Quadrant 1: under saline conditions the DRL 24-s group had more time in Quadrant I than the DRL 72-s group, and amphetamine treatment increased time in Quadrant I to about the same level in both groups. The ANOVA for Quadrant II detected a significant drug effect, F(1,14)=9.638, p<.01; neither the DRL effect nor the interaction effect was significant. In regard to Quadrant III, neither main effect was significant, but DRL parameter and amphetamine interacted significantly, F(1,14)=4.978, p<.05, such that, relative to saline, amphetamine increased time in Quadrant III for the DRL 24-s group, but decreased time in this quadrant for the DRL 72-s group. The ANOVA for Quadrant IV indicated a significant amphetamine-related decrease in percent of time spent about equally for both groups (i.e., neither DRL nor the interaction was significant). Altogether, the data in Fig. 8 show that the space usage was similar, but not identical, for both DRL groups under the saline condition (i.e., by far the greatest amount of time was spent in pre-reinforcement intervals in Quadrant IV). The effect of amphetamine was to redistribute time from Quadrant IV to Quadrant I. The two DRL groups were dissimilar in Quadrant III under both saline (DRL 24-s lower than DRL 72-s) and amphetamine (DRL 24-s higher than DRL 72-s) conditions.
Figure 8.
Group mean percent of time spent in each force-plate quadrant during intervals preceding reinforcement delivery for DRL 24-s and DRL 72-s trained rats that received saline or amphetamine treatment. Graphics conventions are the same as in Fig. 7.
Because data in Fig. 8 are based on the entire interval before RFD responses, they do not unequivocally rule out the possibility that rats may have crossed quadrant boundaries relatively frequently but spent the majority of time in only one quadrant. The data in Fig. 4 (amount of locomotion as a function of time) and Fig. 6 (sample movement tracings), as well as inspection of the 1928 tracings of movement trajectories (described above) were contrary to this supposition.
Discussion
The initial analyses were designed to establish that the combined set of behavioral and pharmacological methods led to the kinds of behavioral results expected when these methods have been used separately. In the case of 5.0 mg/kg amphetamine, the drug induced the same unconditioned behavioral responses (focused stereotypy and subsequent locomotor activation) in an operant conditioning setting that it induced in settings that did not use operant conditioning procedures (Fowler et al. 2003; Fowler et al. 2007). Although behavioral sensitization to amphetamine ordinarily is observed at doses that induce only heightened locomotor activity and not focused stereotypy in rats (e.g., Vezina and Stewart 1989), robust behavioral sensitization can also be documented at higher doses that evoke focused stereotypy followed in time by the expression of hyperlocomotion (Fowler et al. 2003). Accordingly, evidence in support of the assertion that the 5-injection dosing sequence with 5.0 mg/kg d-amphetamine sulfate led to behavioral sensitization was presented in Fig. 1. In the work reported here, operant behavior was almost completely suppressed during focused stereotypy, and the onset of the recovery of locomotion was accompanied by both operant behavior and hyperlocomotion.
After saline treatment, DRL performance, as measured by response rate, reinforcers/hr, and location of the peak of the IRT distributions (see Tables 1 & 2, Fig. 2), was in close agreement with expectations from the literature that used customary lever-switch-closure operanda that protrude into the chamber (e.g., Doughty and Richards 2002; Balcells-Olivero et al. 1998; Balcells-Olivero et al. 1997). In Fig. 2 for the DRL 72-s group, the peaking of the IRT distribution 8 s below the 72-s requirement is a common finding across a broad range of amounts of training experiences in Sprague Dawley rats (40 hr: Balcells-Olivero et al. 1998; 42 hr: O’Donnell and Seiden 1983; 47.5 hr: Balcells-Olivero et al. 1993; 87 hr: Richards et al. 1993; 119 hr: Doughty and Richards, 2002). As in our results for the DRL 24-s requirement, other reports that used lower DRL requirements near 20-s have indicated a peaking of the IRT distribution at, or a few seconds above, the requirement (Pearl and Seiden 1976; Young and McNaughton 2000; Doughty and Richards 2002; Featherstone et al. 2007). Thus the differences between the DRL 24-s and 72-s requirements in the location of the peak in the IRT distribution appear to be related to the requirements per se and not to relative amounts of training on the two contingencies. An especially noteworthy similarity between our results and those reported in the literature was the close correspondence between our obtained 16.2 rft/hr for DRL 72 s and Balcells-Olivero et al.’s 15.8 rft/hr under saline treatment conditions for rats of the same strain from the same supplier. In the current study, the DRL 72-s group’s peak in the IRT distrubution was located near 30 s after amphetamine treatment. These results were also quantitatively close to a previous report (Balcells-Olivero et al. 1997). Therefore, our locating the operandum outside the chamber and our training regimen did not interfere with the expression of DRL-appropriate behavior or with its disruption by amphetamine treatment.
Combining force-plate actometry with operant conditioning methods provided an opportunity to characterize how the DRL contingency controlled collateral behavior and how amphetamine affected that behavior. For example, over the intervals preceding RFD responses and relatively long UNRFD IRTs (Fig. 3), normalized distances were significantly lower for the DRL 72-s schedule compared to the less stringent DRL 24-s schedule. Amphetamine markedly increased distance traveled to approximately the same level for both DRL groups, suggesting that the amphetamine movement-inducing effect was not influenced by the degree of DRL task difficulty.
Distance traveled data examined in 0.1-s bins over the course of the 24-s and 72-s intervals preceding reinforcement (Fig. 4) showed that the rats consistently exhibited a temporal gradient of progressively less spatial movement up to about 8 s before showing a rapidly accelerating increase in movement just before the operant response was executed. Amphetamine abolished this gradient for both DRL groups. Relatively long, but UNRFD, IRTs were also associated with low levels of movement, which was most clearly evident for the DRL 72-s condition (Fig. 5). However, the decline in movement as the end of the UNRFD IRT approached was not as pronounced as in the case of the RFD IRTs. This result for the UNRFD responses probably was the result of the averaging together IRTs that ranged from 48 s to 71.99 s. Obtaining a higher-resolution estimate of the shape of the pre-UNRFD response movement gradient would require much more data than were available in the present data set. Amphetamine treatment raised and flattened the movement gradients for the UNRFD IRTs (Fig. 5), as was the case for the RFD IRTs. The analysis of spatial location under non-drug conditions in the 24-s or 72-s time periods before reinforcement (Figs. 7) showed that the rats predominantly positioned themselves away from the operandum aperture and mostly remained in this location until near the end of the RFD IRT interval. Importantly, data in Fig. 7 suggest a location gradient such that the DRL 72-s rats, on the average on RFD responses, positioned themselves farther away from the operandum aperture than the DRL 24-s rats (note that Quadrant III is further away from the operandum than Quadrant IV). For the UNRFD responses this location gradient was less pronounced, particularly for the DRL 24-s group in which Quadrant I time increased. Amphetamine altered the pattern of space usage (Fig. 8) seen for RFD responses such that the drug treatment resulted in rats in both DRL groups more often positioning themselves closer to the operandum for greater amounts of time than in the saline condition. Taken together, these direct measurements of collateral behavior provide compelling support for the idea that successful DRL performance was associated with staying away from the operandum and remaining nearly motionless in one place while waiting for an unspecified internal state(s) to trigger approach to the operandum. Amphetamine affected the use of floor space during UNRFD responses in the same way that the drug affected usage associated with RFD responses. Note that the 3-way ANOVA comparing RFD and UNRFD responses after amphetamine treatment detected no significant effects beyond a main effect of Quadrant. Thus, the present data do not support previously offered hypothesis that active overt collateral behaviors contribute beneficially to DRL performance (cf., Laties et al. 1965; Staddon 1977). Instead, the current results appear to be consistent with the idea that some aspects of collateral behavior remove the animal from the proximity of the stimuli (operandum aperture in this case) that control the operant response (McGown et al. 1977; McIntire et al. 1983). In addition, the current data are compatible with the view of Richelle and Lejuene (1980) that timing involves waiting (attributed to Pierre Janet 1928) and waiting is the expression of inhibitory processes. The gradients of progressively diminishing lateral movement shown in Fig. 4 for saline conditions suggest the intensification of an active inhibitory process that serves to prevent the expression of locomotion.
Although amphetamine increased rats’ locomotor activity and increased the time spent near the operandum compared to the saline condition, the drug did not substantially increase time in Quadrants II and III. This result provides evidence that the operant conditioning contingency constrained the amphetamine-induced locomotor movements to the side of the chamber allowing access to the operant conditioning fixtures. In addition, amphetamine reduced the expression of the space usage pattern that was successful under non-drug conditions. Together the data suggest that 1) lower doses of amphetamine reflexly (compulsively) induced movement, 2) locomotor movements brought the rat nearer the operandum more frequently, and 3) close proximity to the operandum increased the probability of premature responses (i.e., the operandum aperture is a discriminative stimulus for reaching out and striking the operandum). In other words, the amphetamine compelled locomotion, and locomotion interfered with rats’ spatially-based, impulse control behavior (termed “pre-commitment strategy” by Monterosso & Ainslie 1999), thereby preventing achievement of levels of performance seen in the absence of amphetamine.
One could logically argue that the unconventional, but not unprecented (Fowler et al. 2007; Kuczenski and Segal 1999), method of amphetamine dosing used here limits the generality of the pharmacological findings (i.e., induction of locomotor movement and deterioration of DRL timing performance by amphetamine during the post-focused-stereotypy phase of the amphetamine response). However, arguing against this supposition is the fact that the effects of amphetamine on DRL performance observed here were in close agreement with a previous report (Balcells-Olivero et al. 1997) for unsensitized and sensitized rats on DRL 72-s receiving amphetamine in the 0.5 to 1.5 mg/kg dose range.
Overall, the current data show that DRL 24-s and 72-s contingencies of reinforcement shaped comparatively low levels of bodily movement (movement was significantly lower for the DRL 72-s than for the DRL 24-s schedule) in rats and produced a marked tendency for a rat to establish a spatial position away from the operandum in the intervals before RFD and longer UNRFD responses. It is important to note that this behavioral profile was strongly present before the first amphetamine dose was given, thereby ensuring that prior experience with amphetamine was not prerequisite to the basic behavioral findings.
Acknowledgments
This work was supported by MH43429 and HD02528.
References
- Balcells-Olivero M, Richards JB, Seiden LS. Sensitization to amphetamine on the differential reinforcement of low rate 72-s schedule. Psychopharmacology. 1997;133:207–213. doi: 10.1007/s002130050393. [DOI] [PubMed] [Google Scholar]
- Balcells-Olivero M, Cousins MS, Seiden LS. Holtzman and Harlan Sprague-Dawley rats: differences in DRL 72-sec performance and 8-hydroxy-di-propylamino tetralin-induced hypothermia. Jol Pharmacol Exp Ther. 1998;286:742–752. [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JNP. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behavioral Neuroscience. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Blackman DE. Behavioral tolerance and sensitization. In: Goudie AJ, Emmett-Oglesby MW, editors. Psychtropic drugs: tolerance and sensitization. Humana Press; New Jersey: 1989. pp. 521–545. [Google Scholar]
- Bull E, Reavill C, Hagan JJ, Overend P, Jones DN. Evaluation of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder: acquisition and performance of the DRL-60s test. Behav Brain Res. 2000;109:27–35. doi: 10.1016/s0166-4328(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Costa VC, Bueno JL, Xavier GF. Dentate gyrus-selective colchicine lesion and performance in temporal and spatial tasks. Behav Brain Res. 2005;160:286–303. doi: 10.1016/j.bbr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Doughty AH, Richards JB. Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. J Exp Anal Behav. 2002;78:17–30. doi: 10.1901/jeab.2002.78-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, Fletcher PJ. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: parallels to schizophrenia. Neuropsychopharmacology. 2007;32:483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand B, Vorontsova Chen R, Zarcone TJ. Behavioral sensitization to amphetamine in rats: changes in the rhythm of head movements during focused stereotypies. Psychopharmacology. 2003;170:167–177. doi: 10.1007/s00213-003-1528-5. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Pinkston J, Vorontsova E. Clozapine and prazosin slow the rhythm of head movements during focused stereotypy induced by d-amphetamine in rats. Psychopharmacology. 2007a;192:219–230. doi: 10.1007/s00213-007-0705-3. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Covington HE, Miczek K. Stereotyped and complex motor routines expressed during cocaine self-administration: results from a twenty-four hour binge of unlimited cocaine access in rats. Psychopharmacology. 2007b;192:465–478. doi: 10.1007/s00213-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychol Rev. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of d-amphetamine-induced stereotypy during the acute response. J Pharmacol Exp Ther. 1999;288:699–709. [PubMed] [Google Scholar]
- Laties VG, Weiss B, Clark RL, Reynolds MD. Overt mediating behavior during temporally spaced responding. J Exp Anal Behav. 1965;8:107–116. doi: 10.1901/jeab.1965.8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGown WP, Spencer WB, Neetz R. An investigation of collateral wood-chewing as a time-mediating device. Psychological Reports. 1977;41:1063–1069. [Google Scholar]
- McIntire K, Lundervold D, Calmes H, Jones C, Allard S. Temporal control in a complex environment an analysis of schedule-related behavior. J Exp Anal Behav. 1983;39:465–478. doi: 10.1901/jeab.1983.39-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE. Reinforcement contingencies maintaining collateral responding under DRL schedule. J Exp Anal Behav. 1969;12:413–422. doi: 10.1901/jeab.1969.12-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav Neurosci. 1984;98:3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology. 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Seiden LS. Differential-reinforcement-of-low-rate 72-second schedule: selective effects of antidepressant drugs. J Pharmacol Exp Ther. 1983;224:80–88. [PubMed] [Google Scholar]
- O’Donnell JM, Marek GJ, Seiden LS. Antidepressant effects assessed using behavior maintained under a differential-reinforcement-of-low-rate (DRL) operant schedule. Neurosci Biobehav Rev. 2005;29:785–798. doi: 10.1016/j.neubiorev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Osterhaus GL, Lester A, Fowler SC. Program No. 745.13, Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience; 2007. Microdialysis-measured brain levels of amphetamine in two rats strains expressing focused stereotypy. [Google Scholar]
- Pearl RG, Seiden LS. The existence of tolerance to and cross-tolerance between d-amphetamine and methylphenidate for their effects on milk consumption and on differential-reinforcement-of-low-rate performance in the rat. J Pharmacol Exp Ther. 1976;198:635–647. [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. J Exp Anal Behav. 1993;60:361–385. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelle M, LeJeune H. Time in animal behavior. New York: Pergamon Press; 1980. [Google Scholar]
- Sanabria F, Killeen PR. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;4:7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Zimmerman J. Timing behavior during prolonged treatment with dl-amphetamine. J Exp Anal Behav. 1962;5:327–330. doi: 10.1901/jeab.1961.4-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon JER. Schedule-induced behavior. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Engelwood Cliffs, N.J: Prentice Hall; 1977. pp. 125–152. [Google Scholar]
- van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Young B, McNaughton N. Common firing patterns of hippocampal cells in a differential reinforcement of low rates of response schedule. J Neurosci. 2000;20:7043–7051. doi: 10.1523/JNEUROSCI.20-18-07043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuriff GE. Collateral responding during differential reinforcement of low rates. J Exp Anal Behav. 1969;12:971–976. doi: 10.1901/jeab.1969.12-971. [DOI] [PMC free article] [PubMed] [Google Scholar]