Abstract
Alcohol consumption is a potential trigger for flare in Inflammatory Bowel Disease (IBD) flare because of alcohol’s pro-oxidant effects and its deleterious effects on gut barrier function. The association with alcohol consumption and IBD flare is unclear. To test this hypothesis, we evaluated the pattern of alcohol consumption and its self-reported effect on gastrointestinal (GI) symptoms in patients with IBD. We recruited 129 consecutive patients: 52 patients with Crohn’s Disease, 38 patients with Ulcerative Colitis, and 39 patients with Irritable Bowel Syndrome (IBS). All participants completed a validated questionnaire on disease activity, the CDAI or UCAI respectively, validated questionnaires to quantify alcohol consumption by NIAAA criteria, and two structured questionnaires we designed to access patients’ perception of the effect of alcohol on their GI symptoms and on overall GI symptom severity. The pattern of current, light, moderate, and heavy alcohol consumption in inactive IBD was similar to the general US population. Specifically, 56 of 90 (62%) of inactive IBD patients were current drinkers, compared to 61% in the general US population. Of current drinkers, 75% of IBD (N=42), and 43% of IBS (N=9) reported a worsening of GI symptoms with alcohol consumption (p=0.01); however, overall GI symptom severity did not differ when compared to quantity of alcohol consumed. Patients with inactive IBD drink alcohol in quantities similar to the general population. Current drinkers with inactive IBD are more likely to report worsening of GI symptoms with alcohol than current drinkers with IBS.
Keywords: Crohn’s Disease, Ulcerative Colitis, Alcohol Consumption
Introduction
A therapeutic goal in inflammatory bowel disease (IBD) is to prevent episodes of symptomatic flare and prolong periods of asymptomatic remission. Identifying potentially modifiable factors for disease flare is important to limit the use of steroids, avoid hospitalizations, and improving quality of life. Numerous studies have examined whether environmental factors such as diet modify disease activity in IBD. Studies have shown a link between diet and the development of IBD (Geerling et al., 2000; Reif et al., 1997; Sakamoto et al., 2005), but thus far there is no conclusive evidence that diet can modulate disease activity.
The best established enviromental factor that can affect IBD is cigarette smoking. Cigarette smoking has been shown worsen disease activity in CD (Cottone et al., 1994), and is associated with an increased need for steroids and a higher risk for surgery (Cosnes et al., 2001). However, in UC smoking reduces the risk of colectomy and decreases the extent of disease Green et al., 1998). The exact mechanism of the effect of smoking in IBD is not known, but it does highlight the important role social factors can play in the disease.
Another important social factor is alcohol consumption. Alcohol consumption has been associated with a wide variety of deleterious health effects such as liver disease and cardiomyopathy (Klatsky, 2007). In addition, acute and chronic alcohol consumption have been shown to modify the immune system and could therefore play an important role in IBD. Acutely, alcohol consumption has been shown to inhibit the immune system by decreasing T cell activity and interlukin (IL)-12 levels in healthy controls (Mandrekar et al., 2004). Alcohol can also increases monocyte production of anti-inflammatory cytokines such as IL-10 (Norkina et al., 2007). Chronically, alcohol increases liver Kupffer cell activity and is associated with increased generation of proinflammatory mediators such as TNF-α, IL-1, and IL-6 (Khoruts et al., 1991). Alcohol has also been shown to acutely disrupt gut barrier function, and can increase intestinal permeability in human subjects (Keshavarzian et al., 1994) to which patients with IBD are particularly susceptible (Wyatt et al., 1997).
Even though alcohol has a variety of effects on the immune system, alcohol consumption in IBD has rarely been examined in previous studies. Accordingly, the aims of this current study was to: (1) determine the rate and pattern of alcohol consumption in a cohort of patients with inactive IBD by NIAAA criteria; (2) evaluate the self-reported effect of alcohol consumption on IBD patients GI symptoms and (3) determine if there is any correlation with quantity of alcohol consumed and overall severity of GI symptoms.
Materials and Methods
Study Design
This was a cross-sectional survey of IBD patients done by questionnaire. Patients were recruited from GI and IBD clinics in a metropolitan university practice in Chicago, IL (Rush University gastroenterology) and a private gastroenterology clinic in Macon, GA from July 2004 until 2006. During the one and a half year period, consecutive patients with the established diagnosis of CD and UC who were in clinical remission and patients with the diagnosis of IBS were invited to participate in the study. After signing an informed consent, all patients were given a set of questionnaires to complete. The questionnaire packet included a demographic data form, validated questionnaires on disease activity [Crohn’s disease activity index (CDAI) or ulcerative colitis clinical activity index (UCAI), respectively] and alcohol consumption, as well as a structured questionnaires we developed regarding the effect of alcohol on the GI symptoms and overall severity of GI symptoms. All questionnaire packets were labeled by a sequential patient number to maintain patient confidentiality, and included a post-marked envelope for return to our GI office.
The study was approved by Rush University Investigational Review Board (IRB) (Chicago,IL) and Mercer University IRB (Macon, GA).
Inclusion Criteria and Exclusion Criteria
All IBD patients included in the study had biopsy proven CD or UC. IBS patients had to meet the Rome II criteria for IBS, and have a normal colonoscopy (except for hemorrhoids or diverticuli) and normal random colonic biopsies if the primary symptom was diarrhea. Patients were eligible to be included in the study if the clinician who saw them at the time of inclusion determined that they currently had inactive disease. The status of disease for IBD patients was then confirmed using IBD clinical indices – CDAI < 150 or UCAI < 4 for inactive disease. Patients were excluded from the study if they reported active disease in the three months prior to the enrollment.
Questionnaires
A. Demographic & Medical History Data Form
The demographic variables we collected included age, race, marital status, education, and occupation. In addition patients were asked to indicate different factors regarding the history of GI disease – length of duration of diagnosis, recent disease activity, and family history of GI medical conditions.
B. IBD disease activity indices
We used two validated questionnaires to access CD and UC, the CDAI and UCAI, that have been well described previously (Sandborn et al., 2002; D’Haens et al., 2007). These clinical questionnaires have IBD patients rate their current symptoms based on clinical questions – stool frequency, abdominal pain, general well being, etc.
C. Assessment of alcohol consumption
The National Institute of Alcohol Abuse and Alcoholism (NIAAA) has quantified alcohol consumption in the United Sates as part of the National Health Interview Survey (NHIS) since 1983 (Adams and Schoenborn, 2006). Pattern of alcohol consumption was determined by a validated questionnaire that divides alcohol consumption into a period specific normal week (PSNW). The PSNW has been found to be more accurate and has less underestimation of alcohol consumption than the quantity-frequency alcohol questionnaire (Romelsjo et al., 1995). The questionnaire asked patients to best describe the average amount of alcohol they consumed in a typical week over the last year. The volumes for a standard drink of 12 grams of alcohol were listed just below each type of beverage, to prompt subjects to answer more accurately. Accordingly, we grouped subjects into NIAAA categories as previously described – abstainers (less than 12 drinks per year), current drinkers (more than 12 drinks per year), light drinkers (3 or fewer drinks per week), moderate drinkers (3 to 14 drinks per week), and heavy drinkers (over 14 drinks per week). Binge drinkers were defined as over 5 drinks on one occasion. Subjects were also prompted to mark their “beverage of choice” or the main type of alcohol that they usually consumed.
D. Effect of alcohol consumption on GI symptoms
We developed a structured questionnaire to determine the perceived effects of alcohol consumption on GI symptoms. Subjects were asked if alcohol affected their gastrointestinal symptoms – diarrhea, abdominal pain, or bloating and were prompted to mark worse, the same, or better. Subjects then indicated how many drinks they believed it would take to make their symptoms worse, the same, or improved. In addition, if abstinent from alcohol, patients were asked if the reason for abstinence from alcohol was from worsening GI symptoms.
E. General Gastrointestinal Rating Severity Form
This structured questionnaire asked questions regarding the severity of gastrointestinal symptom based in four categories - overall GI symptoms, abdominal pain, diarrhea and/or constipation, gas/bloating, and heartburn. Subjects were prompted to rate their symptoms as either absent, mild, moderate, or severe. This format is similar to other previously described questionnaires (Svedlund et al., 1988) accessing GI symptom severity.
Statistical Analysis
We used the Pearson’s chi-square test of association to compare proportional differences between groups. We used a p-value of 0.05 for all tests and data was analyzed using, SPSS version 12.0.1 (SPSS, Inc. Chicago, Ill).
Results
Patient Characteristics
A total of 129 subjects completed the study: 52 with CD, 38 with UC, and 39 with IBS. Subject characteristics are listed in Table 1. Mean age was 42, 39, and 53 for CD, UC, and IBS respectively. The mean age was significantly older patients in our IBS subjects. All groups had a predominance of female subjects ranging from 62% in CD to 82% in IBS. There were no other statistical differences in gender, marital status, mean years of GI diagnosis, mean years of education, and family history of GI disease between the three groups.
Table 1.
Patient Characteristics by Group
| CD (N=52) |
UC (N=38) |
IBS (N=39) |
p-value | |
|---|---|---|---|---|
| Mean Age | 42.4 | 38.5 | 53.2 | 0.00 |
| Gender, % female | 62%, (32) | 63%, (24) | 82%,(32) | 0.09 |
| Marital Status, % Married |
55%, (28) | 47%, (18) | 54%, (21) | 0.07 |
| Education, mean yrs |
13.9 | 13.9 | 13.5 | 0.67 |
| GI Diagnosis, mean yrs |
12.7 | 9.5 | 8.9 | 0.11 |
| Family History of GI Disease |
50% | 33% | 62% | 0.27 |
| Abstainers | 38%, (20) | 37%, (14) | 46%, (18) | 0.82 |
| Current Drinkers | 62%, (32) | 63%, (24) | 54%, (21) | 0.79 |
| Light Drinkers | 21%, (11) | 26%, (10) | 26%, (10) | 0.61 |
| Moderate Drinkers | 33%, (17) | 24%, (9) | 18%, (7) | 0.26 |
| Heavy Drinkers | 0%, (0) | 3%, (1) | 3%, (1) | 0.50 |
| Binge Drinkers | 19%, (10) | 16%, (6) | 5%, (2) | 0.15 |
| Beer Drinkers | 31%, (16) | 29%, (11) | 10%, (4) | 0.54 |
| Wine Drinkers | 17%, (9) | 08%, (3) | 23%, (9) | 0.19 |
| Liquor Drinkers | 12%, (6) | 26%, (10) | 18%, (7) | 0.20 |
Alcohol Consumption
The quantity of alcohol consumption by the NIAAA criteria for light, moderate, and heavy drinkers is also listed in Table 1. Patients who abstained from alcohol were 46% of CD, 42% of UC, and 48% of IBS. The rate of current drinkers was 54% in CD, 61% in UC, and 49% in IBS. There was no statistical differences between the rates of abstainers, current drinkers, light, moderate, or heavy drinkers between the three groups.
After quantifying alcohol consumption in our cohort by the NIAAA criteria, a comparison was made with the general US population and is listed in Table 2. Overall pattern and quantity of alcohol consumption was very similar between these two populations. Specifically, the rate of abstainers, the number of current drinkers, and the number of binge or heavy drinkers were comparable. There were slightly more moderate drinkers, 29% vs. 14%, in our IBD cohort.
Table 2.
Quantity of Alcohol Consumption between IBD and US Population
| CD | UC | IBS | US Population1 | |
|---|---|---|---|---|
| Abstainers | 38% | 37% | 46% | 39% |
| Current Drinkers | 62%, | 63% | 54% | 61% |
| Light Drinkers | 21%, | 26% | 26% | 43% |
| Moderate Drinkers | 33%, | 24% | 18% | 14% |
| Heavy Drinkers | 0% | 3% | 3% | 4% |
| Binge Drinkers | 19% | 16% | 5% | 15% |
Estimates calculated by the NIAAA from the 2004 NHIS
The Effect of Alcohol on GI symptoms
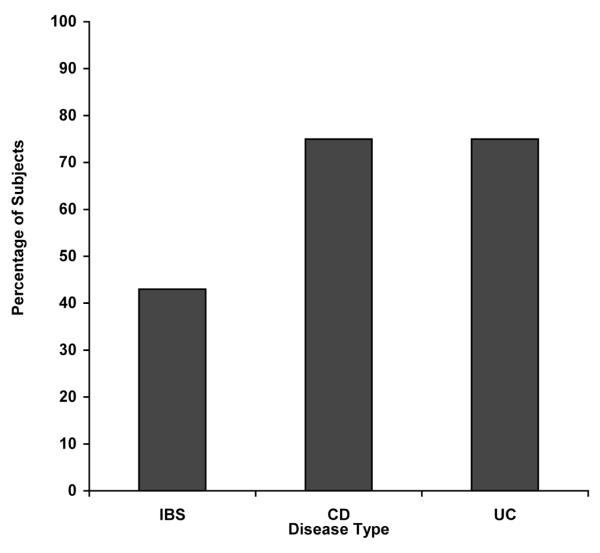
All patients completed the questionnaire on the perceived effect of alcohol on their GI symptoms. Most of the abstainers (n=51) could not recall the effect of alcohol on their GI symptoms, except those who quit specifically to avoid alcohol. The profile of patients that completed the effect of alcohol on their GI symptoms is listed in Table 3. Current drinkers with inactive IBD reported a statistically significant worsening of GI symptoms with alcohol when compared to the current drinkers with IBS (p= 0.01). To represent the data graphically subjects that ranked their symptoms the same or improved were grouped together and are shown in Figure 1.
Table 3.
Questionnaire about Alcohol and GI Symptoms
Abstainers not included
Figure 1.
Percentage of Subjects Reporting Worsening of Gl Symptoms with Alcohol Consumption
We also assessed the severity of GI symptoms in relationship to the quantity of alcohol consumed in Table 4. There was no statistical difference in overall severity of GI symptoms when compared with quantity of alcohol consumed. Similarly was no difference between the severity of other self reported GI symptoms (abdominal pain, diarrhea/constipation, or gas/bloating) and the quantity of alcohol consumed per week in IBD or IBS subjects. The severity of GI symptoms did differ significantly between disease groups, with IBS patients reporting more severe GI symptoms than IBD (p=0.02).
Table 4.
Category of Alcohol Consumption and Ranking of Overall GI symptoms as Severe or Moderate*
| Disease | Light | Moderate | Heavy | p value |
|---|---|---|---|---|
| CD (N=49) |
4%, (2) | 16%, (8) | 0%, (0) | 0.72 |
| UC (N=35) |
6%, (2) | 9%, (3) | 3%, (1) | 0.51 |
| IBS (N=37) |
27%, (10) | 16%, (6) | 3%, (1) | 0.19 |
2 CD, 4 UC, and 4 IBS did not complete the questionnaire on overall GI symptoms
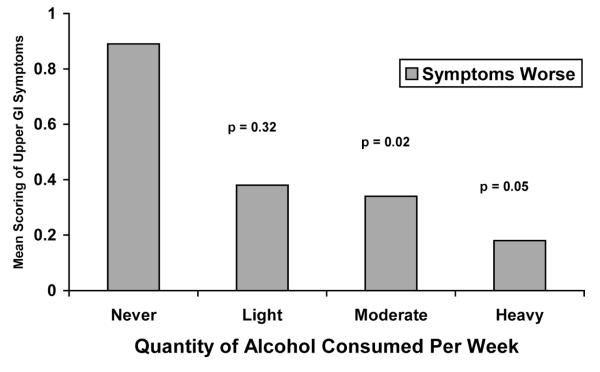
No correlation was observed between the type of alcoholic beverage consumed and GI symptoms in IBD or IBS subjects. However, those with IBD who had heartburn or other upper GI symptoms used significantly less amount of alcohol compared to IBD subjects who did not have these symptoms (Figure 2). This association was not found in subjects with IBS.
Figure 2.
Self-Reported Severity of Upper Gl Symptoms in UC and CD Quantity of Alcohol Consumption
Discussion
The concept that the environment may alter disease activity has been an area of controversy in IBD. Previous clinical studies in diet (Jowett et al., 2004), stress (Mawdsley et al., 2006), and sleep (Keefer et al., 2006) have found a possible relationship, but clinical studies have been disappointing. Currently there is no direct evidence that day to day environmental factors alter the course of disease in IBD, except for one prevalent social habit, cigarette smoking. Alcohol consumption is another prevalent social habit that can also modify immune activity. Alcohol has been previously examined through a prospective detailed diet history in 191 patients with inactive UC over a one year period (Jowett et al., 2004). In that cohort, patients that had a relapse or disease flare over that year consumed 14 grams of alcohol daily compared to 10 grams daily in non-relapsers. In CD, the acute effects of five alcoholic drinks in inactive CD showed that those drinks associated with increased plasma glucose levels but not increased ethanol were associated with worsening GI symptoms (Hey et al., 2007).
The primary goal of the current study was to quantity of alcohol consumption in inactive IBD subjects by NIAAA criteria so the quantity could be compared to national averages. In our cohort, the quantity of alcohol consumed in patients with IBD was very similar to national averages. Specifically, the rate of abstainers, current drinkers, and binge drinkers were comparable. This is an important epidemiological observation that suggests that patients with inactive IBD consume alcohol in an equivalent proportion to the general population. Overall, IBD patients do not seem to avoid alcohol for concern over its effect on GI symptoms.
IBS patients were selected as a comparison group in this study with IBD for two reasons – 1) they have GI symptoms at baseline that could be altered by alcohol, and 2) they had normal colonoscopies without inflammation. Healthy controls were thought to be an inappropriate comparison group as most patients would not have baseline GI symptoms. Our questionnaire on the effect of alcohol on GI symptoms showed that 75% of current drinkers with either inactive UC or inactive CD reported worsening of their GI symptoms with alcohol compared to 43% of IBS, which was statistically different. However, overall GI symptoms did not differ by quantity of alcohol consumed when not directly about alcohol. This is a less sensitive means to access the effect of alcohol on GI symptoms, and it is not surprising that no difference was found between the category of alcohol consumption and the severity of overall GI symptoms.
There are several possible explanations for the finding that alcohol worsened GI symptoms more in IBD than IBS. One possible explanation is that alcohol can alter the immune system and increase the risk of disease flare as was shown in UC (Jowett et al., 2004). The main hypothesis as to how alcohol could affect the luminal immune system is by increasing intestinal permeability and antigen exposure which has been shown previously in animal models (Tamai et al., 2000). Another possible explanation is that alcoholic beverages cause an osmotic diarrhea due to a high sugar content as has also been previously shown in CD (Hey et al., 2007). A retrospective questionnaire study such as this one is not able to differentiate between these hypotheses, and further studies are needed to examine these findings.
There was a significant decrease in the severity of upper GI symptoms as quantity of alcohol consumed increased. This is not surprising as alcohol has been known to worsen GERD by decreasing LES tone and decreasing gastric motility (Castell et al., 2004), and is likely avoided by IBD patients with moderate to severe GERD. Overall GI severity was different among disease states, with our IBS patients reporting worse disease symptoms compared to IBD. This finding is also not surprising, as IBS subjects have been shown to report worse quality of life scores than IBD in previous studies (Pace et al., 2003), and is a common finding between these two disease states.
In summary, we found that inactive IBD patients consume alcohol in a similar quantity and pattern as the general US population. In addition, inactive IBD patients reported worsening of their GI symptoms with alcohol when compared to patients with IBS. This finding may be secondary to alcohol causing a leaky gut and increased luminal immune activity or could be secondary to the high sugar content of most alcoholic drinks. In the future, clinical studies are needed to examine the possible link between alcohol consumption and its effect on disease course and luminal immune activity in IBD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbrugger RW, Brummer RJ. Diet as a risk factor for the development of ulcerative colitis. Am. J. Gastroenterol. 2000;95(4):1008–1013. doi: 10.1111/j.1572-0241.2000.01942.x. [DOI] [PubMed] [Google Scholar]
- Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40(6):754–760. doi: 10.1136/gut.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N, Kono S, Wakai K, et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005;11(2):154–163. doi: 10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in crohn’s disease. Gastroenterology. 1994;106(3):643–648. doi: 10.1016/0016-5085(94)90697-1. [DOI] [PubMed] [Google Scholar]
- Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of crohn’s disease: An intervention study. Gastroenterology. 2001;120(5):1093–1099. doi: 10.1053/gast.2001.23231. [DOI] [PubMed] [Google Scholar]
- Green JT, Rhodes J, Ragunath K, et al. Clinical status of ulcerative colitis in patients who smoke. Am. J. Gastroenterol. 1998;93(9):1463–1467. doi: 10.1111/j.1572-0241.1998.00464.x. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacol. Res. 2007;55(3):237–247. doi: 10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J. Immunol. 2004;173(5):3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Norkina O, Dolganiuc A, Shapiro T, Kodys K, Mandrekar P, Szabo G. Acute alcohol activates STAT3, AP-1, and sp-1 transcription factors via the family of src kinases to promote IL-10 production in human monocytes. J. Leukoc. Biol. 2007 doi: 10.1189/jlb.0207099. [DOI] [PubMed] [Google Scholar]
- Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13(2):267–276. [PubMed] [Google Scholar]
- Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am. J. Gastroenterol. 1994;89(12):2205–2211. [PubMed] [Google Scholar]
- Wyatt J, Oberhuber G, Pongratz S, et al. Increased gastric and intestinal permeability in patients with Crohn’s disease. Am. J. Gastroenterol. 1997;92(10):1891–1896. [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122(2):512–530. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132(2):763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Adams PF, Schoenborn CA. Health behaviors of adults: United states, 2002-04. Vital Health Stat. 2006;10(230):1–140. [PubMed] [Google Scholar]
- Romelsjo A, Leifman H, Nystrom S. A comparative study of two methods for the measurement of alcohol consumption in the general population. Int. J. Epidemiol. 1995;24(5):929–936. doi: 10.1093/ije/24.5.929. [DOI] [PubMed] [Google Scholar]
- Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut. 2004;53(10):1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131(2):410–419. doi: 10.1053/j.gastro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Keefer L, Stepanski EJ, Ranjbaran Z, Benson LM, Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J. Clin. Sleep Med. 2006;2(4):409–416. [PubMed] [Google Scholar]
- Hey H, Schmedes A, Nielsen AA, Winding P, Gronbaek H. Effects of five different alcoholic drinks on patients with crohn’s disease. Scand. J. Gastroenterol. 2007;42(8):968–972. doi: 10.1080/00365520701452241. [DOI] [PubMed] [Google Scholar]
- Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol. Clin. Exp. Res. 2000;24(3):390–394. [PubMed] [Google Scholar]
- Castell DO, Murray JA, Tutuian R, Orlando RC, Arnold R. Review article: The pathophysiology of gastro-oesophageal reflux disease - oesophageal manifestations. Aliment. Pharmacol. Ther. 2004;20(Suppl 9):14–25. doi: 10.1111/j.1365-2036.2004.02238.x. [DOI] [PubMed] [Google Scholar]
- Pace F, Molteni P, Bollani S, et al. Inflammatory bowel disease versus irritable bowel syndrome: A hospital-based, case-control study of disease impact on quality of life. Scand. J. Gastroenterol. 2003;38(10):1031–1038. doi: 10.1080/00365520310004524. [DOI] [PubMed] [Google Scholar]