Abstract
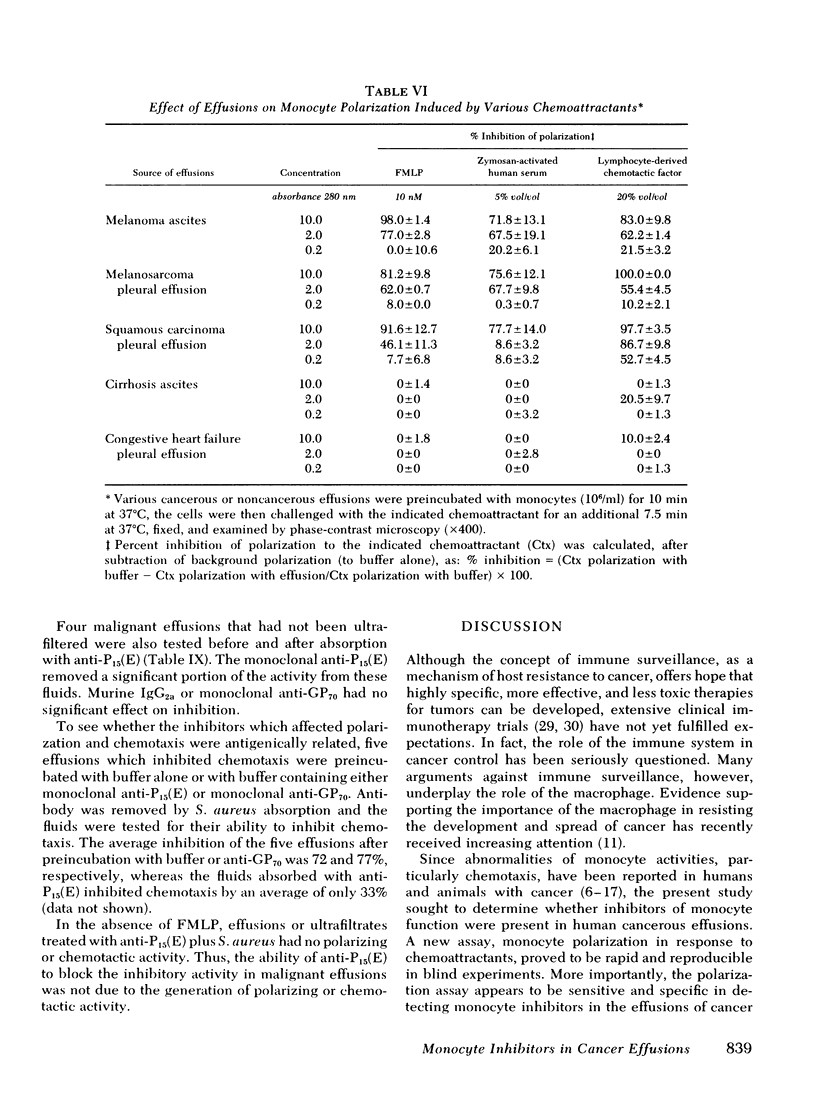
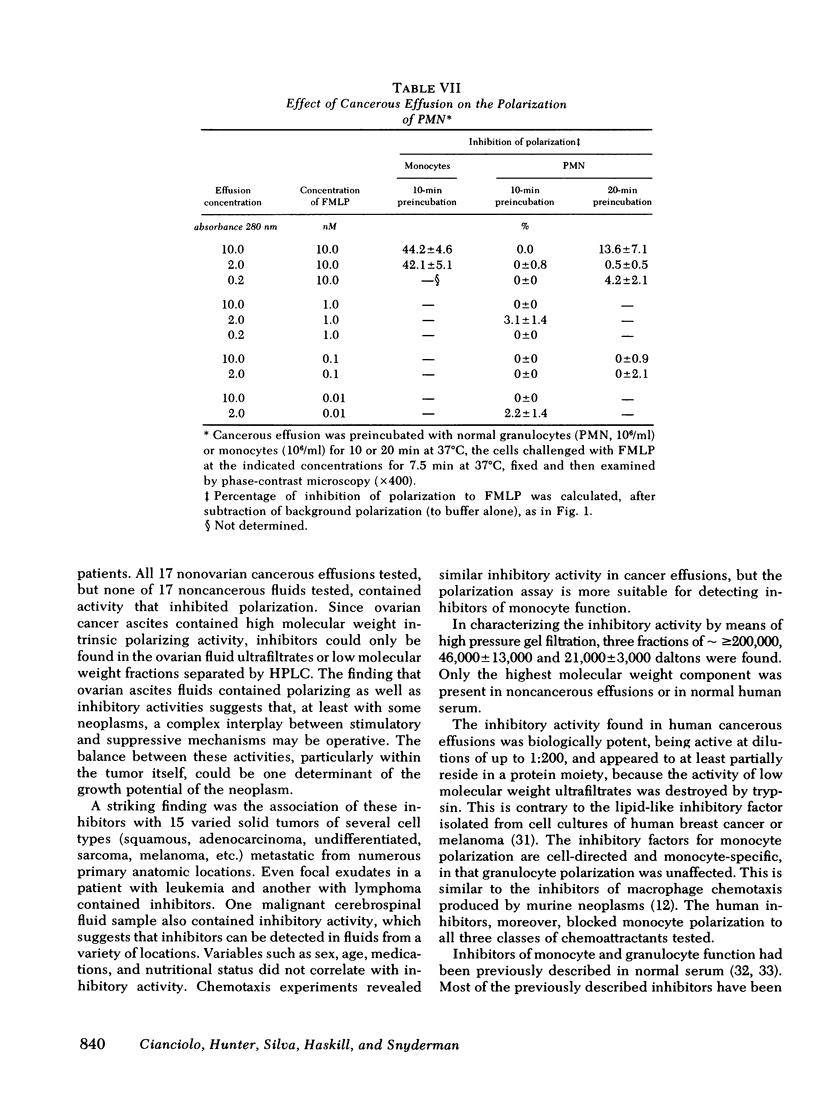
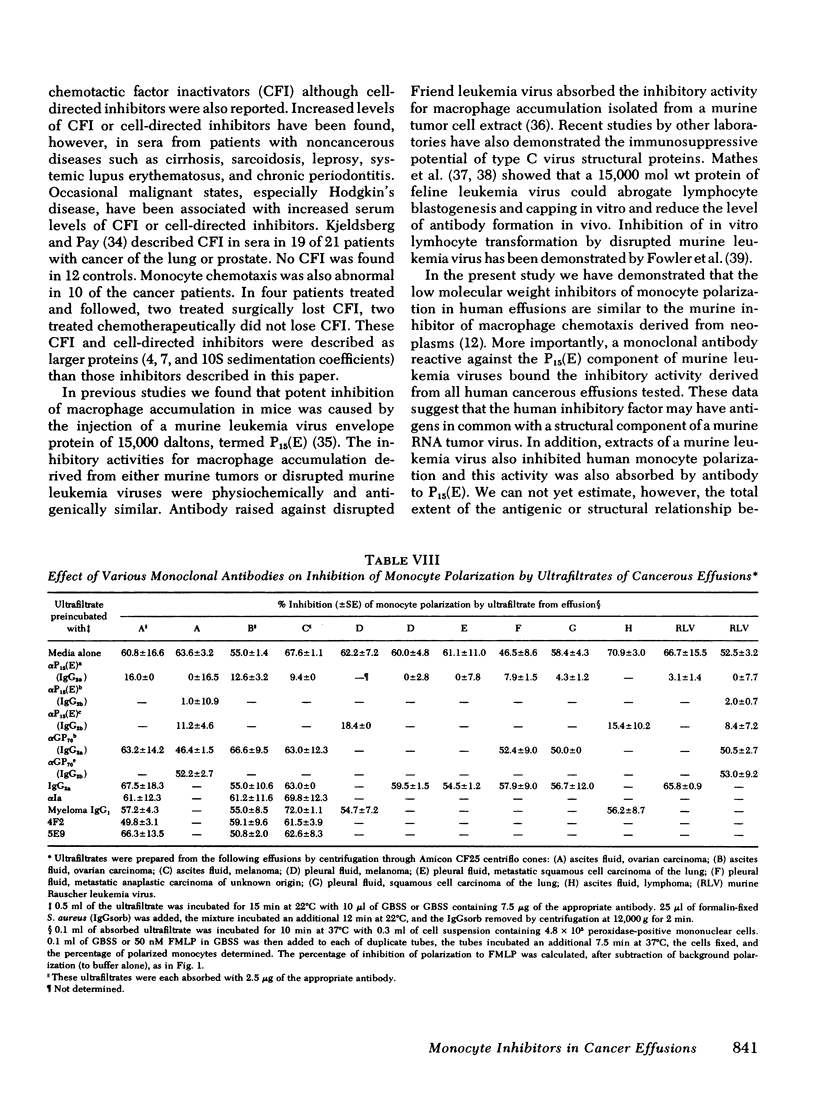
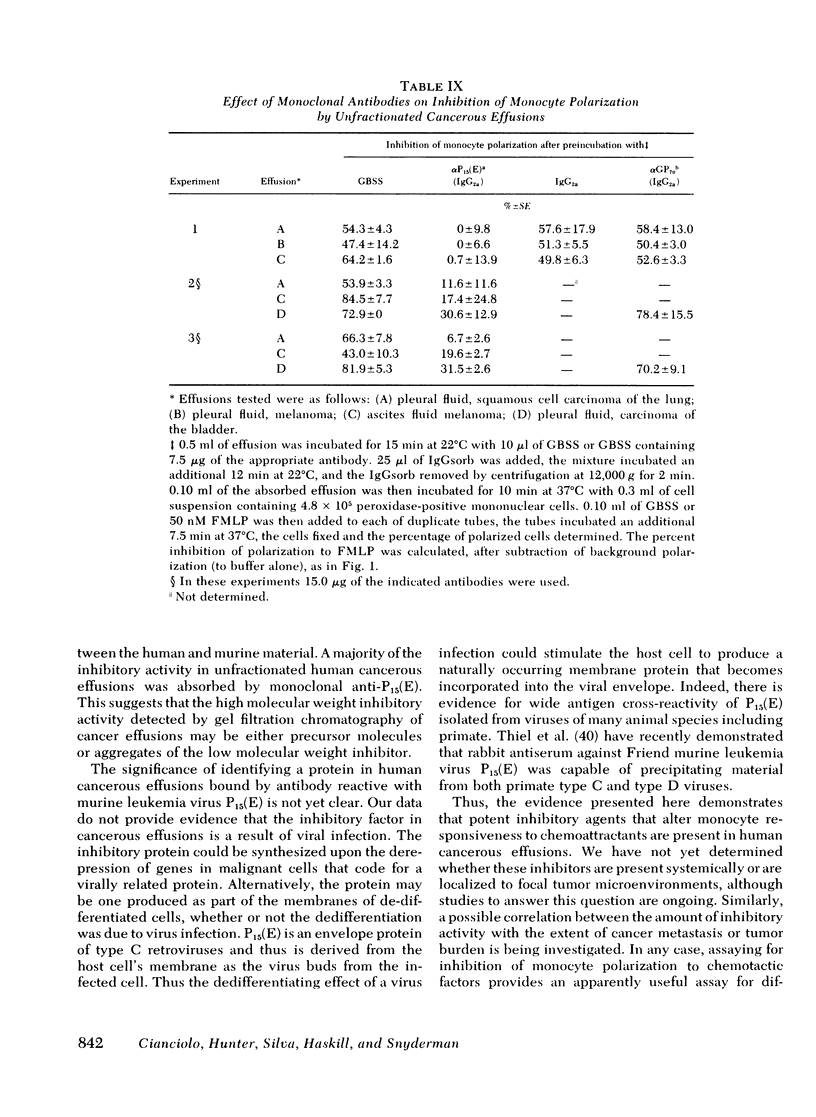
Individuals with cancer have previously been shown to have abnormal chemotactic responsiveness. Surgical removal of the tumor often resulted in normalization of monocyte function, which suggests that human neoplasms might inhibit monocyte chemotaxis by release of soluble mediators. We therefore examined the effects of cancerous effusions on monocyte polarization, i.e., the rapid change in monocyte morphology from round to a triangular "motile" configuration in response to chemoattractants. All 17 malignant effusions, representing 15 tumor types, inhibited monocyte polarization induced by the chemoattractant N-formyl-methionyl-leucyl-phenylalanine by 45-89% (mean 55.9 +/- 12.7%, P less than 0.01) in blinded assays. None of 17 benign effusions signigicantly inhibited polarization (0-15%, mean 6.2 +/- 4.2%). Dilutions of cancerous effusions as low as 1:200 produced inhibition that was time, temperature, and dose dependent . Monocyte polarization induced by activated serum or by chemotactic lymphokine was also blocked by cancerous effusions. The inhibitory activity affected the monocyte directly, and did not destroy the chemoattractant or block the polarization of granulocytes to chemotactic factors. High pressure liquid chromatography of five cancerous fluids revealed three peaks of inhibitory activity: greater than or equal to 200,000, 46,000 +/- 13,000, and 21,000 +/- 3,000 daltons. Fractionation of noncancerous effusions revealed only small amounts of the highest molecular weight inhibitory activity. The inhibitory activity in cancerous effusion was heat stable (56 degrees C, 30 min), trypsin sensitive, and could be absorbed by three different monoclonal antibodies reactive to P15(E), a structural component of type C retroviruses. In contrast, six monoclonal antibodies with other specificities had no effect on the inhibitors of polarization. This study demonstrates that human cancerous effusions contain novel proteins that are potent inhibitors of monocyte function and that are recognized by antibodies reactive to the P15(E) component of retroviruses. By producing such factors, tumor cells may subvert monocyte-mediated surveillance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Snyderman R. Do macrophages destroy nascent tumors? J Natl Cancer Inst. 1979 Jun;62(6):1341–1345. [PubMed] [Google Scholar]
- Alexander P. The functions of the macrophage in malignant disease. Annu Rev Med. 1976;27:207–224. doi: 10.1146/annurev.me.27.020176.001231. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Jr, Zbar B., Borsos T., Rapp H. J. BCG and cancer (first of two parts). N Engl J Med. 1974 Jun 20;290(25):1413–1420. doi: 10.1056/NEJM197406202902506. [DOI] [PubMed] [Google Scholar]
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cianciolo G. J., Matthews T. J., Bolognesi D. P., Snyderman R. Macrophage accumulation in mice is inhibited by low molecular weight products from murine leukemia viruses. J Immunol. 1980 Jun;124(6):2900–2905. [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Inhibition of lymphocyte transformation by disrupted murine oncornavirus. Cancer Res. 1977 Dec;37(12):4529–4531. [PubMed] [Google Scholar]
- Halliday W. J., Koppi T. A., Khan J. M., Davis N. C. Leukocyte adherence inhibition: tumor specificity of cellular and serum-blocking reactions in human melanoma, breast cancer, and colorectal cancer. J Natl Cancer Inst. 1980 Aug;65(2):327–335. [PubMed] [Google Scholar]
- Hausman M. S., Brosman S., Snyderman R., Mickey M. R., Fahey J. Defective monocyte function in patients with genitourinary carcinoma. J Natl Cancer Inst. 1975 Nov;55(5):1047–1054. doi: 10.1093/jnci/55.5.1047. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kjeldsberg C. R., Pay G. D. A qualitative and quantitative study of monocytes in patients with malignant solid tumors. Cancer. 1978 Jun;41(6):2236–2241. doi: 10.1002/1097-0142(197806)41:6<2236::aid-cncr2820410624>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. The role of macrophages in defense against neoplastic disease. Adv Cancer Res. 1974;20:131–163. doi: 10.1016/s0065-230x(08)60110-4. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Maderazo E. G., Anton T. F., Ward P. A. Serum-associated inhibition of leukotaxis in humans with cancer. Clin Immunol Immunopathol. 1978 Feb;9(2):166–176. doi: 10.1016/0090-1229(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P. Abrogation of lymphocyte blastogenesis by a feline leukaemia virus protein. Nature. 1978 Aug 17;274(5672):687–689. doi: 10.1038/274687a0. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P., Adams P. W., Nichols W. S. Immunosuppressive properties of a virion polypeptide, a 15,000-dalton protein, from feline leukemia virus. Cancer Res. 1979 Mar;39(3):950–955. [PubMed] [Google Scholar]
- Nakagawara A., Kayashima K., Tamada R., Onoue K., Ikeda K., Inokuchi K. Sensitive and rapid method for determination of superoxide-generating activity of blood monocytes and its use as a probe for monocyte function in cancer patients. Gan. 1979 Dec;70(6):829–833. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumours. I. Inhibition of delayed-type hypersensitivity reactions by tumour cells and by soluble prducts affecting macrophages. Immunology. 1978 Feb;34(2):277–290. [PMC free article] [PubMed] [Google Scholar]
- Norman S. J., Sorkin E. Cell-specific defect in monocyte function during tumor growth. J Natl Cancer Inst. 1976 Jul;57(1):135–140. doi: 10.1093/jnci/57.1.135. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Cosimi A. B., Goetzl E. J. Defective human mononuclear leukocyte chemotaxis as an index of host resistance to malignant melanoma. Clin Immunol Immunopathol. 1976 Nov;6(3):376–388. doi: 10.1016/0090-1229(76)90091-x. [DOI] [PubMed] [Google Scholar]
- Russell S. W., McIntosh A. T. Macrophages isolated from regressing Moloney sarcomas are more cytotoxic than those recovered from progressing sarcomas. Nature. 1977 Jul 7;268(5615):69–71. doi: 10.1038/268069a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Meadows L., Holder W., Wells S., Jr Abnormal monocyte chemotaxis in patients with breast cancer: evidence for a tumor-mediated effect. J Natl Cancer Inst. 1978 Apr;60(4):737–740. doi: 10.1093/jnci/60.4.737. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. An inhibitor of macrophage chemotaxis produced by neoplasms. Science. 1976 Apr 23;192(4237):370–372. doi: 10.1126/science.946556. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Seigler H. F., Meadows L. Abnormalitieis of monocyte chemotaxis in patients with melanoma: effects of immunotherapy and tumor removal. J Natl Cancer Inst. 1977 Jan;58(1):37–41. doi: 10.1093/jnci/58.1.37. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Meltzer M. S. Depressed chemotactic responses in vitro of peritoneal macrophages from tumor-bearing mice. J Natl Cancer Inst. 1976 Oct;57(4):847–852. doi: 10.1093/jnci/57.4.847. [DOI] [PubMed] [Google Scholar]
- Terry W. D. Immunotherapy of malignant melanoma. N Engl J Med. 1980 Nov 13;303(20):1174–1175. doi: 10.1056/NEJM198011133032010. [DOI] [PubMed] [Google Scholar]
- Thiel H. J., Broughton E. M., Matthews T. J., Schäfer W., Bolognesi D. P. Interspecies reactivity of type C and D retrovirus p 15E and p 15C proteins. Virology. 1981 May;111(1):270–274. doi: 10.1016/0042-6822(81)90671-1. [DOI] [PubMed] [Google Scholar]
- Turner S. R. ACDAS: an automated chemotaxis data acquisition system. J Immunol Methods. 1979;28(3-4):355–360. doi: 10.1016/0022-1759(79)90200-x. [DOI] [PubMed] [Google Scholar]


