Sir, Synchronous neuronal oscillations in the gamma frequency band (30–80 Hz) are implicated in a wide range of cognitive processes, including memory formation (Varela et al., 2001) and sensory processing (Singer, 1993; Gray, 1994). This rapid, temporally co-ordinated activity in spatially distributed areas of cortex is highly energy dependent, producing large changes in blood-oxygen level dependent signals in vivo and showing exquisite sensitivity to hypoxia in vitro (Huchzermeyer, 2008). Gamma oscillations are generated by reciprocal excitation and inhibition in networks of electrically coupled pyramidal cells and GABAergic interneurons (Fisahn et al., 1998; Cunningham et al., 2003; Hájos et al., 2004). Synchronous firing of networks of basket-cell interneurons at gamma frequency produces phasic inhibition of pyramidal cells, the periods when this inhibition is minimal, providing temporal windows for pyramidal cell firing. Pyramidal cell firing in turn provides reciprocal tonic excitation onto interneurons, allowing the cycle to continue (Traub et al., 1996). The research article by Kann et al. (2011) demonstrates the critical dependence of gamma oscillations upon mitochondrial function. However, little is known about the relative energetic requirements of the different cellular components of this network. We have addressed this issue with intracellular recordings of pyramidal cells and interneurons undergoing gamma oscillations under conditions of metabolic stress in vitro.
Rat hippocampal slices (450 μm horizontal sections) were maintained in a recording chamber at the interface between 95% oxygen/5% CO2 and artificial CSF (in mM: 10 glucose, 126 NaCl, 3 KCl, 24 NaHCO3, 1.25 NaH2PO4, 2 MgSO4 and 2 CaCl2). Stable gamma oscillation was induced by adding low dose (100 nM) kainic acid to the perfusate. Local field potential recordings were made from the stratum pyramidale layer in CA3 region of the hippocampus using glass microelectrodes (10–300 Hz band-pass filtered, digitized at 10 kHz). We also performed simultaneous intracellular recordings from CA3 pyramidal cells using sharp microelectrodes (70–130 MΩ) containing 2 M potassium acetate. Cells were recorded in current clamp mode, and identified electrophysiologically on the basis of their low resting firing rate, accommodating response to sustained depolarization and gamma frequency phasic inhibitory post-synaptic potentials. Excitatory post-synaptic potentials (EPSPs) and inhibitory post-synaptic potentials (IPSPs) were recorded at −70 and −20 mV, respectively.
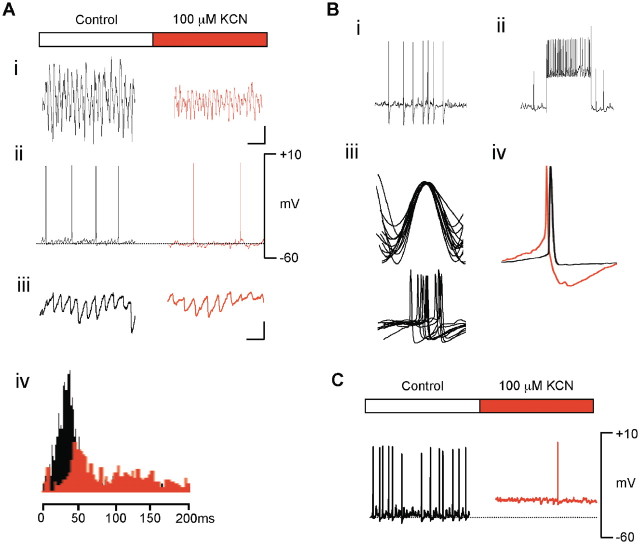
We then measured the effect on gamma oscillation power and corresponding intracellular activity using a variety of mitochondrial respiratory chain inhibitors. Potassium cyanide (KCN; 100 μM), an inhibitor of cytochrome oxidase in complex IV of the mitochondrial respiratory chain, caused a 72.1% reduction in gamma power (1221.3 ± 207.8 μV2 control versus 340.8 ± 70.5 μV2 KCN, n = 9, P < 0.05; Fig. 1A(i)) within 15 min. There was no significant change in the resting membrane potential (−57.9 ± 1.29 mV versus −60.6 ± 2.05 mV KCN, P > 0.05; Fig. 1A(ii)) of pyramidal cells. Despite this, there was a marked reduction in pyramidal cell firing rate during the concurrently recorded gamma frequency oscillation (3.02 ± 0.70 Hz versus 1.43 ± 0.64 Hz KCN, n = 9, P < 0.05). We therefore examined the synaptic inputs to pyramidal cells. There was no significant change in excitatory input (mean EPSP amplitude 3.18 ± 0.70 mV control versus 2.43 ± 0.72 mV KCN, P > 0.05; mean EPSP frequency 12.4 ± 2.6 Hz control versus 11.9 ± 1.9 Hz KCN, P > 0.05). The mean IPSP amplitude was reduced, (6.80 ± 1.66 mV control versus 5.21 ± 0.74 mV KCN, P < 0.05); however, the most striking finding was a marked reduction in the frequency of IPSPs impinging on the pyramidal cells (32.4 ± 2.6 Hz control versus 26.2 ± 3.9 Hz KCN, P < 0.05; Fig. 1A(iv)). We found similar effects on pyramidal cell firing and synaptic inputs using 1 μM Rotenone, an inhibitor of complex I, and 1 μM FCCP, a protonophore that discharges the mitochondrial membrane electrochemical gradient. With all three inhibitors, gamma power and pyramidal cell function returned to control levels on washing out of the inhibitors.
Figure 1.
(A) (i) Local field potential recording, CA3 region of hippocampus. Low dose (100 μM) KCN significantly reduces gamma oscillation power. Scale bar: horizontal 100 ms, vertical 200 μV. (ii) Simultaneous intracellular recording, CA3 stratum pyramidale pyramidal cell. KCN causes reduced resting firing rate with no significant change in resting membrane potential. (iii) Inhibitory post-synaptic potentials recorded at −20 mV. Scale bar: horizontal 50 ms, vertical 2 mV. (iv) Inter-event intervals of IPSPs impinging on pyramidal neuron. Mean frequency of IPSPs declines significantly. (B) (i) CA3 stratum pyramidale interneurons show rapid resting firing rates, (ii) show little accommodation to sustained depolarization, (iii) fire in phase with gamma field potential and, (iv) show prominent after-hyperpolarization potentials (pyramidal cell, black; interneuron, red). (C) Addition of KCN causes sustained depolarization of CA3 interneurons with marked reduction in resting firing rate.
Having demonstrated a predominant effect on the inhibitory input to pyramidal cells, we undertook intracellular recordings from CA3 stratum pyramidale interneurons (n = 3) that contribute to this input. These were identified electrophysiologically as basket cells on the basis of high resting firing rates, lack of accommodation to sustained depolarization, prominent after-hyperpolarization potentials and firing in-phase with the gamma field oscillation. Addition of 100 μM KCN caused a sustained depolarization of the mean membrane potential recorded during gamma oscillations (−50.4 ± 5.37 mV control versus −37.2 ± 2.98 mV KCN, P < 0.05) and produced an almost complete abolition of firing (9.92 ± 1.75 Hz control versus 0.11 ± 0.07 Hz KCN, P < 0.05).
In agreement with the findings of Kann et al. (2011), we demonstrate the extreme sensitivity of gamma oscillations to metabolic stress, and in particular to disruption of either complex I or complex IV in the mitochondrial respiratory chain, or of the mitochondrial membrane potential. Gamma oscillations are dependent on inhibitory input to pyramidal cells, and although these cells receive input from many different sub-types of inhibitory interneurons, it is the input from fast-spiking basket cells that primarily determine the frequency and power of the resulting network oscillations (Fuchs et al., 2007; Middleton et al., 2008). Within this context, our results suggest that the decline in gamma oscillation power seen with inhibitors of the mitochondrial respiratory chain results primarily from effects on these fast spiking interneurons. Concentrations of inhibitor that caused no significant effect on pyramidal cell resting membrane potential, caused a marked and sustained membrane depolarization in fast-firing interneurons. This in turn led to an almost complete cessation of interneuron firing, presumably as a result of inactivation of voltage-gated sodium channels (Martin et al., 2010). This exquisite sensitivity of interneurons is not unexpected; basket cells are known to contain high concentrations of cytochrome oxidase c and greater numbers of mitochondria compared to pyramidal cells (Gulyás et al., 2006), suggesting a heavy reliance on respiratory chain function to maintain their rapid firing rates.
Gamma oscillations are emerging as a fundamental cortical network behaviour. Disruption of fast-spiking interneuron function is observed in a number of neuropsychiatric and neurodegenerative conditions such as schizophrenia, Parkinson’s and Alzheimer’s disease. Importantly, in all of these conditions, disturbances in gamma oscillations dynamics have been observed (Uhlhaas and Singer, 2006). Our findings may offer insight into pathophysiology of cortical dysfunction both in genetically determined diseases of the mitochondrial respiratory chain, but also in other neurodegenerative conditions in which secondary mitochondrial dysfunction is implicated (DiMauro and Schon, 2008).
Funding
This work was supported by the European Union (EUmitocombat) and the Royal Society.
References
- Cunningham MO, Davies CH, Buhl EH, Kopell N, Whittington MA. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J Neurosci. 2003;23:9761–9. doi: 10.1523/JNEUROSCI.23-30-09761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–9. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Gray CM. Synchronous oscillations in neuronal systems: mechanisms and functions. J Comput Neurosci. 1994;1:11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Buzsáki G, Freund TF, Hirase H. Populations of hippocampal inhibitory neurons express different levels of cytochrome c. Eur J Neurosci. 2006;23:2581–94. doi: 10.1111/j.1460-9568.2006.04814.x. [DOI] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–37. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchzermeyer C, Albus K, Gabriel HJ, Otáhal J, Taubenberger N, Heinemann U, et al. Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in pO2 and concomitant changes in mitochondrial redox state. J Neurosci. 2008;28:1153–62. doi: 10.1523/JNEUROSCI.4105-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Huchzermeyer C, Kovács R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134:345–58. doi: 10.1093/brain/awq333. [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005;26:165–71. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dubé CM, Dutton SB, de Haan G, et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–34. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton S, Jalics J, Kispersky T, Lebeau FE, Roopun AK, Kopell NJ, et al. NMDA receptor-dependent switching between different gamma rhythm generating microcircuits in entorhinal cortex. Proc Natl Acad Sci USA. 2008;105:18572–7. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–74. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–4. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–68. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie JF, Lachaux JP, Rodriguez E, et al. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]