Abstract
OBJECTIVES
Different flow patterns and shear forces were shown to cause significantly more luminal narrowing and neointimal tissue proliferation in coronary than in infrainguinal vein grafts. As constrictive external mesh support of vein grafts led to the complete suppression of intimal hyperplasia (IH) in infrainguinal grafts, we investigated whether mesh constriction is equally effective in the coronary position.
METHODS
Eighteen senescent Chacma baboons (28.8 ± 3.6 kg) received aorto-coronary bypass grafts to the left anterior descending artery (LAD). Three groups of saphenous vein grafts were compared: untreated controls (CO); fibrin sealant-sprayed controls (CO + FS) and nitinol mesh-constricted grafts (ME + FS). Meshes consisted of pulse-compliant, knitted nitinol (eight needles; 50 μm wire thickness; 3.4 mm resting inner diameter, ID) spray attached to the vein grafts with FS. After 180 days of implantation, luminal dimensions and IH were analysed using post-explant angiography and macroscopic and histological image analysis.
RESULTS
At implantation, the calibre mismatch between control grafts and the LAD expressed as cross-sectional quotient (Qc) was pronounced [Qc = 0.21 ± 0.07 (CO) and 0.18 ± 0.05 (CO + FS)]. Mesh constriction resulted in a 29 ± 7% reduction of the outer diameter of the vein grafts from 5.23 ± 0.51 to 3.68 ± 0 mm, significantly reducing the calibre discrepancy to a Qc of 0.41 ± 0.17 (P < 0.02). After 6 months of implantation, explant angiography showed distinct luminal irregularities in control grafts (ID difference between widest and narrowest segment 74 ± 45%), while diameter variations were mild in mesh-constricted grafts. In all control grafts, thick neointimal tissue was present [600 ± 63 μm (CO); 627 ± 204 μm (CO + FS)] as opposed to thin, eccentric layers of 249 ± 83 μm in mesh-constricted grafts (ME + FS; P < 0.002). The total wall thickness had increased by 363 ± 39% (P < 0.00001) in CO and 312 ± 61% (P < 0.00001) in CO + FS vs 82 ± 61% in ME + FS (P < 0.007).
CONCLUSIONS
In a senescent non-human primate model for coronary artery bypass grafts, constrictive, external mesh support of saphenous veins with knitted nitinol prevented focal, irregular graft narrowing and suppressed neointimal tissue proliferation by a factor of 2.5. The lower degree of suppression of IH compared with previous infrainguinal grafts coincided with a lesser reduction of calibre mismatch in the coronary grafts.
Keywords: Vein grafts, Coronary bypass, Knitted nitinol mesh, Intimal suppressive
INTRODUCTION
It is estimated that every year, more than 800 000 coronary artery bypass graft (CABG) procedures are performed worldwide. Although most of these procedures utilize at least one arterial graft, the reversed saphenous vein remains the most widely used conduit for coronary revascularization. Despite their central role in coronary surgery, however, saphenous vein grafts have a distinctly higher failure rate than artery grafts. Compared with the excellent 96% 1-year [1] and 83–93% 10-year patencies of internal thoracic artery (ITA) grafts [2, 3], a historical series of saphenous vein grafts from the 1970s and early 1980s reported sobering patencies of 41–71% for the same implantation period [4, 5]. More recent studies were only marginally better, with 56–69% [6]. This difference in long-term patency between arterial and vein grafts is not only reflected in the quality of life, but also in patient survival [7]. As such, improving the long-term performance of saphenous vein grafts in coronary bypass surgery would distinctly affect re-occurrence of angina, re-operation or re-intervention rates, effort tolerance and life expectancy.
The main cause of intermediate to late vein graft failure is attributable to progressive neointimal hyperplasia (IH) with subsequent superimposed arteriosclerosis [8]. Autopsy series demonstrated that, as early as after 6 months of implantation, diffuse IH begins to appear in most of the vein grafts [5, 9]. Beyond 12 months, diffuse IH either remains static or progresses and develops more focally [4, 9]. Between 6 and 10 years, two-thirds of grafts show focal stenoses of between 50 and 75% [5, 9]. As more than 90% of lesions leading to mid-term failure were detectable within the first year, early IH was shown to be the main reason for mid- to long-term failure [10]. Conversely, no late occlusions were found in grafts without diffuse or localized IH present at 1–6 months [8]. Therefore, suppression of IH holds a key to improved mid- to long-term performance of vein grafts.
Among factors such as harvest-related endothelial trauma [11], biomechanical and fluid dynamic forces such as wall tension [12] and low shear stress [13] were shown to be the main triggers of neo-IH in vein grafts. High-flow run-off into large-diameter coronary arteries, for instance, resulted in a 10-year patency of 88% as opposed to 55% seen in low-flow conduits to small-diameter arteries [6]. Similarly, low-flow conditions similar to those resulting from the diameter mismatch between large vein grafts and small run-off arteries were shown to cause significant neo-IH [14]. Experimentally, the reverse principle could also be demonstrated: by using external meshes to constrict femoral vein grafts [15–17] or carotid interposition grafts [18] that were too large in diameter for their run-off arteries, IH could be almost completely suppressed.
In view of the distinctly different remodelling behaviour of infrainguinal and coronary vein grafts [19], however, it remained unclear whether mesh constriction would be equally effective in coronary bypass surgery. While the relatively small dimensions of coronary arteries do not allow the same degree of calibre adjustment of the vein grafts as in femoral grafts, baseline shear forces are more protective in the coronary than femoral circulation [19].
We therefore investigated the mitigating effect of mesh constriction on IH in coronary bypass grafts using a senescent primate model.
MATERIALS AND METHODS
Nitinol meshes of 3.4 mm resting inner diameter [ID; BB Ni–Ti alloy: nickel 56.0 wt%, titanium 43.9365 wt%, carbon 0.033 wt%, oxygen 0.028 wt%, hydrogen 0.0025 wt%; wire thickness: 50 μm (Fort Wayne Metals, Fort Wayne, IN, USA) knitted with an 8-needle head (Lamb, Inc., Chicopee, MA, USA)] were used as external vein graft support of CABGs in a senescent baboon model. The meshes were deployed by gently pulling the veins through an introduction straw on which the mesh was mounted. The veins were then slightly distended against the mesh with heparinized blood and sprayed with a thin layer of fibrin sealant (FS; Tisseel®, Baxter International, Inc.) to attach the mesh. After 6 months of implantation, vein graft remodelling of mesh-supported grafts was compared with that of non-mesh-supported controls [Group 1: controls (CO); Group 2: controls externally sprayed with fibrin sealant (CO + FS); Group 3: mesh-supported and FS-sprayed (ME + FS)]. Group 2 served to exclude the possibility of remodelling differences being due to the external fibrin layer rather than the mesh.
Implant surgery
Eighteen large, senescent Chacma baboons (28.8 ± 3.6 kg) were provided by the breeding and quarantine facilities of the South African Medical Research Council (MRC) at Delft/Cape Town after experiments had been approved by the Animal Ethics Committee of the University of Cape Town. Surgery and pre- and postoperative care complied with the ‘Principles of Laboratory Care’. The similarity of their anatomy to that of humans allowed access through a median sternotomy. All coronary procedures were performed in mild hypothermic arrest [34°C; Infant Blood Oxygenator Bio-2 (Baxter, Bently Laboratories, Irvine, CA, USA); St. Thomas antegrade cardioplegia (Adcock Ingram, Aerton, South Africa)]. Vein harvest methods followed no-touch principles. Each animal received an autologous reversed saphenous vein graft from the ascending aorta to the mid-segment of the LAD (graft length: 9.9 ± 1.2 cm; Fig. 1). Running 7/0 Prolene (Ethicon, Inc., Somerville, NJ, USA) was used for proximal and distal anastomoses. After the first mesh graft showed obvious signs of under-perfusion upon the opening of the cross-clamp, the proximal anastomosis was revised, correcting a retracted vein end that had invaginated as the mesh-supported fibrin glue layer was mistaken for the vein end. Subsequently, the initial fibrin spraying was applied to taper out towards the proximal end of the graft, allowing a separate anastomosis of the straight-cut vein and the mesh to the aorta. Re-cutting of the distal end at an angle permitted the clear identification of the vein, and as such, the mesh continued to be included in the LAD anastomosis, although the wide-spaced mesh loops led to only a few of the anastomotic stitches incorporating the mesh in the suture line. These ‘terminal’ loops all bent slightly outward towards the epicardium, excluding the possibility of causing luminal encroachment.
Figure 1:
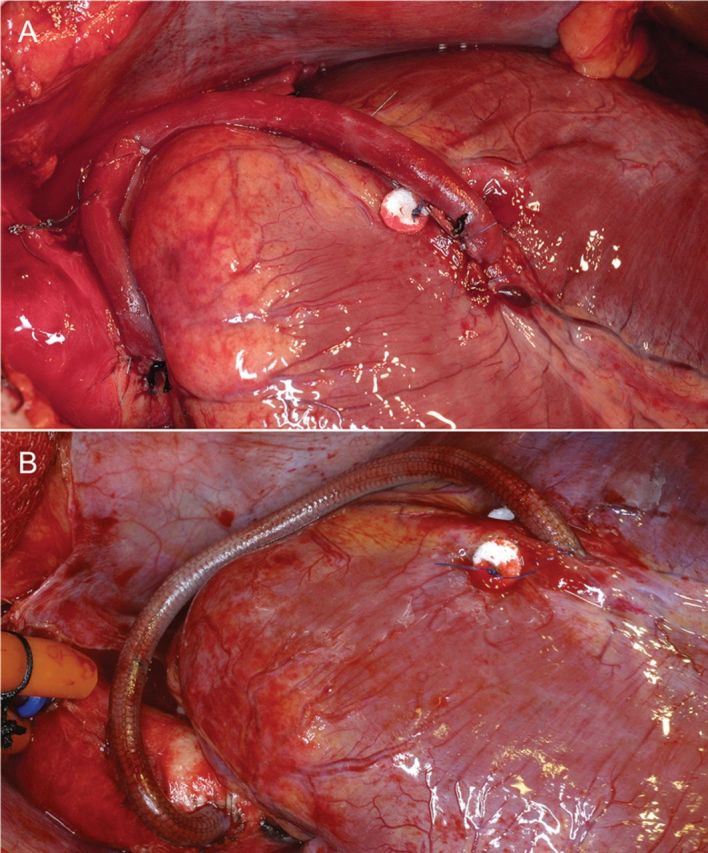
Aorto-coronary vein grafts to the LAD artery (A: Control and B: 3.4 mm ID nitinol mesh-constricted). A pledgeted mattress suture proximal of the distal anastomoses was placed to obliterate competitive flow.
After completion of the proximal vein graft anastomosis, the unattached mesh was briefly slipped back towards the middle of the graft (±2 cm), and the cross-clamp was intermittently opened to allow the measurement of the outer diameter (OD) of the distended vein graft without nitinol mesh support. After a few attachments, stitches were placed through the terminal mesh loops, and a thin layer of FS was also sprayed onto the proximal end of the graft. Competitive flow was prevented by obliterating the LAD 2 cm proximal of the distal anastomotic site with an external pledgeted mattress stitch (4–0 Prolene) followed by probing with a 1-mm probe.
Graft retrieval
All animals were euthanized after 180 days. Subsequently, perfusion fixation was used at 120 mmHg to preserve luminal in vivo dimensions in order to counteract the recoil tendency of non-pressurized blood vessels, and the recoil force integrated into the knitted nitinol mesh (1 l of 10% formalin in phosphate-buffered saline (PBS); iliac cannulation). After no less than 2 h of pressure fixation, the hearts were resected en bloc and transferred to the hospital for post-mortem angiographic assessment. With the aorta clamped proximally and distally to the graft, non-ionic Iopromide contrast medium (e.g. Ultravist® 300) was motor injected with the specimen in both the postero-anterior and right anterior oblique positions. Subsequently, 100 ml of 0.9% NaCl solution was injected, followed by formalin; next, immersion fixation in 10% formalin was conducted for at least another 24 h. Then, the en bloc specimens underwent faxitron analysis (Faxitron MX20; Faxitron X-ray Corporation, IL, USA) using a 35 kV setting to assess the integrity of the nitinol mesh at explantation. Subsequently, a 15-mm long cross-section specimen was centrally excised for macroscopic image analysis prior to embedding in paraffin (Groups 1 and 2) or resin (Group 3). Adjacent ring segments were longitudinally opened and processed for macroscopic assessment and scanning electron microscopy.
Macroscopic graft dimensions
Luminal dimensions of implanted vein grafts were calculated on the basis of ODs and the wall thicknesses of control samples [15, 16]. In the absence of intraoperative angiography facilities, the ID of the LAD at implantation was approximated through gentle insertion of probes with incremental quarter-millimeter diameter steps into the proximal stump.
Macroscopic image analysis of mid-graft sections QWinPro (V2.5; Leica Microsystems Imaging Solutions) was used in conjunction with post-mortem explant angiography performed at Groote Schuur Hospital (University of Cape Town).
The degree of dimensional mismatch between vein grafts and host arteries was expressed as the quotient of cross-sectional areas (Qc = ah/ag, whereby ah represented the cross-sectional area of the host artery and ag that of the interposition graft).
Histology
Three routine stains [haematoxylin and eosin (H&E), modified Elastic Masson trichrome and modified Movat] were used for light microscopy (Nikon E1000M; Nikon, Tokyo). Two series of consecutive 3 μm sections (approximately 300 μm apart) were cut and analysed. For immunofluorescence, vonWillebrand factor and α-actin (Abcam, Cambridge, UK) stains were combined with nuclear counter-stains [4′,6-diamidino-2-phenylindole (DAPI); Invitrogen Molecular Probes, Carlsbad, USA] and viewed under a Nikon 90i microscope (Nikon, Tokyo, Japan).
For resin histology, mid-graft sections were infiltrated with methyl-methacrylate resin (Sigma-Aldrich, Steinheim, Germany) [15, 16] and cut on a Leica Polycut using a tungsten carbide blade. Then, the resin was removed with 2-methoxyethylacetate to enable staining [20].
Microscopic image analysis
Composite images were assembled from digital single-frames (Nikon Eclipse 90i) and analysed using Eclipse Net (Laboratory Imaging, Prague, Czech Republic) software. The neointimal tissue (IH tissue) was easily discernable by its typical loose arrangement of smooth muscle cells (SMCs) embedded in abundant extracellular matrix and often additionally by demarcating internal elastic membranes (IEMs). Graft shrinkage or dilatation was assessed on the basis of ‘sub-intimal diameters’ (SIDs).
Scanning electron microscopy
After critical point drying (CPD7501, Polaron, Watford, UK) of specimens that were post-fixed in 2% glutaraldehyde, they were viewed in a JEOL JSM5200 (Jeol, Tokyo, Japan). Scanning electron microscopy images were digitally captured by Orion V5.20 software (Orion, Brussels, Belgium) at ×15 magnifications. Scion Image Software (NIH, Bethesda, USA) was used for the quantification of surface endothelialization.
Statistical analysis
Patency data were compared using Fisher's exact test. For between-group comparisons, one-way analysis of variance (overall P = 0.0006), followed by the post hoc Tukey–Kramer HSD test, was applied using ‘Statistics’ (JMP version 10, SAS, Cary, NC, USA). Normal distribution was confirmed by the Shapiro–Wilk test. Two-tailed P-values <0.05 were regarded as demonstrating statistical significance.
RESULTS
In both control groups, one of the six grafts were occluded, while all mesh-supported grafts (6/6) were patent after 180 days of implantation.
Graft pathology
Upon explantation, control grafts—either untreated or sprayed with FS—were thick walled, with irregular luminal diameters. In contrast, mesh-supported grafts were delicate and thin walled with even luminal dimensions (Fig. 2). While the wall thickness had increased by 363 ± 39% (P < 0.00001) in the untreated controls and 312 ± 61% in the fibrin-sprayed control group (P < 0.00001), it was only 82 ± 61% in the mesh-supported group (P < 0.007). Compared with mesh-supported grafts, the wall thickness at explantation was 2.3 times thicker in controls (P < 0.00001) and 2.0 times thicker in fibrin-sprayed controls (P < 0.0001).
Figure 2:
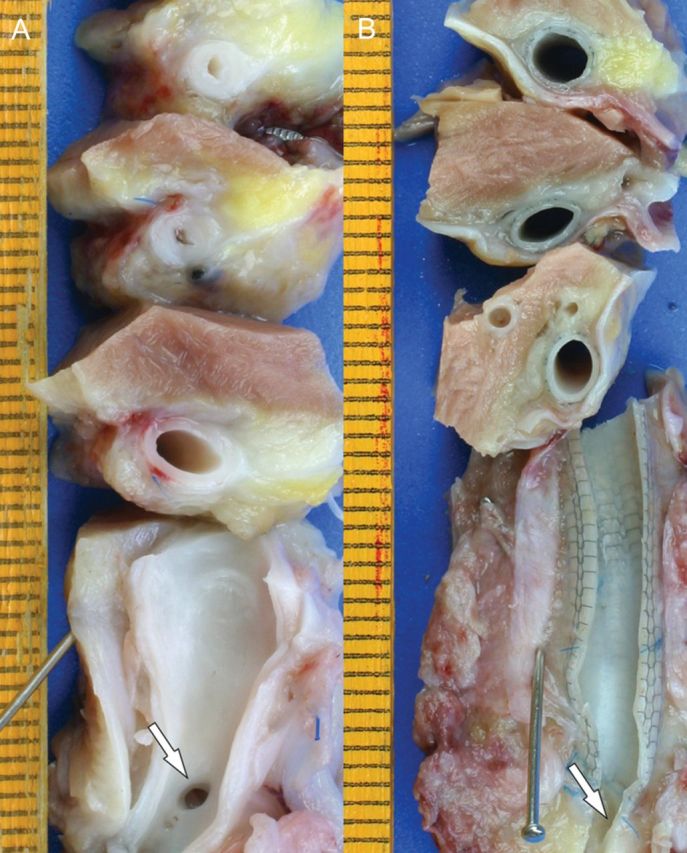
Macroscopic explant morphology of a control graft (A) and a mesh-constricted graft (B) 6 months after implantation. The origins of the proximal LAD stumps are indicated with an arrow where the distal LAD has been longitudinally opened. The distinct wall thickness and luminal irregularities of the control graft are visible on the series of successive cross-sections. The mesh-constricted graft shows constant luminal dimensions and a thin wall thickness that allows the external mesh to be visible through the vein wall. Near the anastomosis with the LAD, transanastomotic neointimal outgrowth led to a slightly thicker, opaque vein wall without causing luminal encroachment.
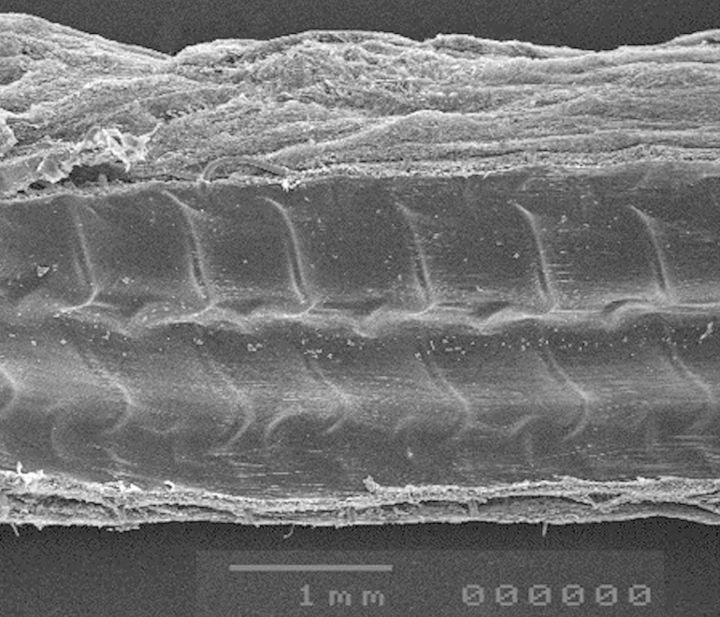
All grafts had a glistening, thrombus-free blood surface [endothelial coverage 91 ± 12% (CO); 75 ± 22% (CO + FS) and 92 ± 8% (ME + FS); NS]. In mesh-constricted grafts, the tissue layer between the mesh and the endothelium was mostly so delicate that the relief of the mesh was recognizable on the inner surface (Fig. 3).
Figure 3:
Scanning electron micrograph of the mid-section of a mesh-constricted saphenous vein graft to the LAD. The wall thickness including neointimal tissue is so delicate that the struts of the nitinol mesh show their imprint on the blood surface.
In all control grafts, concentric, thick neointimal tissue was present [600 ± 63 μm (CO) and 627 ± 204 μm (CO + FS); NS]. In contrast, IH was distinctly less pronounced and typically eccentric in the mesh-supported grafts [249 ± 83 μm; CO vs ME + FS P = 0.002; CO + FS vs ME + FS P = 0.001; Figs 4 and 5]. The data were normally distributed (confirmed by the Shapiro–Wilk test). Related to intimal thickness at the time of implantation, it had increased by 298 ± 42% in controls and 315 ± 135% in fibrin-sprayed controls, but only 65 ± 54% in the mesh-constricted group (P < 0.003 vs both control groups). Compared with mesh-constricted grafts, the cross-sectional area of neointimal tissue was 155% larger in controls (P < 0.0005) and 152% in fibrin-sprayed controls (P < 0.001). IH accounted for 56 ± 6% of the total wall thickness in non-fibrin-treated grafts; 66 ± 14% in fibrin-treated grafts and 59 ± 4% in mesh-supported grafts with no significant difference between any of the groups.
Figure 4:
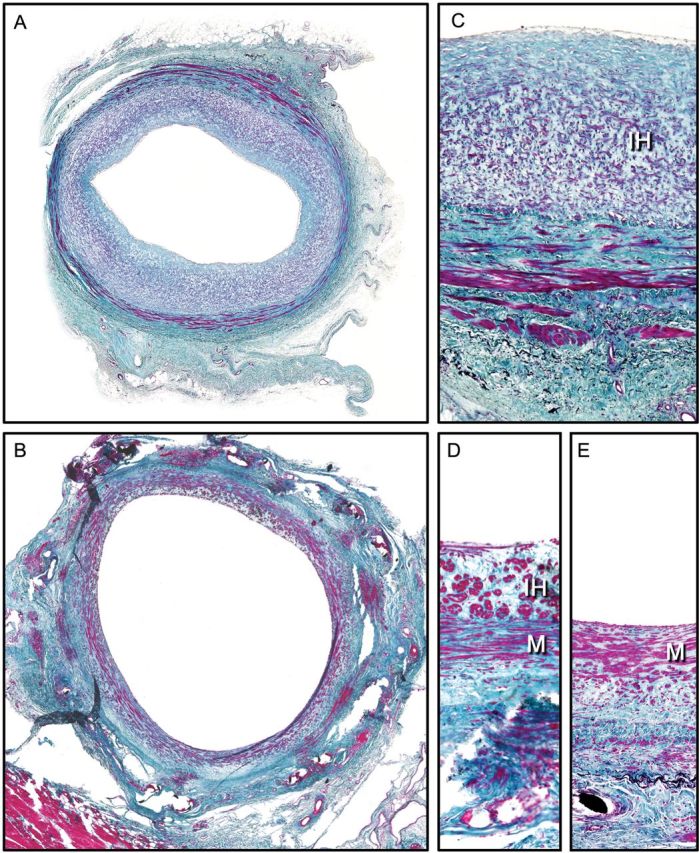
Histological comparison of mid-sections of control grafts [A and C paraffin sections; Masson's Trichrome stain (1.2× and 20× objectives)] and nitinol-constricted vein grafts [B, D and E methyl-methacrylate resin sections; Masson's Trichrome stain (1.2× and 40× objectives)]. Control grafts regularly showed pronounced neo-IH (A and C). The muscle bundles of the media (M) were separated by abundant collagen-rich extracellular matrix. Mesh-constricted grafts had larger luminal dimensions (B) and a relatively compact and smooth muscle cell-rich media. Some areas showed thin to moderate layers of neointimal tissue (D), while others had the endothelium directly overlying the media without any intima hyperplasia tissue (E).
Figure 5:
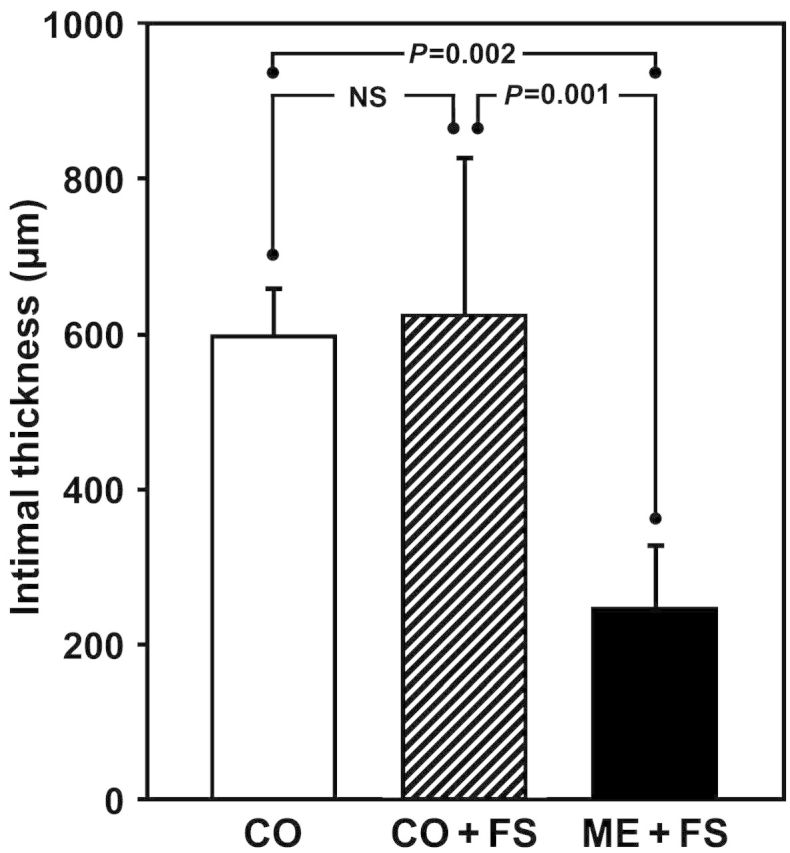
Neointimal thickness in mid-graft sections. Control grafts—untreated (CO) or CO + FS—had thick layers of IH tissue accounting for more than 1.2 mm of luminal encroachment. Mesh constriction resulted in a significant 2.5-fold mitigation of intimal proliferation.
Luminal dimensions
At the time of implantation, the mean ID of all LADs was 1.89 ± 0.30 mm. Upon release of the cross-clamp, the OD/ID of untreated controls was 4.75 ± 0.73/4.27 ± 0.69 mm; of the fibrin-sprayed control 5.18 ± 0.72/4.67 ± 0.71 mm and of the mesh group prior to mesh constriction 5.23 ± 0.51/4.77 ± 0.55 mm with no significant difference between the groups. The mesh-constricted grafts had IDs of 3.02 ± 0.08 mm under arterial pressures. As such, cross-sectional quotients (Qc's) between vein grafts and target arteries were Qc = 0.21 ± 0.07 for untreated controls; Qc = 0.18 ± 0.05 for FS controls and Qc = 0.41 ± 0.17 for mesh-constricted grafts (P < 0.02 vs CO and P < 0.01 vs CO + FS).
After 6 months of implantation, explant angiography showed distinct luminal irregularities in control graft, whereby the ID at the narrowest and the widest segment differed by as much as 74 ± 45%. Localized narrowings were as long as 2 cm (Fig. 6). In contrast, diameter fluctuations were mild in the mesh-constricted grafts and appeared attributable to slight oval mesh deformation at sites of marked bending.
Figure 6:
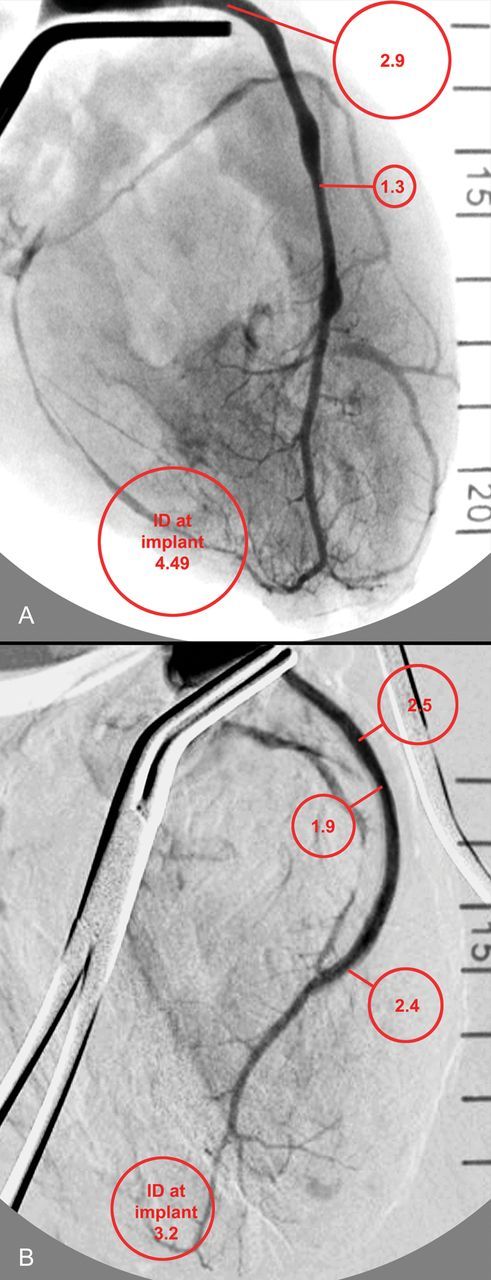
Explant angiographies with contrast medium pressure-injected into the aortic root between two cross-clamps. Control grafts (A) that had a relatively constant calibre at implantation overall shrinkage overlayered by long stretches of narrowings alternating with ‘ectatic’ areas. Mesh-constricted grafts (B) also showed a mild degree of postimplantation shrinkage, but a relatively constant calibre. The mid-graft area appearing slightly narrowed coincided with the slight oval bending deformation seen on the explant in Fig. 3.
The analysis of mid-graft cross-sections showed that all grafts had shrunk significantly in SID (CO: −28.8 ± 17.4%, P < 0.01; CO + FS: −40.0 ± 13.7%, P < 0.002; ME + FS: −20.6 ± 10.1%, P < 0.003). Diameter shrinkage did not differ significantly between any of the groups. As a result, Qc's increased by a factor 6.2, 6.3 and 1.9 to Qc = 1.30 ± 0.85 (CO, P < 0.01); Qc = 1.13 ± 0.85 (CO + FS, P < 0.02) and Qc = 0.78 ± 0.59 (ME + FS; P < 0.05), respectively.
The faxitron analysis showed that only one of the six grafts showed mild signs of wire breakage (Fig. 7).
Figure 7:
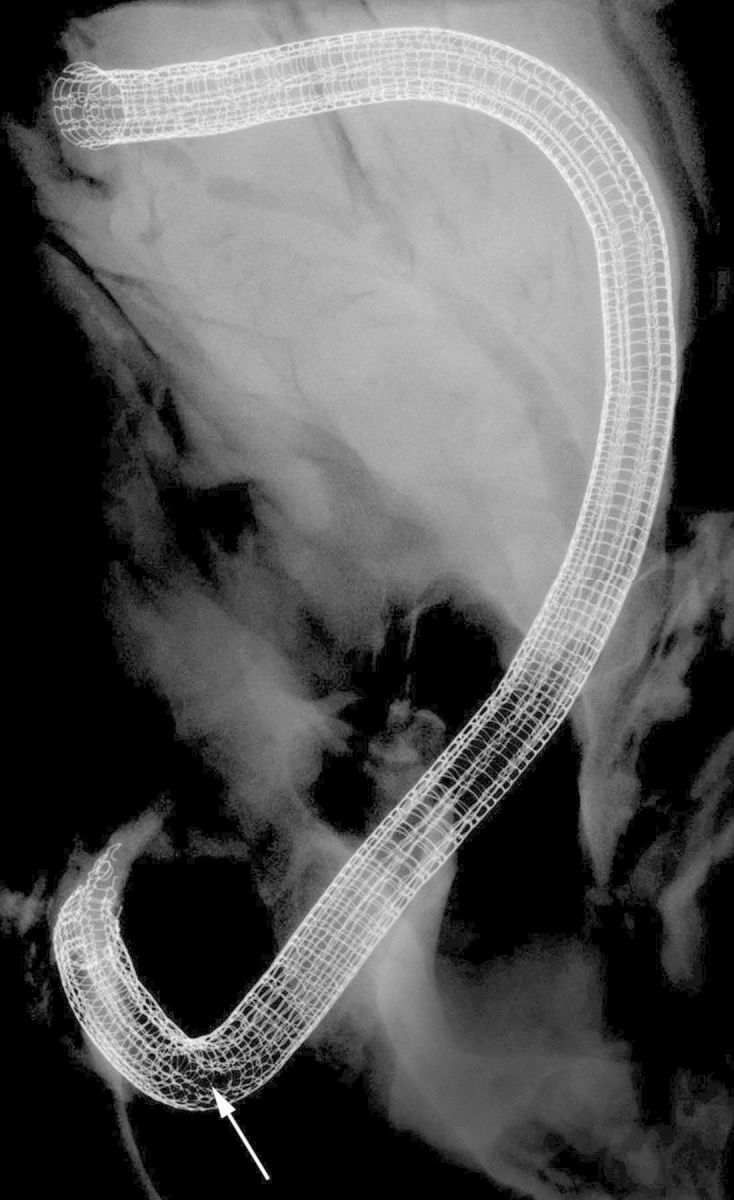
Faxitron picture of a mesh-constricted graft after 6 months of implantation. Neither the mesh end incorporated in the aortic anastomosis (top) nor the one included in the coronary anastomosis showed any loose struts. The displayed mesh was the only one of the current series that showed signs of wire breakage. It was limited to a few wires (arrow) at the outside curvature of the strongest bent segment of the graft.
DISCUSSION
Using a senescent, non-human primate model, we could demonstrate that external constriction of coronary vein grafts with a knitted nitinol meshes suppresses IH and prevents the formation of localized luminal narrowings. After 180 days of implantation, control grafts showed thick layers of neointimal tissue as opposed to the largely delicate neointima found on mesh-constricted grafts. Moreover, luminal irregularities in control vein grafts—varying between extended segments of narrowing and sometimes ectatic areas—were opposed by a relatively constant ID in mesh-sheathed grafts.
Although the vascular tissue response in senescent Chacma baboons is still less deferred than in humans, it has previously been shown to reflect vascular injury and remodelling better than other animal models [21, 22]. As such, a half-year observation period is likely to correspond [21] with the mid-term graft failure period of clinical CABG. Since a majority of clinical graft failures occurring beyond the first year have been shown to be attributable to pre-existing diffuse or localized IH [8], it seems justified to project the results of the present study to the clinical mid- to long-term performance of mesh-constricted vein grafts.
However, while the suppression of neointimal tissue formation was still distinct and significant, it was less pronounced than in previously implanted infrainguinal grafts using the same animal model. In contrast to the near-complete abolition of IH in the infrainguinal model, mesh-constricted coronary vein grafts regularly showed thin but clearly discernable areas of IH along the inner circumference. Apart from different flow patterns [19], the main difference between infrainguinal and coronary bypass grafts is the degree of calibre mismatch between the vein grafts and target arteries.
In the senescent Chacma baboon, this calibre mismatch between coronary vein grafts and the LAD is 3.6 times that of infrainguinal grafts, resulting in a Qc of 0.16 as opposed to 0.62. Calibre-mismatched vein grafts that are larger than their target arteries experience flow deceleration. Notwithstanding differences in flow patterns, pulse volumes and run-off at different anatomical sites, the lower shear stress resulting from this flow deceleration is known to trigger IH and graft shrinkage [19]. Corresponding with the more pronounced calibre mismatch in coronary vein grafts, the maximum flow velocity was found to be 58% and the mean velocity 62% lower than in femoral vein grafts [19]. As such, the resulting mean shear stress was shown to be 2.4 dynes/cm2 in coronary vein grafts as opposed to 5.8 dynes/cm2 in infrainguinal grafts using the same saphenous vein as a conduit [19]. The threshold for the suppression of IH had been estimated to be 5 dynes/cm2 [23]. In a previous study, we concluded that the threshold Qc for the complete suppression of IH seemed to be at a Qc of >0.45 [15]. Given the fact that a 3.4-mm mesh constriction of baboon coronary vein grafts only partially mitigated their calibre mismatch with the LAD from a Qc of 0.18 to a Qc of 0.41, it seems plausible that 5 dynes/cm2 were only marginally reached. In contrast, a similar constriction of infrainguinal vein grafts with 3.4 mm ID meshes allowed even an over-correction of the calibre mismatch to a Qc of >1. The resulting high shear forces were unequivocally suppressive for IH [15, 16]. The diameter of a coronary vein graft that corresponds with this degree of adaptive constriction would have needed to be <1.8 mm ID. Such small diameters are not a problem per se. As ITA grafts show, small diameters are ideal if they can be achieved through the use of naturally occurring arteries. The much larger vein grafts, though, have limits of diameter constriction. While most surgeons would have a high threshold towards using a vein graft of <3 mm diameter, there are also handling issues and aspects of vein redundancy. Although we have previously shown that longitudinal pleating of the nitinol mesh allows significant down-sizing of the cross-sectional area without reduction of the circumference [16] and as such would take care of the excess of vein wall relative to the small diameter, there are limits of diameter reduction when it comes to the atraumatic insertion of longer veins into the mesh. As the insertion resistance increases with decreasing mesh diameter, the postinsertion retraction forces increase. As a consequence, the vein end may retract under the cover of the thin layer of FS used for the attachment of the mesh. If the suture of the aortic anastomosis mistakes the fibrin for the vein, acute inflow occlusions of the graft are possible. Therefore, after a short learning curve, we took care to re-cut the mesh to align it with the vein graft end. Moreover, mesh-constricted vein grafts have a low tolerance towards additional luminal encroachment. Therefore, side branches were cut-off and over-sewn with 7–0 Prolene to prevent the ligated stumps from compressing the vein. In a study of 39 human saphenous veins inserted in 3.4 mm meshes, the luminal encroachment by ligated branches was >40% (manuscript in preparation). This distinct additional narrowing of a lumen that had already been reduced by almost 40% through the mesh constriction was the obvious reason for a dismal 9-month patency of 28% in a clinical pilot study [24].
However, while distinctly smaller mesh sizes would be required to achieve completely suppressive shear forces in coronary vein grafts of baboons, constriction with an identical 3.4-mm mesh would likely achieve these forces in humans where the diameter of the saphenous vein is 15% smaller and that of the LAD 38% larger than in baboons [19, 25]. As such, a 3.4-mm (ID) mesh that only led to an increase in Qc from <0.20 to 0.41 in the present study would have achieved a Qc of >0.6 under clinical circumstances [15].
Apart from the remodelling behaviour of the sheathed vein grafts, the meshes themselves behaved differently in the coronary compared with the infrainguinal position. While 63% of meshes had shown local signs of wire breakage in patent infrainguinal vein grafts [17], only one of the six mesh-constricted coronary grafts showed any wire breakage in the present study, restricted to a few single struts in the area of strongest graft bending. The differences between the previously investigated femoral and the present coronary locations were three-fold: for one, the inserted vein was the saphenous vein in the coronary position, while it was the significantly larger femoral vein in infrainguinal grafts. However, since we have previously shown that saphenous veins dilate under femoral flow conditions [19] while having an even thinner wall thickness than femoral veins [15], this difference seems unlikely to be the reason for the wire breakages. Alternatively, one could argue that the breakages all happened in a mechanically strained area as opposed to the protected anatomy behind the sternum. Yet, the fact that breakages only happened in patent grafts makes this explanation unlikely to be a single-factor cause of material tiring. Lastly, differences in pulse volume and amplitude could account for higher wire-stress in the femoral position. Alternatively, a combination of all three factors could be the case.
In conclusion, we could show in a senescent non-human primate model that constrictive, external mesh support of saphenous vein grafts with knitted nitinol suppresses neointimal tissue proliferation in CABGs by a factor of 2.5. Although distinct and significant, this was clearly less pronounced than the mitigation of IH by a factor of 8.9, which we had previously observed in the high-flow and better diameter-adapted infrainguinal position [17]. However, the milder calibre mismatch between saphenous veins and the coronary arteries in humans makes it likely that the effect of mesh constriction on coronary vein grafts will be more pronounced when clinically applied. Notwithstanding, even the more moderate mesh effect observed in the present study resulted in a clear outcome with regard to larger and constant luminal graft dimensions and the mitigation of focal and diffuse IH. The fact that the meshes showed hardly any signs of wire breakage should alleviate previous concerns.
Funding
This work was supported by the ‘Cape Heart Group’ grant of the South African Medical Research Council (MRC; no grant number); a ‘Technology and Human Resources for Industry Program’ grant of the National Research Foundation in South Africa (NRF) and a Research Collaboration grant by Medtronic Inc. (Minneapolis, Minnesota) to the University of Cape Town.
Conflict of interest: Deon Bezuidenhout, Thomas Franz, Michael F. Wolf, Paul Human and Peter Zilla are inventors on patents for the technology described in this study. At the time of the study, Michael F. Wolf was an employee of Medtronic.
ACKNOWLEDGEMENTS
The authors thank Adcock Ingram Critical Care for the generous donation of the Baxter Tisseel® fibrin kits used in this study.
REFERENCES
- 1.Fukui T, Tabata M, Manabe S, Shimokawa T, Takanashi S. Graft selection and one-year patency rates in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2010;89:1901–5. doi: 10.1016/j.athoracsur.2010.02.016. doi:10.1016/j.athoracsur.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. doi:10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 3.Tatoulis J. Giant leaps in surgical myocardial revascularisation. Heart Lung Circ. 2011;20:149–56. doi: 10.1016/j.hlc.2010.07.011. doi:10.1016/j.hlc.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.FitzGibbon G, Leach A, Kafka H, Keon W. Coronary bypass graft fate: long-term angiographic study. J Am Coll Cardiol. 1991;17:1075–80. doi: 10.1016/0735-1097(91)90834-v. doi:10.1016/0735-1097(91)90834-V. [DOI] [PubMed] [Google Scholar]
- 5.Campeau L, Enjalbert M, Lesperance J, Vaislic C, Grondin CM, Bourassa MG. Atherosclerosis and late closure of aortocoronary saphenous vein grafts: sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation. 1983;68:II1–7. [PubMed] [Google Scholar]
- 6.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–56. doi: 10.1016/j.jacc.2004.08.064. doi:10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 7.Grondin CM, Campeau L, Lesperance J, Enjalbert M, Bourassa MG. Comparison of late changes in internal mammary artery and saphenous vein grafts in two consecutive series of patients 10 years after operation. Circulation. 1984;70:I208–12. [PubMed] [Google Scholar]
- 8.Campeau L, Lesperance J, Corbara F, Hermann J, Grondin CM, Bourassa MG. Aortocoronary saphenous vein bypass graft changes 5 to 7 years after surgery. Circulation. 1978;58:I170–5. [PubMed] [Google Scholar]
- 9.Lie JT, Lawrie GM, Morris GC., Jr Aortocoronary bypass saphenous vein graft atherosclerosis. Anatomic study of 99 vein grafts from normal and hyperlipoproteinemic patients up to 75 months postoperatively. Am J Cardiol. 1977;40:906–14. doi: 10.1016/0002-9149(77)90041-8. doi:10.1016/0002-9149(77)90041-8. [DOI] [PubMed] [Google Scholar]
- 10.Mills JL, Bandyk DF, Gahtan V, Esses GE. The origin of infrainguinal vein graft stenosis: a prospective study based on duplex surveillance. J Vasc Surg. 1995;21:16–22. doi: 10.1016/s0741-5214(95)70240-7. discussion 22–5 doi:10.1016/S0741-5214(95)70240-7. [DOI] [PubMed] [Google Scholar]
- 11.Souza DS, Johansson B, Bojo L, Karlsson R, Geijer H, Filbey D, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg. 2006;132:373–8. doi: 10.1016/j.jtcvs.2006.04.002. doi:10.1016/j.jtcvs.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz L, O'Donohoe DM, Purut C, Mikat E, Hagen P, McCann R. Myointimal thickening in experimental vein grafts is dependent on wall tension. J Vasc Surg. 1992;15:176–86. doi: 10.1067/mva.1992.33805. doi:10.1016/0741-5214(92)70026-H. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Chandran KB, Bower TJ, Corson JD. Flow dynamics across end-to-end vascular bypass graft anastomoses. Ann Biomed Eng. 1993;21:311–20. doi: 10.1007/BF02368624. doi:10.1007/BF02368624. [DOI] [PubMed] [Google Scholar]
- 14.Kohler T, Kirkman T, Kraiss L, Zierler B, Clowes A. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991;69:1557–65. doi: 10.1161/01.res.69.6.1557. doi:10.1161/01.RES.69.6.1557. [DOI] [PubMed] [Google Scholar]
- 15.Zilla P, Human P, Wolf M, Lichtenberg W, Rafiee N, Bezuidenhout D, et al. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. J Thorac Cardiovasc Surg. 2008;136:717–25. doi: 10.1016/j.jtcvs.2008.02.068. doi:10.1016/j.jtcvs.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 16.Zilla P, Wolf M, Rafiee N, Moodley L, Bezuidenhout D, Black M, et al. Utilization of shape memory in external vein-graft meshes allows extreme diameter constriction for suppressing intimal hyperplasia: a non-human primate study. J Vasc Surg. 2009;49:1532–42. doi: 10.1016/j.jvs.2009.01.068. doi:10.1016/j.jvs.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 17.Zilla P, Moodley L, Wolf MF, Bezuidenhout D, Sirry MS, Rafiee N, et al. Knitted nitinol represents a new generation of constrictive external vein graft meshes. J Vasc Surg. 2011;54:1439–50. doi: 10.1016/j.jvs.2011.05.023. doi:10.1016/j.jvs.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Klesius AA, Konerding MA, Knez P, Dzemali O, Schmitz-Rixen T, Ackermann H, et al. External stenting with a new polyester mesh reduces neointimal hyperplasia of vein grafts in a sheep model. Int J Artif Organs. 2007;30:930–8. doi: 10.1177/039139880703001011. [DOI] [PubMed] [Google Scholar]
- 19.Zilla P, Moodley L, Scherman J, Krynauw H, Kortsmit J, Human P, et al. Remodeling leads to distinctly more intimal hyperplasia in coronary than in infrainguinal vein grafts. J Vasc Surg. 2012;55:1734–41. doi: 10.1016/j.jvs.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 20.Rippstein P, Black M, Boivin M, Veinot J, Ma X, Chen Y, et al. Comparison of processing and sectioning methodologies for arteries containing metallic stents. J Histochem Cytochem. 2006;54:673–81. doi: 10.1369/jhc.5A6824.2006. doi:10.1369/jhc.5A6824.2006. [DOI] [PubMed] [Google Scholar]
- 21.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–27. doi: 10.1016/j.biomaterials.2007.07.017. doi:10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Zilla P, Preiss P, Groscurth P, Rosemeier F, Deutsch M, Odell J, et al. In vitro-lined endothelium: initial integrity and ultrastructural events. Surgery. 1994;116:524–34. [PubMed] [Google Scholar]
- 23.Sho E, Nanjo H, Sho M, Kobayashi M, Komatsu M, Kawamura K, et al. Arterial enlargement, tortuosity, and intimal thickening in response to sequential exposure to high and low wall shear stress. J Vasc Surg. 2004;39:601–12. doi: 10.1016/j.jvs.2003.10.058. doi:10.1016/j.jvs.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 24.Schoettler J, Jussli-Melchers J, Grothusen C, Stracke L, Schoeneich F, Stohn S, et al. Highly flexible nitinol mesh to encase aortocoronary saphenous vein grafts: first clinical experiences and angiographic results nine months postoperatively. Interact CardioVasc Thorac Surg. 2011;13:396–400. doi: 10.1510/icvts.2010.265116. doi:10.1510/icvts.2010.265116. [DOI] [PubMed] [Google Scholar]
- 25.Human P, Franz T, Scherman J, Moodley L, Zilla P. Dimensional analysis of human saphenous vein grafts: implications for external mesh support. J Thorac Cardiovasc Surg. 2009;137:1101–8. doi: 10.1016/j.jtcvs.2008.10.040. doi:10.1016/j.jtcvs.2008.10.040. [DOI] [PubMed] [Google Scholar]