Abstract
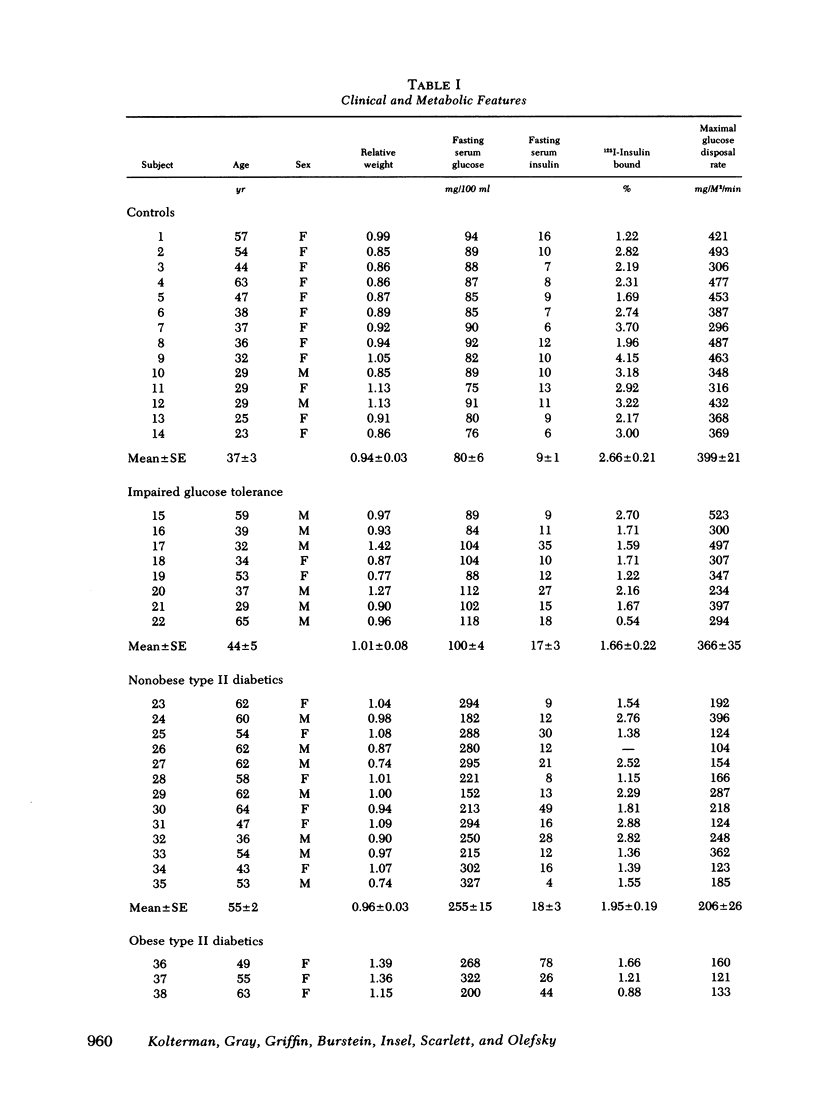
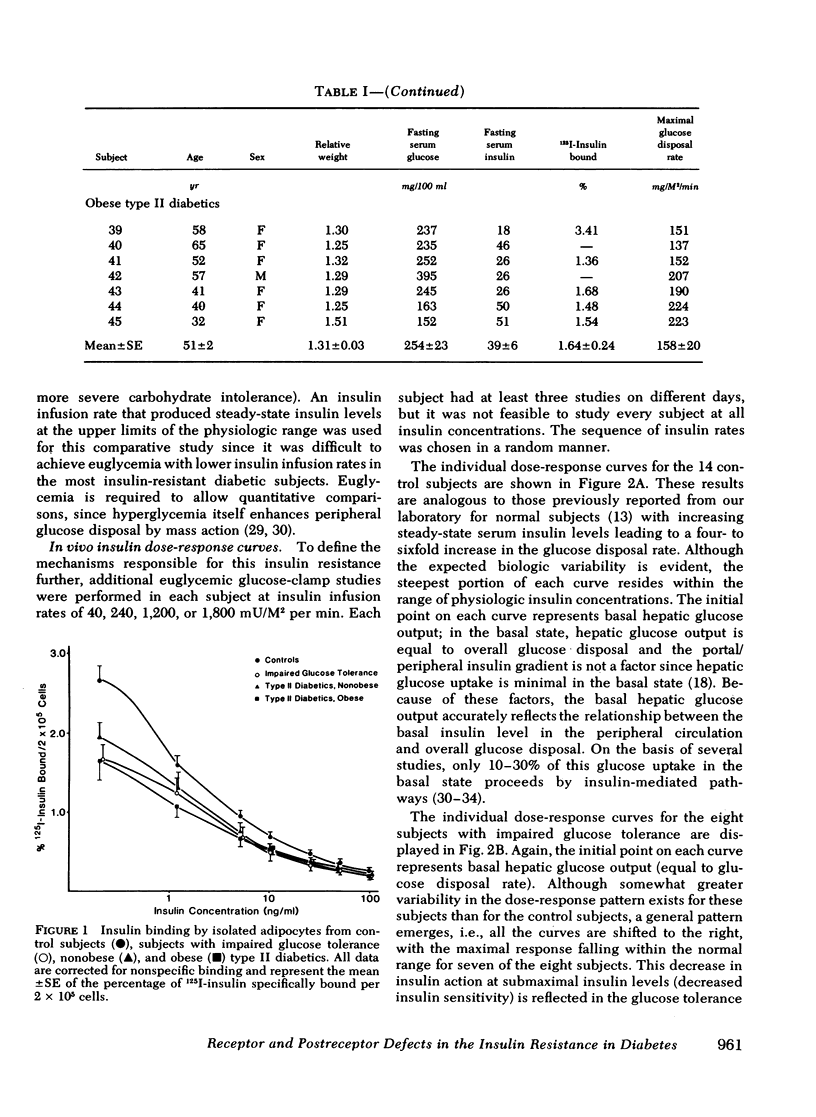
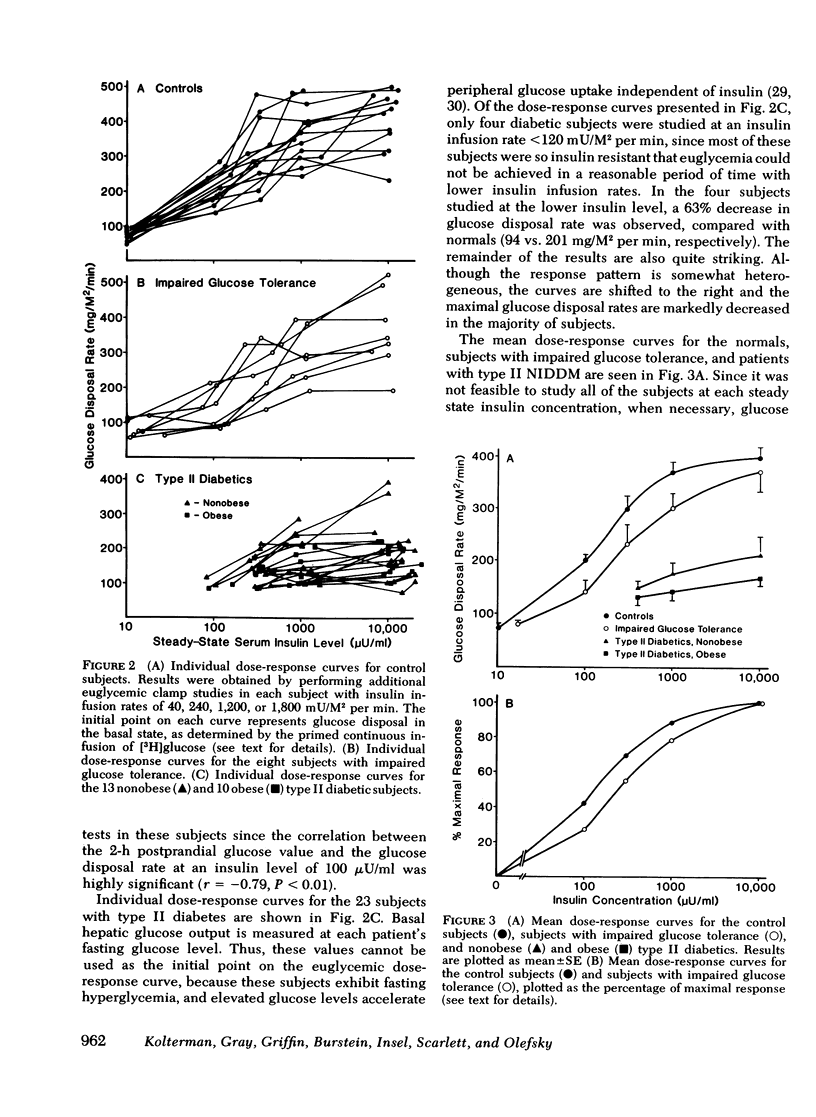
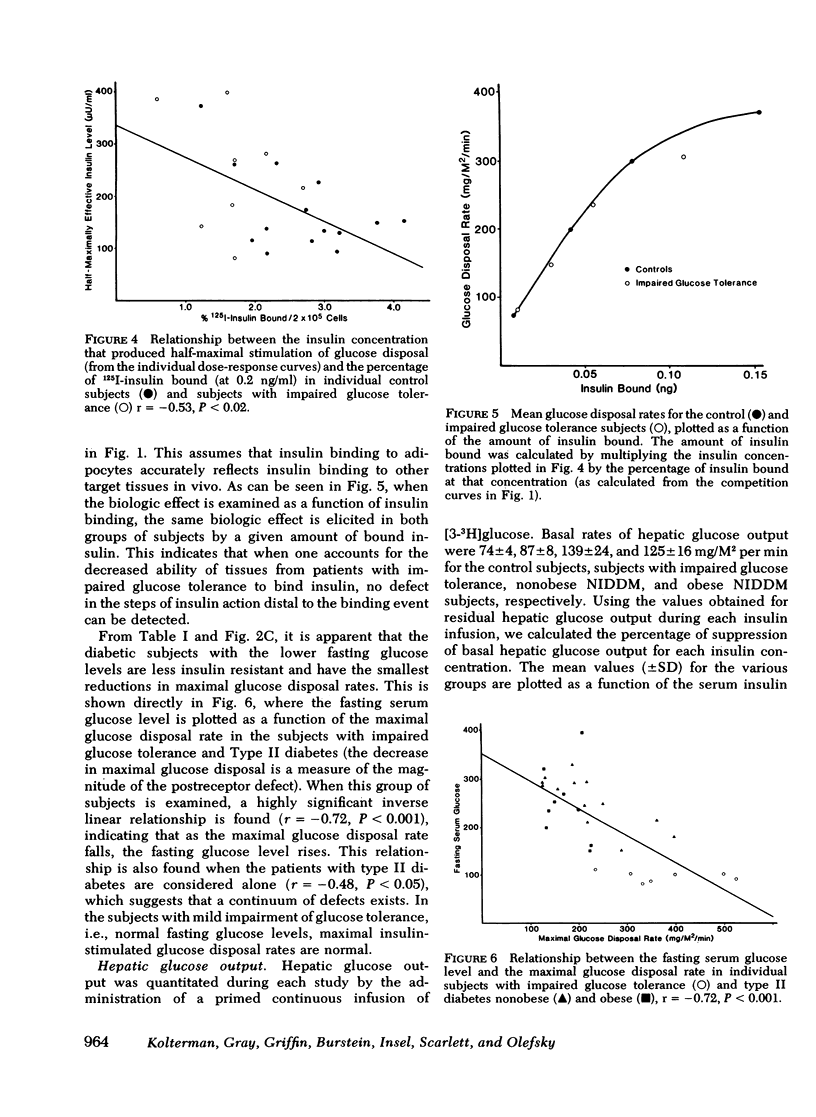
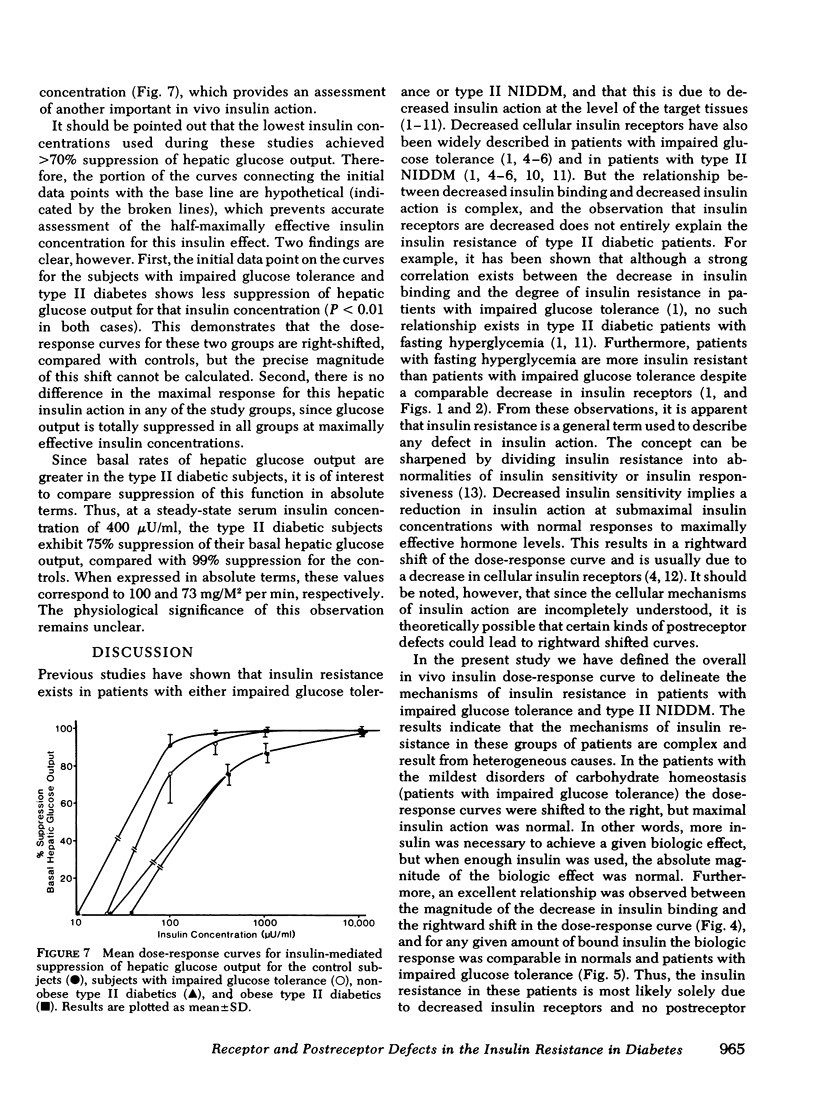
We have assessed the mechanisms involved in the pathogenesis of the insulin resistance associated with impaired glucose tolerance and Type II diabetes mellitus by exploring, by means of the euglycemic glucose-clamp technique, the in vivo dose-response relationship between serum insulin and the overall rate of glucose disposal in 14 control subjects; 8 subjects with impaired glucose tolerance, and 23 subjects with Type II diabetes. Each subject had at least three studies performed on separate days at insulin infusion rates of 40, 120, 240, 1,200, or 1,800 mU/M2 per min. In the subjects with impaired glucose tolerance, the dose-response curve was shifted to the right (half-maximally effective insulin level 240 vs. 135 microunits/ml for controls), but the maximal rate of glucose disposal remained normal. In patients with Type II diabetes mellitus, the dose-response curve was also shifted to the right, but in addition, there was a posal. This pattern was seen both in the 13 nonobese and the 10 obese diabetic subjects. Among these patients, an inverse linear relationship exists (r = -0.72) so that the higher the fasting glucose level, the lower the maximal glucose disposal rate. Basal rates of hepatic glucose output were 74 +/- 4, 82 +/- 7, 139 +/- 24, and 125 +/- 16 mg/M2 per min for the control subjects, subjects with impaired glucose tolerance, nonobese Type II diabetic subjects, and obese Type II diabetic subjects, respectively. Higher serum insulin levels were required to suppress hepatic glucose output in the subjects with impaired glucose tolerance and Type II diabetics, compared with controls, but hepatic glucose output could be totally suppressed in each study group. We conclude that the mechanisms of insulin resistance in patients with impaired glucose tolerance and in patients with Type II noninsulin-dependent diabetes are complex, and result from heterogeneous causes. (a) In the patients with the mildest disorders of carbohydrate homeostasis (patients with impaired glucose tolerance) the insulin resistance can be accounted for solely on the basis of decreased insulin receptors. (b) In patients with fasting hyperglycemia, insulin resistance is due to both decreased insulin receptors and postreceptor defect in the glucose mechanisms. (c) As the hyperglycemia worsens, the postreceptor defect in peripheral glucose disposal emerges and progressively increases. And (d) no postreceptor defect was detected in any of the patient groups when insulin's ability to suppress hepatic glucose output was measured.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck-Nielsen H. The pathogenic role of an insulin-receptor defect in diabetes mellitus of the obese. Diabetes. 1978 Dec;27(12):1175–1181. doi: 10.2337/diab.27.12.1175. [DOI] [PubMed] [Google Scholar]
- Bergman R. N. Integrated control of hepatic glucose metabolism. Fed Proc. 1977 Feb;36(2):265–270. [PubMed] [Google Scholar]
- Bowen H. F., Moorhouse J. A. Glucose turnover and disposal in maturity-onset diabetes. J Clin Invest. 1973 Dec;52(12):3033–3045. doi: 10.1172/JCI107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Harris M. S. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978 Jul;27(7):787–791. doi: 10.1016/0026-0495(78)90213-5. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Forbath N., Hetenyi G., Jr Glucose dynamics in normal subjects and diabetic patients before and after a glucose load. Diabetes. 1966 Nov;15(11):778–789. doi: 10.2337/diab.15.11.778. [DOI] [PubMed] [Google Scholar]
- Ginsberg H., Kimmerling G., Olefsky J. M., Reaven G. M. Demonstration of insulin resistance in untreated adult onset diabetic subjects with fasting hyperglycemia. J Clin Invest. 1975 Mar;55(3):454–461. doi: 10.1172/JCI107951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. C., Phelps M. E., Hoffman E. J., Sideris K., Selin C. J., Kuhl D. E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980 Jan;238(1):E69–E82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS G., REICHARD G., GOODMAN E. H., Jr, FRIEDMANN B., WEINHOUSE S. Action of insulin and tolbutamide on blood glucose entry and removal. Diabetes. 1958 Sep-Oct;7(5):358–364. doi: 10.2337/diab.7.5.358. [DOI] [PubMed] [Google Scholar]
- KALANT N., CSORBA T. R., HELLER N. EFFECT OF INSULIN ON GLUCOSE PRODUCTION AND UTILIZATION IN DIABETES. Metabolism. 1963 Dec;12:1100–1111. [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Jen P., Reaven G. M., Alto P. Insulin binding to isolated human adipocytes. Diabetes. 1974 Jul;23(7):565–571. doi: 10.2337/diab.23.7.565. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M. Ability of circulating insulin to chronically regulate the cellular glucose transport system. Metabolism. 1978 Dec;27(12 Suppl 2):1917–1929. doi: 10.1016/s0026-0495(78)80009-2. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding in diabetes. Relationships with plasma insulin levels and insulin sensitivity. Diabetes. 1977 Jul;26(7):680–688. doi: 10.2337/diab.26.7.680. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Sperling M. A., Reaven G. M. Does glucagon play a role in the insulin resistance of patients with adult non-ketotic diabetes? Diabetologia. 1977 Aug;13(4):327–330. doi: 10.1007/BF01223274. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor: its role in insulin resistance of obesity and diabetes. Diabetes. 1976 Dec;25(12):1154–1162. doi: 10.2337/diab.25.12.1154. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R., DeFronzo R., Wahren J., Felic P. Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci U S A. 1977 Jan;74(1):348–352. doi: 10.1073/pnas.74.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk C. A., Rizza R. A., Gerich J. E. Effects of plasma glucose concentration on glucose utilization and glucose clearance in normal man. Diabetes. 1981 Jun;30(6):535–537. doi: 10.2337/diab.30.6.535. [DOI] [PubMed] [Google Scholar]
- Vranic M., Wrenshall G. A. Matched rates of insulin infusion and secretion and concurrent tracer-determined rates of glucose appearance and disappearance in fasting dogs. Can J Physiol Pharmacol. 1968 May;46(3):383–390. doi: 10.1139/y68-058. [DOI] [PubMed] [Google Scholar]



