Abstract
Background
L. tropica can cause both cutaneous and visceral leishmaniasis in humans. Although the L. tropica-induced cutaneous disease has been long known, its potential to visceralize in humans was recognized only recently. As nothing is known about the genetics of host responses to this infection and their clinical impact, we developed an informative animal model. We described previously that the recombinant congenic strain CcS-16 carrying 12.5% genes from the resistant parental strain STS/A and 87.5% genes from the susceptible strain BALB/c is more susceptible to L. tropica than BALB/c. We used these strains to map and functionally characterize the gene-loci regulating the immune responses and pathology.
Methods
We analyzed genetics of response to L. tropica in infected F2 hybrids between BALB/c×CcS-16. CcS-16 strain carries STS-derived segments on nine chromosomes. We genotyped these segments in the F2 hybrid mice and tested their linkage with pathological changes and systemic immune responses.
Principal Findings
We mapped 8 Ltr (Leishmania tropica response) loci. Four loci (Ltr2, Ltr3, Ltr6 and Ltr8) exhibit independent responses to L. tropica, while Ltr1, Ltr4, Ltr5 and Ltr7 were detected only in gene-gene interactions with other Ltr loci. Ltr3 exhibits the recently discovered phenomenon of transgenerational parental effect on parasite numbers in spleen. The most precise mapping (4.07 Mb) was achieved for Ltr1 (chr.2), which controls parasite numbers in lymph nodes. Five Ltr loci co-localize with loci controlling susceptibility to L. major, three are likely L. tropica specific. Individual Ltr loci affect different subsets of responses, exhibit organ specific effects and a separate control of parasite load and organ pathology.
Conclusion
We present the first identification of genetic loci controlling susceptibility to L. tropica. The different combinations of alleles controlling various symptoms of the disease likely co-determine different manifestations of disease induced by the same pathogen in individual mice.
Author Summary
Leishmaniasis, a disease caused by Leishmania ssp. is among the most neglected infectious diseases. In humans, L. tropica causes cutaneous form of leishmaniasis, but can damage internal organs too. The reasons for this variability are not known, and its genetic basis was never investigated. Therefore, analysis of genes affecting host's responses to this infection can elucidate the characteristics of individual host-parasite interactions. Recombinant congenic strain CcS-16 carries 12.5% genes from the mouse strain STS/A on genetic background of the strain BALB/c, and it is more susceptible than BALB/c. In F2 hybrids between BALB/c and CcS-16 we detected and mapped eight gene-loci, Ltr1-8 (Leishmania tropica response 1-8) that control various manifestations of disease: skin lesions, splenomegaly, hepatomegaly, parasite numbers in spleen, liver, and inguinal lymph nodes, and serum level of CCL3, CCL5, and CCL7 after L. tropica infection. These loci are functionally heterogeneous - each influences a different set of responses to the pathogen. Five loci co-localize with the previously described loci that control susceptibility to L. major, three are species-specific. Ltr2 co-localizes not only with Lmr14 (Leishmania major response 14), but also with Ir2 influencing susceptibility to L. donovani and might therefore carry a common gene controlling susceptibility to leishmaniasis.
Introduction
Leishmaniasis is endemic in 98 countries on 5 continents, causing 20,000 to 40,000 deaths per year [1]. In the past decade the number of endemic regions have expanded, prevalence has increased and the number of unrecorded cases must have been substantial, because notification has been compulsory in only 32 of the 98 countries where 350 million people are at risk [1], [2]. Infection represents an important global health problem, as no safe and effective vaccine currently exists against any form of human leishmaniasis, and the treatment is hampered by serious side effects [3].
The disease is caused by obligate intracellular vector-borne parasites of the genus Leishmania. In the vertebrate host organism, Leishmania parasites infect so-called professional phagocytes (neutrophils, monocytes and macrophages) [4], as well as dendritic cells [5], immature myeloid precursor cells, sialoadhesin-positive stromal macrophages of the bone marrow, hepatocytes and fibroblasts [6]. Leishmaniasis includes asymptomatic infection and three main clinical syndromes. In the dermis, parasites cause the cutaneous form of the disease, which can be localized or diffuse; in the mucosa, they cause mucocutaneous leishmaniasis, and the metastatic spread of infection to the spleen and liver leads to visceral leishmaniasis (also known as kala-azar or black fever). Parasites can also enter other organs, such as lymph nodes, bone marrow and lungs, and in rare cases, can even reach the brain [4]. One of the major factors determining the type of pathology is the species of Leishmania [7]. However, the transmitting vector, as well as genotype, nutritional status of the host, and environmental and social factors also have a large impact on the outcome of the disease [4], [7]. That is why even patients infected by the same species of Leishmania develop different symptoms [7] and may differ in response to therapy [3]. The basis of this heterogeneity is not well understood [8], but part of this variation is likely genetic [4].
The search for loci and genes controlling leishmaniasis included candidate-gene approach, genome-wide linkage and association mapping. Genotyping of candidate genes, which have been chosen on the basis of previous immunological studies (hypothesis-driven approach) detected influence of polymorphism in HLA-Cw7, HLA-DQw3, HLA-DR, TNFA (tumor necrosis factor alpha), TNFB, IL4, IFNGR1 (interferon gamma receptor 1) [reviewed in [4]], TGFB1 (transforming growth factor, beta 1) [9], IL1 [10], IL6 [11], CCL2/MCP1 (chemokine (C-C motif) ligand 2) [12], CXCR1 (chemokine (C-X-C motif) receptor 1) [13], CXCR2 (chemokine (C-X-C motif) receptor 2) [14], FCN2 (ficolin-2) [15] and MBL2 (mannose-binding lectin (protein C) 2) [16] on response to different human leishmaniases.
Hypothesis-independent search for susceptibility genes included genome-wide linkage and association mapping. Bucheton and coworkers [17] performed a genome-wide linkage scan, identified a major susceptibility locus that controls the susceptibility to L. donovani on chromosome 22q12 [17] and found that polymorphism in IL2RB (interleukin 2 receptor, beta chain) in this chromosomal region is associated with susceptibility to visceral leishmaniasis [18]. Genome-wide search with the subsequent analysis of a putative susceptibility locus on chromosome 6q27 revealed that polymorphism in DLL1 (delta-like 1 (Drosophila)), the ligand for NOTCH3 (Neurogenic locus notch homolog protein 3) [19] is associated with susceptibility to visceral leishmaniasis caused by L. donovani and L. infantum chagasi. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells [20]. GWAS (genome-wide association study) established that common variants in the HLA-DRB1-HLA-DQA1 HLA class II region contribute to susceptibility to L. donovani and L. infantum chagasi [21].
Genome-wide linkage in mouse revealed susceptibility genes Nramp1 (Natural resistance-associated macrophage protein 1)/Slc11a1 (solute carrier family 11 (proton-coupled divalent metal ion transporters), member 1) [22] and Fli1 (Friend leukaemia virus integration 1) [23] and the role of these genes has been also established in humans [13], [24], [25]. NRAMP1, which controls susceptibility to L. donovani and L. infantum functions as a divalent metal pH-dependent efflux pump at the phagosomal membrane of macrophages and neutrophils [26]. It is also expressed in dendritic cells and influences major histocompatibility complex class II expression and antigen-presenting cell function [27]. Susceptible mouse allele carries a “null” mutation that abolishes gene function (it is a natural knockout) [28], whereas polymorphisms in the promoter, exon3 and the intron of human SLC11A1 [24], are expected to have a smaller impact on gene function. The Friend leukaemia virus integration gene, linked with wound healing, influences cutaneous leishmaniasis caused by L. major in mouse [23] and by L. braziliensis in human [25]. It remains to be tested, whether natural polymorphisms detected in mouse genes bg (beige)/Lyst (lysosomal trafficking regulator) [29] and cationic amino acid transporter Slc7a2 (solute carrier family 7 (cationic amino acid transporter, y+ system), member 2) [30] influencing response to L. donovani [31] and L. major [30], respectively, plays role also in humans. However, nothing is known about genes controlling L. tropica-induced disease in humans.
L. tropica causes cutaneous leishmaniasis in humans, but it can also visceralize. Although cutaneous disease due to L. tropica is known for a long time, its potential to visceralize in humans has been recognized only relatively recently [32]. Visceralized L. tropica was also identified as the cause of an initially not understood systemic illness in veterans returning from endemic areas in the Middle East [33]. This finding stimulated interest in less typical symptoms induced by this parasite. It was found that L. tropica caused visceral disease in Kenya [34], as well as classical visceral leishmaniasis (kala-azar) in India [35], [36] and in Iran [37], and disseminated cutaneous leishmaniasis accompanied with visceral leishmaniasis in Iran [38]. L. tropica was also implicated in development of mucosal leishmaniasis in Iran [39]. The reasons of this variability are not known.
A suitable animal model for study of this parasite would therefore contribute to genetic dissection of the functional and clinical manifestations of infection. Golden hamsters (Mesocricetus auratus) have been considered to be the best model host for L. tropica infection, but this host is not inbred and therefore not suitable for genetic dissection. Fortunately, several L. tropica strains from Afghanistan, India [40], and Turkey [41] have been reported to cause cutaneous disease in inbred BALB/c mice. Extension of analysis to the strains C57BL/6J, C57BL/10SgSnAi and gene-deficient mice on their backgrounds indicated role of IL-10 and TGFβ in regulation of parasite numbers in ears of infected mice [42].
We studied susceptibility to L. tropica using BALB/c-c-STS/A (CcS/Dem) recombinant congenic (RC) strains [43], which differ greatly in susceptibility to L. major [44], [45]. Parental strains BALB/c, STS and RC strains CcS-3, CcS-5, CcS-11, CcS-12, CcS-16, CcS-18, and CcS-20 were infected with L. tropica and skin lesions, cytokine and chemokine levels in serum, splenomegaly, hepatomegaly, and parasite numbers in organs were measured [46]. These experiments revealed that manifestations of the disease after infection with L. tropica are strongly influenced by genotype of the host. We have found that females of the RC strain CcS-16 that contains 12.5% genes of the resistant donor strain STS and 87.5% genes of the susceptible strain BALB/c [43], [47] developed the largest skin lesions and exhibited a unique systemic chemokine reaction, characterized by additional transient early peaks of CCL3 and CCL5, which were present neither in CcS-16 males nor in any other tested RC strain [46]. In order to establish the genetic basis of these differences, we prepared F2 hybrids between BALB/c and CcS-16, infected them with L. tropica and measured their skin lesions, splenomegaly, hepatomegaly, parasite numbers in spleen, liver and inguinal lymph nodes, and serum level of CCL3, CCL5 and CCL7 during the transient early peak. The strain CcS-16 carries STS-derived segments on nine chromosomes. They were genotyped in the F2 hybrid mice and their linkage with pathological symptoms and systemic immune responses was determined, which revealed eight controlling genes.
Materials and Methods
Mice
Females of strains BALB/c (16 infected, 16 uninfected) and CcS-16 (15 infected, 11 uninfected) were 8 to 19 weeks old (mean age 12 weeks, median age 12 weeks) at the time of infection. When used for these experiments, strain CcS-16 was in more than 90 generations of inbreeding. The parts of its genome inherited from the BALB/c or STS parents were defined [48]. 247 female F2 hybrids between CcS-16 and BALB/c (age 9 to 16 weeks at the time of infection, mean age 13 weeks, median 13 weeks) were produced at the Institute of Molecular Genetics AS CR, v.v.i.. Mice were kept in individually ventilated cages (Ehret, Emmendingen, Germany) and tested in two experimental groups. Both groups of F2 hybrids were derived from the same F1 parents; second experiment started seven weeks after the first. 2 mice died shortly after inoculation and were excluded from experiments. Among analyzed F2 hybrids, first experiment consisted of 111 mice, of which 51 mice originated from a cross (BALB/c×CcS-16)F2 (mean age 11.9 weeks, median 12 weeks; 3 mice died before the end of an experiment), 60 mice originated from a cross (CcS-16×BALB/c)F2 (mean age 12.6 weeks, median age 13 weeks; 1 mouse died before the end of an experiment). According to the nomenclature rules, the first strain listed in the cross symbol is the female parent, the second the male. The second experiment contained 134 mice, of which 64 mice originated from a cross (BALB/c×CcS-16)F2 (mean age 12.6 weeks, median 16 weeks; 2 mice died before the end of an experiment), 70 mice originated from a cross (CcS-16×BALB/c)F2 (mean age 13.4 weeks, median age 13 weeks; 6 mice died before the end of an experiment). The numbers of mice analyzed for individual phenotypes are given in Supplementary Table S1.
Ethics statement
All experimental protocols utilized in this study comply with the Czech Government Requirements under the Policy of Animal Protection Law (No. 246/1992) and with the regulations of the Ministry of Agriculture of the Czech Republic (No. 207/2004), which are in agreement with all relevant European Union guidelines for work with animals and were approved by the Institutional Animal Care Committee of the Institute of Molecular Genetics AS CR and by Departmental Expert Committee for the Approval of Projects of Experiments on Animals of the Academy of Sciences of the Czech Republic (permission Nr. 37/2007).
Parasite
Leishmania tropica from Urfa, Turkey (MHOM/1999/TR/SU23) was used for infecting mice. Amastigotes were transformed to promastigotes using SNB-9 [49], and 1×107 stationary phase promastigotes from subculture 2 were inoculated in 50 µl of sterile Phosphate Buffer Saline (PBS) s.c. into the tail base, with promastigote secretory gel (PSG) collected from the midgut of L. tropica-infected Phlebotomus sergenti females (laboratory colony originating from L. tropica focus in Urfa). PSG was collected as described [50]. The amount corresponding to one sand fly female was used per mouse.
Disease phenotype
The size of the skin lesions was measured every second week using the Profi LCD Electronic Digital Caliper Messschieber Schieblehre Messer (Shenzhen Xtension Technology Co., Ltd. Guangdong, China), which has accuracy 0.02 mm. Blood was collected every 2 weeks in volume from 60 to 180 µl, and serum was frozen at −30°C for further analysis. The mice were killed 43 weeks after inoculation. Blood, spleen, liver and inguinal lymph nodes were collected for later analysis.
Quantification of parasite load
Parasite load was measured in frozen lymph nodes, spleen, and liver samples using PCR-ELISA according to the previously published protocol [51]. Briefly, total DNA was isolated using a TRI reagent (Molecular Research Center, Cincinnati, USA) standard procedure (http://www.mrcgene.com/tri.htm). For PCR, two primers (digoxigenin-labeled F 5′-ATT TTA CAC CAA CCC CCA GTT-3′ and biotin-labeled R 5′-GTG GGG GAG GGG CGT TCT-3′ (VBC Genomics Biosciences Research, Austria) were used for amplification of the 120-bp conservative region of the kinetoplast minicircle of Leishmania parasite, and 50 ng of extracted DNA was used per each PCR reaction. For a positive control, 20 ng of L. tropica DNA per reaction was amplified as a highest concentration of standard. A 30-cycle PCR reaction was used for quantification of parasites in lymph nodes; 33 cycles for spleen, and 40 cycles for liver. Parasite load was determined by analysis of the PCR product by the modified ELISA protocol (Pharmingen, San Diego, USA). Concentration of Leishmania DNA was determined using the ELISA Reader Tecan and the curve fitter program KIM-E (Schoeller Pharma, Prague, Czech Republic) with least squares-based linear regression analysis.
Chemokines and cytokine levels
Levels of GM-CSF (granulocyte-macrophage colony-stimulating factor), CCL2 (chemokine ligand 2)/MCP-1 (monocyte chemotactic protein-1), CCL3/MIP-1α (macrophage inflammatory protein-1α), CCL4/MIP-1β (macrophage inflammatory protein-1β), CCL5/RANTES (regulated upon activation, normal T-cell expressed, and secreted) and CCL7/MCP-3 (monocyte chemotactic protein-3) in serum were determined using Mouse chemokine 6-plex kit (eBioscience, Vienna, Austria). The kit contains two sets of beads of different size internally dyed with different intensities of fluorescent dye. The set of small beads was used for GM-CSF, CCL5/RANTES and CCL4/MIP-1β and the set of large beads for CCL3/MIP-1α, CCL2/MCP-1 and CCL7/MCP-3. The beads are coated with antibodies specifically reacting with each of the analytes (chemokines) to be detected in the multiplex system. A biotin secondary antibody mixture binds to the analytes captured by the first antibody. Streptavidin-phycoerythrin binds to the biotin conjugate and emits a fluorescent signal. The test procedure was performed in the 96 well filter plates (Millipore, USA) according to the protocol of manufacturer. Beads were analyzed on flow cytometer LSR II (BD Biosciences, San Jose, USA). Lyophilized GM-CSF and chemokines (CCL2/MCP-1, CCL3/MIP1α, CCL4/MIP1β, CCL5/RANTES, CCL7/MCP-3) supplied in the kit were used as standards. Concentration was evaluated by Flow Cytomix Pro 2.4 software (eBioscience, Vienna, Austria). The limit of detection of each analyte was determined to be for GM-CSF 12.2 pg/ml, CCL2/MCP-1 42 pg/ml, CCL7/MCP-3 1.4 pg/ml, CCL3/MIP-1α 1.8 pg/ml, CCL4/MIP-1β 14.9 pg/ml, and for CCL5/RANTES 6.1 pg/ml.
Genotyping of F2 mice
DNA was isolated from tails using a proteinase procedure [52] with modifications described in [51]. The strain CcS-16 differs from BALB/c at STS-derived regions on nine chromosomes [48 and unpublished results]. These differential regions were typed in the F2 hybrid mice between CcS-16 and BALB/c using 23 microsatellite markers (Generi Biotech, Hradec Králové, Czech Republic): D2Mit156, D2Mit389, D2Nds3, D2Mit257, D2Mit283, D2Mit52, D3Mit25, D3Mit11, D4Mit153, D6Mit48, D6Mit320, D10Mit67, D10Mit103, D11Mit139, D11Mit242, D11Nds18, D11Mit37, D16Mit126, D17Mit38, D17Mit130, D18Mit35, D18Mit40 and D18Mit49 (Supplementary Table S2). The maximum distance between any two markers in the chromosomal segments derived from the strain STS or from the nearest BALB/c derived markers was 14.16 cM. The DNA genotyping by PCR was performed as described elsewhere [53]. The genotyping for microsatellite markers with fragment length difference of less than 10 bp was performed by using ORIGINS Elchrom Scientific electrophoresis (Elchrom Scientific AG, Cham, Switzerland) according to manufacturer's instruction. Briefly, DNA was amplified as described in [53]. Each PCR product was mixed with 5 µl of loading buffer and electrophoresed using Spreadex EL300 gel and Spreadex EL400 gel (Elchrom Scientific AG, Cham, Switzerland) for product with size of less than 150 bp or more than 150 bp, respectively. The best gel and proper running time was selected using ElQuantTM Software (Elchrom Scientific AG, Cham, Switzerland). 30 mM TAE buffer was used as a running buffer. Running temperature was set to 20°C and to 50°C, when voltage was set to 120 V and 100 V, respectively. After finishing the electrophoresis gel was stained by ethidium bromide and the results were read by GENE bio-imaging system (Syngene, Cambridge, UK).
Statistical analysis
The role of genetic factors in control of the tested pathological and immunological symptoms was examined with ANOVA using the program Statistica for Windows 8.0 (StatSoft, Inc., Tulsa, Oklahoma, USA). Marker, grandparent-of-origin effect and age were fixed factors and the experiment was considered as a random factor. In order to obtain normal distribution of the analyzed parameters, the obtained values were transformed, each as required by its distribution, as shown in the legends of the Tables. Markers and interactions with P<0.05 were combined in a single comparison.
To obtain whole genome significance values (corrected P-values) the observed P-values (αT) were adjusted according to Lander and Schork [54] using the formula:
where G = 1.75 Morgan (the length of the segregating part of the genome: 12.5% of 14 M); C = 9 (number of chromosomes segregating in cross between CcS-16 and BALB/c, respectively); ρ = 1.5 for F2 hybrids; h(T) = the observed statistic (F ratio).
The percent of total phenotypic variance accounted for by a QTL and its interaction terms was computed by subtracting the sums of squares of the model without this variable from the sum of squares of the full model and this difference divided by the total regression sums of squares:
Results
Genetic control of skin lesions development
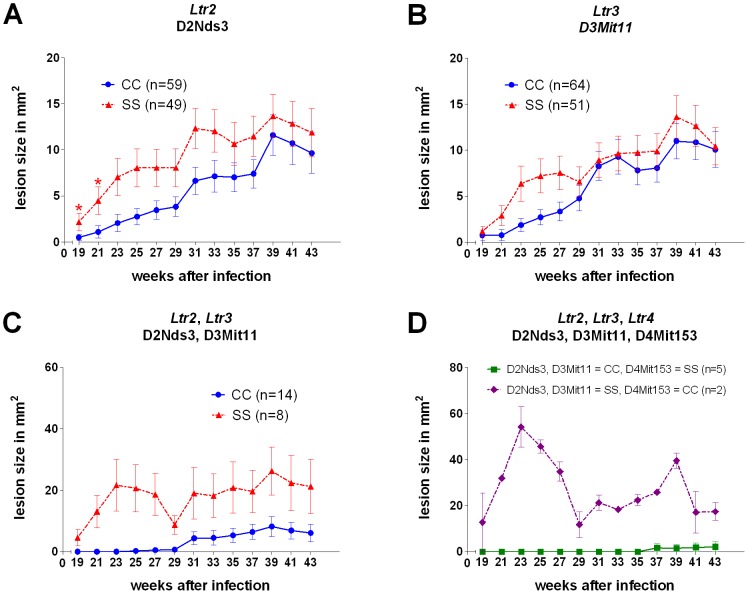
Differences in skin lesions development between strains BALB/c and CcS-16 are controlled by two loci, which are not dependent on or influenced by interaction with other genes (main effects) (Table 1, Figure 1). Ltr2 (Leishmania tropica response 2) linked to D2Nds3 (Figure 1A) and D2Mit389 influences lesion size at week 19 (corrected P = 0.004, Bonferroni corr. P = 0.049), 21 (corrected P = 0.0020, Bonferroni corr. P = 0.024) and 31 (corrected P = 0.0152, Bonferroni corr. P = 0.18) after infection, Ltr3 that controls lesion size at week 21 after infection is linked to D3Mit11 (corrected P = 0.042, Bonferroni corr. P = 0.5) (Figure 1B). STS allele of both Ltr2 and Ltr3 determines larger lesions. STS allele of Ltr4 marked by D4Mit153 (which also controls parasite numbers in liver and in lymph nodes) has an opposite effect on the studied trait; its STS allele is associated with smaller lesions at week 27 after infection. Figure 1C and Figure 1D show the strong additive effects of Ltr2 and Ltr3, and Ltr2, Ltr3 and Ltr4, respectively. However, Ltr3 and Ltr4 effects on skin lesions (nominal P value = 0.00048 and 0.00096, respectively, corr. P value = 0.024 and 0.045, respectively) were not significant after Bonferroni correction for number of tested weeks of infection and for whole genome significance. Although lesions were larger in the second experiment, no significant interaction between experimental group and markers was observed.
Table 1. Loci that control skin lesion development.
| Phenotype | Locus | Marker | Genotype | P value | corr. P value | Bonfer. corr. P value | % of explained variance | ||||||||
| CC | CS | SS | |||||||||||||
| lesion wk 19 | Ltr2 | D2Nds3 | 0.00 | 0.002±0.001 | (n = 60) | 0.35 | 0.004±0.001 | (n = 127) | 1.65 | 0.010±0.001 | (n = 53) | 6.6×10−5 | 0.004 | 0.049 | 20.90 |
| lesion wk 19 | Ltr4 | D4Mit153 | 1.31 | 0.008±0.001 | (n = 54) | 0.23 | 0.003±0.001 | (n = 130) | 0.38 | 0.004±0.001 | (n = 56) | 0.0018 | 0.077 | 0.93 | 9.66 |
| lesion wk 21 | Ltr2 | D2Nds3 | 0.20 | 0.003±0.002 | (n = 60) | 0.36 | 0.004±0.001 | (n = 127) | 2.27 | 0.012±0.002 | (n = 53) | 2.9×10−5 | 0.002 | 0.024 | 10.93 |
| lesion wk 21 | Ltr3 | D3Mit11 | 0.39 | 0.004±0.002 | (n = 64) | 0.42 | 0.004±0.001 | (n = 122) | 1.94 | 0.011±0.002 | (n = 54) | 0.00088 | 0.042 | 0.50 | 12.48 |
| lesion wk 23 | Ltr3 | D3Mit11 | 0.25 | 0.149±0.010 | (n = 64) | 0.28 | 0.152±0.008 | (n = 122) | 1.29 | 0.203±0.012 | (n = 54) | 0.00053 | 0.026 | 0.32 | 6.59 |
| lesion wk 25 | Ltr3 | D3Mit11 | 0.52 | 0.069±0.008 | (n = 64) | 0.48 | 0.067±0.007 | (n = 122) | 2.21 | 0.110±0.009 | (n = 54) | 0.00048 | 0.024 | 0.29 | 12.02 |
| lesion wk 27 | Ltr4 | D4Mit153 | 1.75 | 0.101±0.008 | (n = 54) | 0.59 | 0.071±0.006 | (n = 130) | 0.30 | 0.060±0.008 | (n = 55) | 0.00096 | 0.045 | 0.54 | 7.53 |
| lesion wk 31 | Ltr2 | D2Mit389 | 1.32 | 0.092±0.012 | (n = 55) | 1.57 | 0.097±0.007 | (n = 134) | 5.53 | 0.148±0.011 | (n = 48) | 0.00028 | 0.015 | 0.18 | 10.77 |
Lesions were measured every second week. In order to normalize distribution of the observed values, the natural logarithm of the lesion size (mm2) at each measured week (value+1.5) raised to the power of 0.04 was further raised to the power of 1.5 for weeks 19, 21, 23, 25; and to the power of 0.75 for week 31. The table shows means and SE calculated by analysis of variance. Non-transformed values of mean are given in bold. Number of tested mice is shown in brackets. Only P values significant after correction for genome-wide analysis and Bonferroni correction (multiplied by the number of tested weeks) are given. C and S indicate the presence of BALB/c and STS allele, respectively.
Figure 1. Differential lesion development in F2 hybrid mice carrying one, two and three Ltr loci after infection with L. tropica.
A. F2 hybrids carrying BALB/c or STS homozygous (resistant or susceptible) alleles in Ltr2 (D2Nds3); B. F2 hybrids carrying BALB/c or STS homozygous (resistant or susceptible) alleles in Ltr3 (D3Mit11); C. F2 hybrids carrying BALB/c or STS homozygous (both resistant or both susceptible) alleles in both Ltr2 (D2Nds3) and Ltr3 (D3Mit11); D. F2 hybrids carrying BALB/c homozygous (both resistant) alleles in Ltr2 and Ltr3 and STS (resistant) homozygous alleles in Ltr4 (D4Mit153), and F2 hybrids carrying STS homozygous (both susceptible) alleles in Ltr2 and Ltr3 and BALB/c (susceptible) homozygous alleles in Ltr4. n, number of mice. Graphs summarize data from two independent experimental groups and give non-normalized lesion sizes. Lesions were measured every second week. CC and SS indicate the homozygosity of BALB/c and STS allele, respectively. Please note different scales of Figures 1A,B, 1C and 1D.
Genetic control of parasite numbers in organs and visceral pathology
Parasite numbers in spleen and splenomegaly are controlled by different sets of genes
Parasite numbers in spleen are controlled by two loci (Table 2). STS allele of Ltr3 linked with D3Mit25 (corrected P = 0.0085) determines lower parasite load, whereas STS allele of Ltr6 (linked with D11Mit37) (corrected P = 0.014) is associated with higher parasite numbers. These P-values for Ltr3 and Ltr6 were significant only in cross (BALB/c×CcS-16)F2 (where mother of the F1 hybrids was BALB/c and father was CcS-16), but not in cross (CcS-16×BALB/c)F2 (where mother was CcS-16 and father was BALB/c). Interaction between the cross and marker D3Mit25 is highly significant (corr. P = 0.0013). Younger mice (from 9 to 12 weeks, mean = 11 weeks) have higher parasite load than the older (from 13 to 16 weeks, mean = 14 weeks) mice, but interaction between the marker and age was not significant (nominal P = 0.86).
Table 2. Main effect loci: control of parasite load in spleen and in liver, and visceral pathology.
| Phenotype | Locus | Marker | Genotype | P value | corr. P value | % of expl. variance | ||||||||
| CC | CS | SS | ||||||||||||
| Parasites in spleen | ||||||||||||||
| Both crosses | Ltr3 | D3Mit25 | 0.80 | 4.38±0.16 | (n = 61) | 0.63 | 4.15±0.13 | (n = 108) | 0.48 | 3.87±0.17 | (n = 62) | 0.094 | NS | NA |
| (BALB/c×CcS-16)F2 | Ltr3 | D3Mit25 | 1.72 | 5.15±0.22 | (n = 29) | 0.75 | 4.32±0.21 | (n = 43) | 0.43 | 3.76±0.22 | (n = 37) | 0.00014 | 0.0085 | 19.38 |
| (CcS-16×BALB/c)F2 | Ltr3 | D3Mit25 | 0.38 | 3.63±0.21 | (n = 32) | 0.49 | 3.89±0.15 | (n = 65) | 0.61 | 4.11±0.23 | (n = 25) | 0.304 | NS | NA |
| Parasites in spleen | ||||||||||||||
| Both crosses | Ltr6 | D11Mit37 | 0.57 | 4.04±0.16 | (n = 65) | 0.46 | 3.84±0.12 | (n = 105) | 0.96 | 4.56±0.16 | (n = 62) | 0.0028 | 0.113 | NA |
| (BALB/c×CcS-16)F2 | Ltr6 | D11Mit37 | 0.65 | 4.17±0.24 | (n = 31) | 0.45 | 3.81±0.21 | (n = 46) | 1.75 | 5.17±0.23 | (n = 32) | 0.00024 | 0.014 | 29.58 |
| Splenomegaly | Ltr8 | D18Mit49 | 5.28 | 1.70±0.06 | (n = 74) | 4.67 | 1.57±0.05 | (n = 106) | 3.60 | 1.30±0.07 | (n = 53) | 0.00022 | 0.012 | 18.59 |
| Parasites in liver | Ltr2 | D2Nds3 | 0.61 | 4.12±0.11 | (n = 60) | 0.83 | 4.42±0.08 | (n = 123) | 1.25 | 4.83±0.14 | (n = 49) | 0.00056 | 0.028 | 9.50 |
| Hepatomegaly | Ltr2 | D2Mit389 | 45.76 | 37.41±0.81 | (n = 55) | 42.28 | 34.66±0.52 | (n = 131) | 48.31 | 39.42±0.86 | (n = 46) | 4.3×10−6 | 0.00033 | 13.83 |
Parasite numbers (week 43) were estimated by PCR–ELISA. Means, SE and P values for splenomegaly (week 43), hepatomegaly (week 43) and concentration of parasite DNA (ng/µl) in isolates from lymph nodes, spleen and liver were calculated by analysis of variance. Normal distribution was obtained for splenomegaly (spleen-to-body weight ratio×1000) by raising values to the power of 0.00002. Hepatomegaly (liver-to-body weight ratio×1000) was normalized by raising values to the power of 0.0125. To obtain normal distribution for parasite load in organs, the following transformations were used: natural logarithm of (value×100). The numbers in bold give the average non-transformed values. Only P values significant after correction for genome-wide testing are given. Number of tested mice is shown in brackets. C and S indicate the presence of BALB/c and STS allele, respectively. NS – not significant, NA – not applicable.
Splenomegaly is controlled by five loci (Table 2, 3). Ltr8 linked with D18Mit49 (corr. P = 0.012) has a main effect, its BALB/c allele is associated with a larger spleen to body weight ratio. Ltr2, Ltr3, Ltr5 and Ltr7 affect splenomegaly in gene-gene interactions. Ltr2 linked to D2Mit257 influences splenomegaly in interaction with Ltr3 linked to D3Mit11 (corrected P = 0.010). F2 mice with homozygous STS (SS) alleles at both Ltr2 and Ltr3 have the smallest splenomegaly. Ltr5 linked to D10Mit103 influences splenomegaly in interaction with Ltr8 linked to D18Mit49 (corrected P = 0.029). F2 mice with homozygous STS (SS) alleles at both Ltr5 and Ltr8 have the smallest splenomegaly. Ltr5 also influences splenomegaly in interaction with Ltr7 linked to D17Mit30 (corrected P = 0.029). F2 mice with homozygous BALB/c (CC) alleles at Ltr5 and homozygous STS (SS) alleles at Ltr7 have the most severe splenomegaly, the other genotypes show no pronounced differences.
Table 3. Interaction between loci that control splenomegaly after 43 weeks of L. tropica infection.
| P = 0.00026 | Corrected P = 0.010 | % of explained variance = 9.05 | ||||||
| D2Mit257 (Ltr2) | ||||||||
| CC | CS | SS | ||||||
| D3Mit11 | CC | 3.98 | 1.41±0.09 | 4.58 | 1.55±0.06 | 5.24 | 1.69±0.08 | |
| ( Ltr3 ) | (n = 14) | (n = 33) | (n = 17) | |||||
| CS | 4.51 | 1.54±0.06 | 4.35 | 1.50±0.04 | 5.32 | 1.71±0.08 | ||
| (n = 36) | (n = 63) | (n = 19) | ||||||
| SS | 4.37 | 1.50±0.10 | 5.23 | 1.69±0.06 | 3.12 | 1.16±0.13 | ||
| (n = 13) | (n = 31) | (n = 7) | ||||||
Means, SE and P values for splenomegaly were calculated by analysis of variance. Normal distribution was obtained for splenomegaly (spleen-to-body weight ratio×1000) by raising values to the power of 0.00002. The numbers in bold give the average non-transformed values. Only P values significant after correction for genome-wide significance are given. Number of tested mice is shown in brackets. C and S indicate the presence of BALB/c and STS allele, respectively.
Parasite numbers in liver are controlled by Ltr2, Ltr4 and Ltr8, whereas hepatomegaly is influenced by Ltr2 only
Parasite numbers in liver are controlled by three genes (Table 2, 4). Ltr2 linked to D2Nds3 (corrected P = 0.028) has a main effect on parasite numbers in liver. Its STS allele is associated with a higher parasite load (Table 2). Ltr4 linked to D4Mit153 influences parasite load in liver in interaction with Ltr8 linked to D18Mit40 (corrected P = 0.021). F2 mice with homozygous BALB/c (CC) alleles at Ltr4 and heterozygous at Ltr8 have the highest parasite burden in liver.
Table 4. Interaction between loci controlling parasite burden in lymph nodes and liver 43 weeks after infection.
| Interaction between loci that control parasite burden in liver | ||||||||
| P = 0.00059 | Corrected P = 0.021 | % of explained variance = 8.11 | ||||||
| D18Mit40 (Ltr8) | ||||||||
| CC | CS | SS | ||||||
| D4Mit153 | CC | 0.85 | 4.44±0.2 | 1.19 | 4.78±0.2 | 0.51 | 3.93±0.21 | |
| ( Ltr4 ) | (n = 14) | (n = 25) | (n = 14) | |||||
| CS | 1.00 | 4.61±0.14 | 0.65 | 4.17±0.09 | 1.05 | 4.65±0.18 | ||
| (n = 33) | (n = 72) | (n = 19) | ||||||
| SS | 0.67 | 4.21±0.2 | 0.73 | 4.28±0.18 | 0.85 | 4.44±0.20 | ||
| (n = 13) | (n = 22) | (n = 20) | ||||||
Means, SE and P values for concentration of parasite DNA (ng/µl) in isolates from lymph nodes and liver were computed by analysis of variance. The following transformations were used to obtain normal distribution: natural logarithm of (value×100). Hepatomegaly (liver-to-body weight ratio×1000) was normalized by raising values to the power of 0.0125. The numbers in bold give the average non-transformed values. Only P values significant after correction for genome-wide significance are given. Number of tested mice is shown in brackets. C and S indicate the presence of BALB/c and STS allele, respectively.
Hepatomegaly is determined by locus Ltr2 linked to D2Mit389 (corrected P = 0.00033) (Table 2). Less severe hepatomegaly was observed in heterozygotes.
Genetic control of parasite load in inguinal lymph nodes
Parasite numbers in inguinal lymph nodes are influenced by interaction between Ltr1 linked to D2Mit156 and Ltr4 linked to D4Mit153 (corrected P = 0.032). Highest parasite load is observed in F2 mice with homozygous STS (SS) alleles at Ltr4 and homozygous BALB/c (CC) alleles at Ltr1 (Table 4). There was no interaction between experimental group and markers (nominal P = 0.89).
Genetic control of early peak of chemokines level in serum of infected mice
Genetic analysis of F2 hybrids has revealed identical genetic control of serum levels of CCL3 and CCL5 at week 7 after infection (Table 5, 6). Ltr3 linked to D3Mit11 determines levels of both CCL3 (corrected P = 0.0046) and CCL5 (corrected P = 0.010), its BALB/c allele is associated with higher chemokine levels (Table 5). Ltr3 has not only individual (main) effect on chemokines levels, but also influences levels of CCL3 (corrected P = 0.014) and CCL5 (corrected P = 0.0012) in interaction with Ltr7 linked to D17Mit130. The largest effect is seen by Ltr3 when Ltr7 is SS. In that genotypic situation the Ltr3 CC alleles cause more than 300×higher levels of CCL3 and 28×higher levels of CCL5 than the Ltr3 SS alleles (Table 6). It is likely that this very large size of this effect in Ltr7 SS mice makes the Ltr3 effects visible as a main effect, although smaller, in F2 hybrids irrespective of their Ltr7 genotype.
Table 5. Main effect of loci controlling serum chemokine level after 7 weeks of infection.
| Phenotype | Locus | Marker | Genotype | P value | corr. P value | % of explained variance | ||||||||
| CC | CS | SS | ||||||||||||
| CCL3 | Ltr3 | D3Mit11 | 711.42 | 3.72±0.18 | (n = 64) | 371.57 | 3.27±0.12 | (n = 118) | 94.68 | 2.49±0.21 | (n = 53) | 7.5×10−5 | 0.0046 | 4.56 |
| CCL5 | Ltr3 | D3Mit11 | 2724.44 | 5.15±0.08 | (n = 64) | 1805.94 | 4.98±0.05 | (n = 117) | 861.34 | 4.66±0.09 | (n = 53) | 0.00018 | 0.010 | 3.99 |
| CCL7 | Ltr2 | D2M52 | 566.41 | 6.34±0.05 | (n = 48) | 590.60 | 6.38±0.03 | (n = 127) | 740.99 | 6.61±0.05 | (n = 60) | 3×10−5 | 0.002 | 9.06 |
| CCL7 | Ltr8 | D18M40 | 766.86 | 6.64±0.05 | (n = 60) | 602.67 | 6.40±0.04 | (n = 118) | 613.11 | 6.42±0.06 | (n = 55) | 0.00024 | 0.013 | 11.38 |
In order to normalize distribution of the observed values (in pg/ml), the following transformations were used: the power of 0.2 (concentration value+1) – CCL3/MIP1α; natural logarithm – CCL7/MCP-3; the power of −0.117545 followed by subtraction with 1 – CCL5/RANTES. In case of CCL5/RANTES, the calculated value was further divided by −0.117545. The Table gives mean of non-transformed (in bold) and transformed concentration and SE of the transformed values calculated by analysis of variance. Only P values significant after correction for genome-wide significance are given. Number of tested mice is shown in brackets. C and S indicate the presence of BALB/c and STS allele, respectively.
Table 6. Interaction between loci that control chemokines level after 7 weeks of L. tropica infection.
| A. CCL3/MIP-1α | P = 0.00036 | Corrected P = 0.014 | % of variance = 3.33 | |||||
| D3Mit11 (Ltr3) | ||||||||
| CC | CS | SS | ||||||
| D17Mit130 ( Ltr7 ) | CC | 298.60 | 3.13±0.36 | 377.81 | 3.28±0.22 | 172.79 | 2.81±0.30 | |
| (n = 12) | (n = 32) | (n = 17) | ||||||
| CS | 358.96 | 3.25±0.20 | 248.17 | 3.02±0.17 | 288.44 | 3.11±0.23 | ||
| (n = 40) | (n = 58) | (n = 30) | ||||||
| SS | 2511.51 | 4.79±0.36 | 531.34 | 3.51±0.24 | 8.15 | 1.56±0.51 | ||
| (n = 12) | (n = 28) | (n = 6) | ||||||
In order to normalize distribution of the observed values, the concentration in pg/ml was raised to the power of 0.2 (concentration value+1) – CCL3/MIP1α; to the power of −0.117545 followed by subtraction with 1 – CCL5/RANTES. In case of CCL5/RANTES, the calculated value was further divided by −0.117545. The Table gives mean of non-transformed (in bold) and transformed concentration and SE of the transformed values calculated by analysis of variance. Only P values significant after correction for genome-wide significance are given. Number of tested mice is shown in brackets. C and S indicate the presence of BALB/c and STS allele, respectively.
CCL7 level is controlled with two loci with an opposite effect on the studied trait. The homozygosity for the STS allele of Ltr2 (SS) determines higher CCL7 level (corrected P = 0.002), whereas homozygosity for the BALB/c allele of Ltr8 (CC) is associated with higher level of this chemokine (corrected P = 0.013) (Table 5). No significant interaction between experimental group and marker was observed. Older mice had higher levels of CCL7 in serum than the younger ones, but we did not observe any interactions between marker and age (nominal P (Ltr2) = 0.80, nominal P (Ltr8) = 0.64). Levels of CCL7 in serum of infected mice are also influenced by interaction of Ltr2 linked to D2Mit257 and Ltr6 linked to D11Mit37 (corrected P = 0.016), the highest CCL7 levels are observed in STS allele (SS) homozygotes in Ltr6 in combination with heterozygotes (CS) or STS allele (SS) homozygotes in Ltr2 (Table 6).
Although chemokine levels were higher in the first experiment, no significant interaction between experimental group and markers was observed.
No linkage was found for GM-CSF, CCL2/MCP-1 and CCL4/MIP-1β.
Discussion
The present study provides the first insight into the genetic architecture of susceptibility to L. tropica. We have described eight loci on seven chromosomes (Figure 2 [10], [12], [55]–[83]) and shown that the presence of individual symptoms of disease is controlled by different subsets of host's genes. The identification of host's genes responsible for the specific symptoms of the disease induced by different Leishmania species will contribute to the understanding of mechanisms of pathogenesis of leishmaniasis, similarly as comparative parasite genomics led to identification of differentially distributed genes in Leishmania species inducing different pathology [84], [85], and analysis of specific virulence factors revealed how different Leishmania species subvert or circumvent host's defenses [7]. Such analysis will provide description of individual predisposition to specific symptoms of disease and its probable course. Moreover, the possibility to compare genetics of response to several Leishmania species will further help to understand the genetic basis of general and species-specific responses of the host. This will synergize with the future information about genome sequence of L. tropica and about interaction of its specific virulence factors with the immune system.
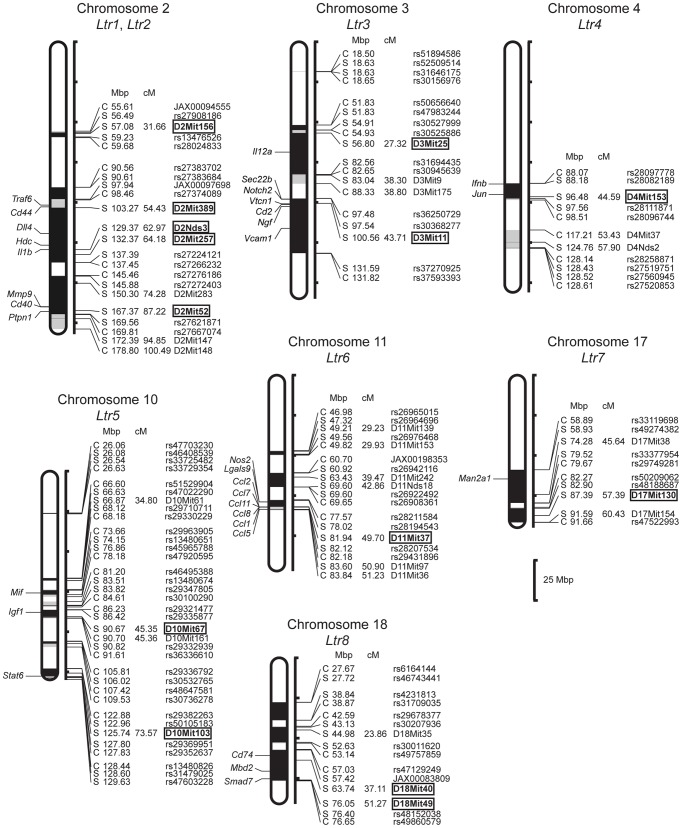
Figure 2. Position of the loci that control response to L. tropica in strain CcS-16.
The regions of STS and BALB/c origin are represented as dark and white, respectively; the boundary regions of undetermined origin are shaded. Only the markers and SNPs defining the boundaries the STS-derived segment and the markers that were tested for linkage are shown. The markers that exhibit significant P values (corrected for genome-wide search) are shown in bold. Abbreviations show genes that have been reported to be involved in response to Leishmania ssp.: Ccl1 (chemokine (C-C motif) ligand 1) [55], Ccl11 (chemokine (C-C motif) ligand 11) [56], Ccl2 (chemokine (C-C motif) ligand 2), Ccl5 (chemokine (C-C motif) ligand 5) [57], Ccl7 [58], Cd2 (CD2 antigen) [59], Cd40 (CD40 antigen) [60], Cd44 (CD44 antigen) [61], Cd74 (CD74 antigen) [62], Dll4 (Delta-like 4) [63], Hdc (histidine decarboxylase) [64], Ifnb1 (interferon beta 1) [65], Igf1 (insulin-like growth factor 1) [66], Il1 (interleukin 1) [67], Il12a (Interleukin 12a) [68], Jun (Jun oncogene) [69], Lgals9 (lectin, galactose binding, soluble 9) [70], Man2a1 (mannosidase 2, alpha 1) [71], Mbd2 (methyl-CpG binding domain protein 2) [72], Mif (macrophage inhibitory factor) [73], Mmp9 (matrix metalopeptidase 9) [74], Ngf (nerve growth factor) [75], Nos2 (nitric oxide synthase 2, inducible) [76], Notch2 (notch 2) [77], Ptpn1 (protein tyrosine phosphatase, non-receptor type 1) [78], Sec22b (SEC22 vesicle trafficking protein homolog B (S. cerevisiae)) [79], Smad7 (SMAD family member 7) [80], Stat6 (Signal transducer and activator of transcription-6) [81], Traf6 (TNF receptor associated factor 6) [60], Vcam1 (vascular cell adhesion molecule 1) [82], Vtcn1 (V-set domain containing T cell activation inhibitor 1) [83]. (Genes IDs are shown in Supplementary Table S3).
Response to L. tropica is controlled by multiple genes with heterogeneous effects
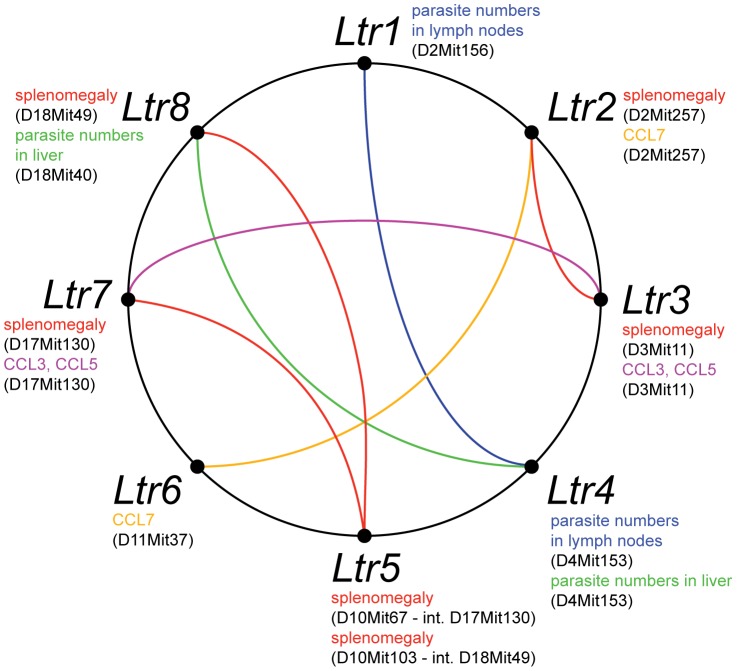
Our data show that interaction of mice with L. tropica parasites is complex and involves numerous genes and responses (Table 7). We have detected eight loci that in the strain CcS-16 control host-parasite interaction (Table 7, Figure 2). All eight Ltr loci are involved in gene-gene interactions (Figure 3), four loci (Ltr2, Ltr3, Ltr6, Ltr8) have also individual effect, while effects of Ltr1, Ltr4, Ltr5 and Ltr7 are seen only in interaction with other Ltr loci. This is not surprising, as the average proportion of genetic variation explained by epistatic QTLs in mice in different systems was estimated to be 49% [86] and gene-gene interactions were observed also in response to other pathogens such as L. major [87]–[89], Trypanosoma brucei brucei [53], Salmonella enteritidis [90], Plasmodium falciparum [91] and Mycobacterium leprae [92].
Table 7. Summary of loci that control response to L. tropica.
| chr. | locus | marker | Phenotype controlled |
| 2 | Ltr1 | D2Mit156 | parasites in lymph nodes (int. Ltr4 - D4Mit153) |
| 2 | Ltr2 | D2Mit389; D2Nds3/Il1b; D2Mit257; D2Mit52 | skin lesions wk 19; skin lesions wk 21; splenomegaly (int. Ltr3 - D3Mit11); parasites in liver; hepatomegaly; CCL7; CCL7 (int. Ltr6 - D11Mit37) |
| 3 | Ltr3 | D3Mit25; D3Mit11 | splenomegaly (int. Ltr2 - D2Mit257); parasites in spleen (transgenerational parental effect); CCL3; CCL3 (int. Ltr7 - D17Mit130); CCL5; CCL5 (int. Ltr7 - D17Mit130) |
| 4 | Ltr4 | D4Mit153 | parasites in lymph nodes (int. Ltr1 - D2Mit156); parasites in liver (int. Ltr8 - D18Mit40) |
| 10 | Ltr5 | D10Mit67; D10Mit103 | splenomegaly (int. Ltr7 - D17Mit130); splenomegaly (int. Ltr8 - D18Mit49) |
| 11 | Ltr6 | D11Mit37 | parasites in spleen (transgenerational parental effect); CCL7 (int. Ltr2 - D2Mit257) |
| 17 | Ltr7 | D17Mit130 | splenomegaly (int. Ltr5 - D10Mit67); CCL3 (int. Ltr3 - D3Mit11); CCL5 (int. Ltr3 - D3Mit11) |
| 18 | Ltr8 | D18Mit40; D18Mit49 | splenomegaly; splenomegaly (int. Ltr5 - D10Mit103); parasites in liver (int. Ltr4 - D4Mit153); CCL7 |
Figure 3. Interactions among loci that control response to L. tropica.
Phenotypes controlled by each locus are shown at its symbol in different colors. The colored lines connecting the loci indicate interactions controlling the specific phenotypes.
The loci described here have heterogeneous effects (Table 7). Ltr1 on chromosome 2 controls in interaction with Ltr4 only parasite numbers in lymph nodes, whereas the more distal Ltr2 on the same chromosome influences development of skin lesions, splenomegaly (in interaction with Ltr3), hepatomegaly, parasite load in liver and level of CCL7 in serum. Multiple functions are also exerted by Ltr3 on chromosome 3, which controls splenomegaly (in interaction with Ltr2), parasite numbers in spleen, and levels of CCL3 and CCL5 in serum. We have analyzed genetic control of early levels of chemokines, as there is a unique early peak in the CcS-16 females [46]. However, comparison of genetic control of CCL3 and CCL5 levels with genetic control of development of skin lesions indicates that there is no simple correlation between the chemokines levels and manifestations of disease. Ltr4 on chromosome 4 controls in interaction with Ltr1 and Ltr8 parasite numbers in lymph nodes and in liver, respectively. Ltr5 on chromosome 10 influences in interaction with Ltr7 or Ltr8 splenomegaly. Ltr6 influences parasite numbers in spleens and level of CCL7 in serum (in interaction with Ltr2). Ltr7 controls splenomegaly (in interaction with Ltr5) and in interaction with Ltr3 level of both CCL3 and CCL5 in serum. Ltr8 controls splenomegaly (as a main effect gene and in interaction with Ltr5), parasite numbers in liver (in interaction with Ltr4) and level of CCL7 in serum. Ltr1 and Ltr5 control only one parameter, whereas other loci have multiple effects. Some multiple effects could reflect causal relationship – e.g. CCL7 influences recruitment of monocytes to spleen [93], which could contribute to splenomegaly. The observed multiple effects of some Ltr loci might also suggest that some such loci might represent complexes of two or more closely linked Ltr genes. This issue will be resolved by future recombinational analysis.
We have detected also loci that control symptoms, such as splenomegaly, in which the strains BALB/c and CcS-16 do not differ [46]. This is because in an inbred strain the final outcome of response is exerted by multiple genes, which often have opposite effects, masking each other. In the F2 hybrids these genes segregate and can be therefore detected.
Reliability and validity of the described loci is supported by the fact that they have been detected by analysis of different phenotypes and their statistical significance was corrected for whole genome testing and where appropriate also by conservative Bonferroni correction. The relatively high proportion of variance explained by the mapped loci (Table 1–6) might be partly due to a limited variability of the tested manifestations of the disease.
Susceptibility alleles carried by a resistant strain
Most inbred mouse strains that were produced without intentionally selectively bred for a specific quantitative phenotype (like susceptibility to specific infections) inherited from their non-inbred ancestors randomly susceptible alleles at some loci and resistant alleles at others, so that their overall susceptibility phenotype depends on the relative number of both. STS is resistant to L. tropica and does not develop skin lesions [24], however some STS-derived segments carried by CcS-16 on chromosome 2 (Ltr2) and possibly also on chromosome 3 (Ltr3) are associated with larger lesions. Similarly, STS-derived alleles of Ltr2 and Ltr6 are associated with higher parasite load in liver and spleen, respectively. This finding is not unique as susceptibility alleles originating from resistant strains were found in studies of colon cancer [94] and L. major [95] susceptibility; a low-responder allele was identified in a strain exhibiting high response to IL-2 [96] or producing a high level of IFNγ [97], whereas a high responder allele was found in a strain producing low level of IL-4 [98].
Transgenerational parental effect
Loci Ltr3 and Ltr6 influencing parasite numbers in spleen (Table 2) were significant only in the cross (BALB/c×CcS-16)F2, but not in the cross (CcS-16×BALB/c)F2, hence the outcome in these crosses that are theoretically genetically identical depends on the strain of the female or male used originally to produce the F1 hybrids, which were then crossed with each other to produce the F2 hybrids for the tests. Thus, this is a special type of a transgenerational parental effect as the mothers and fathers of the F2 hybrids were genetically identical. Recently, examples of transgenerational parental effects have been described in several species [reviewed in [99]] and several possible mechanisms have been proposed. Our observation may reflect a parental effect due to modification of the developing immune system of fetuses or youngs by maternal environment, maternal nutritional effects, or epigenetic effects, and it offers a possibility to characterize the transgenerationally regulated functional pathways.
Control of parasite load is predominantly organ specific
Control of parasite elimination differs among organs: the loci Ltr1 and Ltr4 interact to control parasite numbers in inguinal lymph nodes, while Ltr4 in interaction with Ltr8 influences parasite load in liver (Table 4). Parasite load in liver is also controlled by Ltr2 (Table 2), whereas parasite burden in spleen is influenced by Ltr3 and Ltr6 (Table 2). These data show that parasite elimination in lymph nodes, liver and spleen are controlled differently, suggesting a predominantly organ specific control of parasite load. Mechanistic studies analyzing response to L. tropica in different organs are not yet available, but generally organ specific responses described here are compatible with the mechanistic studies of other parasites. The enzymes inducible nitric oxide synthase and phagocyte NADPH oxidase, which are required for the control of L. major, display organ- and stage-specific anti-Leishmania effects [76], [100]. Inducible nitric oxide synthase has been shown to control resistance to parasites in skin and draining lymph nodes, but not in spleen of the resistant strain C57BL/6 [100]. On the other hand, activity of phagocyte NADPH oxidase is essential for the clearance of L. major in the spleen, but it is dispensable for the resolution of the acute skin lesions and it exerted only a limited effect on the containment of the parasites in the draining lymph node [76]. Similarly, bg/Lyst (lysosomal trafficking regulator) is involved in control of parasite numbers of L. donovani in spleen, but not in liver [31]. On the other hand VCAM-1 (vascular cell adhesion molecule-1) and VLA-4 (very late antigen-4) interactions influenced early L. donovani burden in liver, but not in spleen [82].
Different control of parasite elimination and organ pathology
Comparison of genetic control of parasite numbers in spleen and splenomegaly, or parasite numbers in liver and hepatomegaly shows that control of parasites elimination and organ pathology overlap only partially. For example Ltr3 controls both parasite numbers in spleen and splenomegaly, but Ltr6 is involved in control of parasite numbers in spleen, but not in splenomegaly, whereas Ltr2, Ltr8, Ltr5, and Ltr7 are involved only in control of splenomegaly (Table 2, 3, 7). Similarly, Ltr2 influences both parasite load in liver and hepatomegaly, but parasite load in liver is controlled also by interaction of Ltr4 with Ltr8. The differences in genetic control of parasite numbers and organ pathology induced by the parasites are probably due to the fact that during a chronic disease the organ damage is a combined result of speed of elimination of parasite on one hand and changes caused by reaction to parasite (such as influx of immune cells, inflammatory responses) and healing processes on the other hand. It is therefore likely that these processes are regulated by different sets of genes.
It is important to understand that as in any QTL study, failure to find a linkage between a phenotype and a marker does not rule out that such linkage may exist, although its phenotypic effect are likely smaller than in the detected linkages. So for a QTL, which affects several but not all parameters of a complex disease, this indicates that it has predominant effects on some parameters, although it might modify to a lesser extent other parameters as well.
Comparison of genetic control of response to several pathogens
Comparison of loci that control response to L. tropica and L. major – indication of common and species-specific genes
Comparison of genetic control of response to L. tropica and L. major might indicate some common and some distinct mechanisms in response to these two parasites. We compared genetic relationship between the Ltr (this study) and Lmr [88], [95], [101] loci detected in the strain CcS-16. Loci Ltr1 (chromosome 2), Ltr4 (chromosome 4) and Ltr7 (chromosome 17) appear to be species-specific and do not overlap with loci controlling response to L. major. Ltr2 (chromosome 2) co-localizes with Lmr14, Ltr5 (chromosome 10) with Lmr5, Ltr6 (chromosome 11) with Lmr15, and Ltr8 (chromosome 18) with Lmr13. Ltr2 controls visceral pathology in both species and is also involved in additional responses, which are unique for each parasite. Moreover, Ltr2 and Lmr14 overlap with Ir2, which controls visceral pathology after infection with L. donovani [4]. The other co-localizing loci also influence different sets of symptoms and are often involved in different interactions. This might indicate either the presence of the same controlling genes, which function differently under exposure to L. tropica and L. major, or less likely, a chance coincidence – presence of different controlling genes on the same chromosomal segment.
Ltr3 on chromosome 3 co-localizes with Lmr11, which was detected in the strain CcS-20, but not in the CcS-16, and which exhibits a single gene effect on IL-6 level in serum [88] and in interaction with Lmr8 on chromosome 1 influences serum IgE level in L. major-infected mice [101].
Some loci affect susceptibility to several pathogens
Some loci affect responses to a very broad spectrum of pathogens. For example, locus Ltr2 co-localizes also with Bb15, which controls specific and total IgG in serum after infection with Borrelia burgdorferi [102]. The most obvious potential candidate gene in this chromosomal segment is Il1 (interleukin 1). IL-1β was found to be up-regulated in dermal lesions of patients with cutaneous leishmaniasis caused by L. tropica and decreased after therapy [103], IL-1 was also found to regulate visceral manifestation of murine leishmaniasis after infection with L. major [67], and polymorphism in IL1B was linked with disease severity in patients infected with L. mexicana [10]. IL-1 was also described to influence IgG level in autoimmunity [104], which might suggest its involvement in response to B. burgdorferi.
Potential candidate genes
Usually, a standard inbred-strain mapping experiment using F2 hybrids will map a QTL into a 20- to 40-cM interval [105]. In the RC strains 54% of their donor strain genome reside in segments of medium length (5–25 cM) [106]. However, RC strains can carry on some chromosomes very short segments of the donor strain origin. This feature of the RCS system allowed us previously to narrow the location of Lmr9 (Leishmania major response 9) on chromosome 4 to a segment of 1.9 cM (6.79 Mb) without any additional crosses [101]. The short length of this segment, which controls levels of serum IgE in L. major infected mice, enabled us to detect a human homolog of this locus on human chromosome 8q12 and show that it controls susceptibility to atopy [107]. In another study, we were able to precisely map Tbbr2 (Trypanosoma brucei brucei response 2) to 2.15 Mb [53].
In the present F2 mapping experiment the shortest locus Ltr1 is 4.07 Mb long (Figure 2). Although most Ltr loci contain several possible candidate genes, here we list (Figure 2)[10], [12], [55]–[83] only those that have been shown previously to influence infection with Leishmania ssp.. However, the effects of many of Ltr loci might be caused by genes that are at the present not considered as candidates. Currently we are producing mice with recombinant haplotypes that carry individual Ltr loci in a very short segment on chromosome. The testing of these strains will restrict the present number of the candidate genes to the most likely ones.
Conclusion
We present the first description of genetic architecture of response to L. tropica in any species. We observed organ specific control of infection and distinct control of parasite load and organ pathology, the typical characteristics of immune response to many pathogens observed in all infections where multiple disease parameters were studied (L. major [4], L. donovani [4], Borrelia burgdorferi [102], Toxoplasma gondii [108], Trypanosoma congolense [109], and Chlamydia psittaci [110]). In addition, the genetic control of response to L. tropica exhibits heterogeneity of gene effects, gene-gene interactions, and trans-generational parental effects. These complexities of genetic control have been invoked [111] to explain the very large fraction of heritability that has not been detectable in genome-wide association studies (GWAS) [112], a power deficiency that likely cannot be ameliorated by further increases of the number of tested SNPs or by whole genome sequencing. Identification of these complexities in the present study will open way to elucidation of their functional basis and detection of homologous processes in humans.
Supporting Information
Numbers of mice analyzed in individual phenotypes.
(XLS)
Chromosomal positions of typed markers.
(XLS)
ID numbers of potential candidate genes localized in Ltr loci.
(XLS)
Acknowledgments
We thank Dr. Alan Hutson from the Department of Biostatistics of Roswell Park Cancer Institute for advice on analysis of transgenerational parental effects.
Funding Statement
This work was supported by grants GACR 310/08/1697 (http://www.gacr.cz/en/), MEYS, LH12049 LH-KONTAKT (http://www.msmt.cz/index.php?lang=2), and RVO68378050 (http://www.cas.cz/index.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3365071&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ready PD (2010) Leishmaniasis emergence in Europe. Euro Surveill 15: 19505 Available: http://www.ncbi.nlm.nih.gov/pubmed/20403308. [PubMed] [Google Scholar]
- 3. Kobets T, Grekov I, Lipoldová M (2012) Leishmaniasis: prevention, parasite detection and treatment. Curr Med Chem 19: 1443–1474 Available: http://www.ncbi.nlm.nih.gov/pubmed/22360481. [DOI] [PubMed] [Google Scholar]
- 4. Lipoldová M, Demant P (2006) Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet 7: 294–305 Available: http://www.ncbi.nlm.nih.gov/pubmed/16543933. [DOI] [PubMed] [Google Scholar]
- 5. Terrazas CA, Terrazas LI, Gómez-García L (2010) Modulation of dendritic cell responses by parasites: a common strategy to survive. J Biomed Biotechnol 2010: 357106 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2829630&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogdan C (2008) Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol 10: 1221–1234 Available: http://www.ncbi.nlm.nih.gov/pubmed/18363880. [DOI] [PubMed] [Google Scholar]
- 7. McMahon-Pratt D, Alexander J (2004) Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev 201: 206–224 Available: http://www.ncbi.nlm.nih.gov/pubmed/15361243. [DOI] [PubMed] [Google Scholar]
- 8. Herwaldt BL (1999) Leishmaniasis. Lancet 354: 1191–1199 Available: http://www.ncbi.nlm.nih.gov/pubmed/10513726. [DOI] [PubMed] [Google Scholar]
- 9. Frade AF, Oliveira LC de, Costa DL, Costa CHN, Aquino D, et al. (2011) TGFB1 and IL8 gene polymorphisms and susceptibility to visceral leishmaniasis. Infection, genetics and evolution: Infect Genet Evol 11: 912–916 Available: http://www.ncbi.nlm.nih.gov/pubmed/21376140. [DOI] [PubMed] [Google Scholar]
- 10. Fernández-Figueroa EA, Rangel-Escareño C, Espinosa-Mateos V, Carrillo-Sánchez K, Salaiza-Suazo N, et al. (2012) Disease severity in patients infected with Leishmania mexicana relates to IL-1β. PLoS Negl Trop Dis 6: e1533 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3358333&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castellucci L, Menezes E, Oliveira J, Magalhaes A, Guimaraes LH, et al. (2006) IL6 −174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. J Infect Dis 194: 519–527 Available: http://www.ncbi.nlm.nih.gov/pubmed/16845637. [DOI] [PubMed] [Google Scholar]
- 12. Ramasawmy R, Menezes E, Magalhães A, Oliveira J, Castellucci L, et al. (2010) The −2518 bp promoter polymorphism at CCL2/MCP1 influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. Infect Genet Evol 10: 607–613 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2878927&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castellucci L, Jamieson SE, Miller EN, Menezes E, Oliveira J, et al. (2010) CXCR1 and SLC11A1 polymorphisms affect susceptibility to cutaneous leishmaniasis in Brazil: a case-control and family-based study. BMC Med Genet 11: 10 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2823618&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehrotra S, Fakiola M, Oommen J, Jamieson SE, Mishra A, et al. (2011) Genetic and functional evaluation of the role of CXCR1 and CXCR2 in susceptibility to visceral leishmaniasis in north-east India. BMC Med Genet 12: 162 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3260103&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assaf A, Hoang TV, Faik I, Aebischer T, Kremsner PG, et al. (2012) Genetic evidence of functional ficolin-2 haplotype as susceptibility factor in cutaneous leishmaniasis. PloS One 7: e34113 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3311577&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alonso DP, Ferreira AFB, Ribolla PEM, de Miranda Santos IKF, do Socorro Pires e Cruz M, et al. (2007) Genotypes of the mannan-binding lectin gene and susceptibility to visceral leishmaniasis and clinical complications. J Infect Dis 195: 1212–1217 Available: http://www.ncbi.nlm.nih.gov/pubmed/17357060. [DOI] [PubMed] [Google Scholar]
- 17. Bucheton B, Abel L, El-Safi S, Kheir MM, Pavek S, et al. (2003) A major susceptibility locus on chromosome 22q12 plays a critical role in the control of kala-azar. Am J Hum Genet 73: 1052–1060 Available: http://www.gpubmedcentral.nih.gov/articlerender.fcgi?artid=1180485&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bucheton B, Argiro L, Chevillard C, Marquet S, Kheir MM, et al. (2007) Identification of a novel G245R polymorphism in the IL-2 receptor beta membrane proximal domain associated with human visceral leishmaniasis. Genes Immun 8: 79–83 Available: http://www.ncbi.nlm.nih.gov/pubmed/17108990. [DOI] [PubMed] [Google Scholar]
- 19. Fakiola M, Miller EN, Fadl M, Mohamed HS, Jamieson SE, et al. (2011) Genetic and functional evidence implicating DLL1 as the gene that influences susceptibility to visceral leishmaniasis at chromosome 6q27. J Infect Dis 204: 467–477 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3132144&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, et al. (2003) Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity 19: 549–559 Available: http://www.ncbi.nlm.nih.gov/pubmed/14563319. [DOI] [PubMed] [Google Scholar]
- 21. Fakiola M, Strange A, Cordell HJ, Miller EN, Pirinen M, et al. (2013) Common variants in the HLA-DRB1-HLA-DQA1 HLA class II region are associated with susceptibility to visceral leishmaniasis. Nat Genet 45: 208–213 Available: http://www.ncbi.nlm.nih.gov/pubmed/23291585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vidal SM, Malo D, Vogan K, Skamene E, Gros P (1993) Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg . Cell 73: 469–485 Available: http://www.ncbi.nlm.nih.gov/pubmed/8490962. [DOI] [PubMed] [Google Scholar]
- 23. Sakthianandeswaren A, Curtis JM, Elso C, Kumar B, Baldwin TM, et al. (2010) Fine mapping of Leishmania major susceptibility Locus lmr2 and evidence of a role for Fli1 in disease and wound healing. Infect Immun 78: 2734–2744 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2876540&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bucheton B, Abel L, Kheir MM, Mirgani A, El-Safi SH, et al. (2003) Genetic control of visceral leishmaniasis in a Sudanese population: candidate gene testing indicates a linkage to the NRAMP1 region. Genes Immun 4: 104–109 Available: http://www.ncbi.nlm.nih.gov/pubmed/12618857. [DOI] [PubMed] [Google Scholar]
- 25. Castellucci L, Jamieson SE, Miller EN, de Almeida LF, Oliveira J, et al. (2011) FLI1 polymorphism affects susceptibility to cutaneous leishmaniasis in Brazil. Genes Immun 12: 589–594 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3297968&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fortier A, Min-Oo G, Forbes J, Lam-Yuk-Tseung S, Gros P (2005) Single gene effects in mouse models of host: pathogen interactions. J Leukoc Biol 77: 868–877 Available: http://www.ncbi.nlm.nih.gov/pubmed/15653750. [DOI] [PubMed] [Google Scholar]
- 27. Stober CB, Brode S, White JK, Popoff J-F, Blackwell JM (2007) Slc11a1, formerly Nramp1, is expressed in dendritic cells and influences major histocompatibility complex class II expression and antigen-presenting cell function. Infect Immun 75: 5059–5067 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2044529&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malo D, Vogan K, Vidal S, Hu J, Cellier M, et al. (1994) Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics 23: 51–61 Available: http://www.ncbi.nlm.nih.gov/pubmed/7829102. [DOI] [PubMed] [Google Scholar]
- 29. Perou CM, Moore KJ, Nagle DL, Misumi DJ, Woolf EA, et al. (1996) Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet 13: 303–308 Available: http://www.ncbi.nlm.nih.gov/pubmed/8673129. [DOI] [PubMed] [Google Scholar]
- 30. Sans-Fons G, Yeramian A, Pereira-Lopes S, Santamaría-Babi L, Modolell M, et al. (2013) Arginine transport is impaired in C57BL/6 mouse macrophages as a result of a deletion in the promoter of slc7a2 (CAT2) and Leishmania infection is reduced. J Infect Dis 207(11): 1684–93 Available: http://www.ncbi.nlm.nih.gov/pubmed/23460752. [DOI] [PubMed] [Google Scholar]
- 31. Kirkpatrick CE, Farrell JP (1982) Leishmaniasis in beige mice. Infect Immun 38: 1208–1216 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=347877&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacobson RL (2003) Leishmania tropica (Kinetoplastida: Trypanosomatidae)–a perplexing parasite. Folia Parasitol 50: 241–250 Available: http://www.ncbi.nlm.nih.gov/pubmed/14971592. [DOI] [PubMed] [Google Scholar]
- 33. Magill AJ, Grögl M, Gasser RA, Sun W, Oster CN (1993) Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N Engl J Med 328: 1383–1387 Available: http://www.ncbi.nlm.nih.gov/pubmed/8292114. [DOI] [PubMed] [Google Scholar]
- 34. Mebrahtu Y, Lawyer P, Githure J, Were JB, Muigai R, et al. (1989) Visceral leishmaniasis unresponsive to pentostam caused by Leishmania tropica in Kenya. Am J Trop Med Hyg 41: 289–294 Available: http://www.ncbi.nlm.nih.gov/pubmed/2552850. [DOI] [PubMed] [Google Scholar]
- 35. Sacks DL, Kenney RT, Kreutzer RD, Jaffe CL, Gupta AK, et al. (1995) Indian kala-azar caused by Leishmania tropica . Lancet 345: 959–961 Available: http://www.ncbi.nlm.nih.gov/pubmed/7715298. [DOI] [PubMed] [Google Scholar]
- 36. Khanra S, Datta S, Mondal D, Saha P, Bandopadhyay SK, et al. (2012) RFLPs of ITS, ITS1 and hsp70 amplicons and sequencing of ITS1 of recent clinical isolates of Kala-azar from India and Bangladesh confirms the association of L. tropica with the disease. Acta Trop 124: 229–234 Available: http://www.ncbi.nlm.nih.gov/pubmed/22960646. [DOI] [PubMed] [Google Scholar]
- 37. Alborzi A, Rasouli M, Shamsizadeh A (2006) Leishmania tropica-isolated patient with visceral leishmaniasis in southern Iran. Am J Trop Med Hyg 74: 306–307 Available: http://www.ncbi.nlm.nih.gov/pubmed/16474088. [PubMed] [Google Scholar]
- 38. Alborzi A, Pouladfar GR, Fakhar M, Motazedian MH, Hatam GR, et al. (2008) Isolation of Leishmania tropica from a patient with visceral leishmaniasis and disseminated cutaneous leishmaniasis, southern Iran. Am J Trop Med Hyg 79: 435–437 Available: http://www.ncbi.nlm.nih.gov/pubmed/18784238. [PubMed] [Google Scholar]
- 39. Shirian S, Oryan A, Hatam G-R, Daneshbod K, Daneshbod Y (2012) Molecular diagnosis and species identification of mucosal leishmaniasis in Iran and correlation with cytological findings. Acta Cytol 56: 304–309 Available: http://www.ncbi.nlm.nih.gov/pubmed/22555534. [DOI] [PubMed] [Google Scholar]
- 40. Lira R, Méndez S, Carrera L, Jaffe C, Neva F, et al. (1998) Leishmania tropica: the identification and purification of metacyclic promastigotes and use in establishing mouse and hamster models of cutaneous and visceral disease. Exp Parasitol 89: 331–342 Available: http://www.ncbi.nlm.nih.gov/pubmed/9676711. [DOI] [PubMed] [Google Scholar]
- 41. Girginkardeşler N, Balcioğlu IC, Yereli K, Ozbilgin A, Ozbel Y (2001) Cutaneous leishmaniasis infection in Balb/c mice using a Leishmania tropica strain isolated from Turkey. J Parasitol 87: 1177–1178 Available: http://www.ncbi.nlm.nih.gov/pubmed/11695390. [DOI] [PubMed] [Google Scholar]
- 42. Anderson CF, Lira R, Kamhawi S, Belkaid Y, Wynn TA, et al. (2008) IL-10 and TGF-beta control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. J Immunol 180: 4090–4097 Available: http://www.ncbi.nlm.nih.gov/pubmed/18322219. [DOI] [PubMed] [Google Scholar]
- 43. Demant P (2003) Cancer susceptibility in the mouse: genetics, biology and implications for human cancer. Nat Rev Genet 4: 721–734 Available: http://www.ncbi.nlm.nih.gov/pubmed/12951573. [DOI] [PubMed] [Google Scholar]
- 44. Demant P, Lipoldová M, Svobodová M (1996) Resistance to Leishmania major in mice. Science (New York, NY) 274: 1392 Available: http://www.ncbi.nlm.nih.gov/pubmed/17772041. [PubMed] [Google Scholar]
- 45. Lipoldová M, Svobodová M, Havelková H, Krulová M, Badalová J, et al. (2002) Mouse genetic model for clinical and immunological heterogeneity of leishmaniasis. Immunogenetics 54: 174–183 Available: http://www.ncbi.nlm.nih.gov/pubmed/12073146. [DOI] [PubMed] [Google Scholar]
- 46. Kobets T, Havelková H, Grekov I, Volkova V, Vojtíšková J, et al. (2012) Genetics of host response to Leishmania tropica in mice - different control of skin pathology, chemokine reaction, and invasion into spleen and liver. PLoS Negl Trop Dis 6: e1667 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3367980&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Démant P, Hart AA (1986) Recombinant congenic strains - a new tool for analyzing genetic traits determined by more than one gene. Immunogenetics 24: 416–422 Available: http://www.ncbi.nlm.nih.gov/pubmed/3793154. [DOI] [PubMed] [Google Scholar]
- 48. Stassen AP, Groot PC, Eppig JT, Demant P (1996) Genetic composition of the recombinant congenic strains. Mamm Genome 7: 55–58 Available: http://www.ncbi.nlm.nih.gov/pubmed/8903730. [DOI] [PubMed] [Google Scholar]
- 49. Grekov I, Svobodová M, Nohýnková E, Lipoldová M (2011) Preparation of highly infective Leishmania promastigotes by cultivation on SNB-9 biphasic medium. J Microbiol Methods 87: 273–277 Available: http://www.ncbi.nlm.nih.gov/pubmed/21889549. [DOI] [PubMed] [Google Scholar]
- 50. Rogers ME, Ilg T, Nikolaev AV, Ferguson MAJ, Bates PA (2004) Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature 430: 463–467 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2835460&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kobets T, Badalová J, Grekov I, Havelková H, Svobodová M, et al. (2010) Leishmania parasite detection and quantification using PCR-ELISA. Nat Prot 5: 1074–1080 Available: http://www.ncbi.nlm.nih.gov/pubmed/20539283. [DOI] [PubMed] [Google Scholar]
- 52. Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, et al. (1991) Simplified mammalian DNA isolation procedure. Nucl Acids Res 19: 4293 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=328579&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Šíma M, Havelková H, Quan L, Svobodová M, Jarošíková T, et al. (2011) Genetic control of resistance to Trypanosoma brucei brucei infection in mice. PLoS Negl Trop Dis 5: e1173 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3110168&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lander ES, Schork NJ (1994) Genetic dissection of complex traits. Science 265: 2037–2048 Available: http://www.ncbi.nlm.nih.gov/pubmed/8091226. [DOI] [PubMed] [Google Scholar]
- 55. Nascimento MS, Albuquerque TD, Do-Valle-Matta MA, Caldas IS, Diniz LF, et al. (2013) Naturally Leishmania infantum-infected dogs display an overall impairment of chemokine and chemokine receptor expression during visceral leishmaniasis. Vet Immunol Immunopathol 153 ((3–4)): 202–8 Available: http://www.sciencedirect.com/science/article/pii/S0165242713000871. [DOI] [PubMed] [Google Scholar]
- 56. Machado PR, Rosa ME, Costa D, Mignac M, Silva JS, et al. (2011) Reappraisal of the immunopathogenesis of disseminated leishmaniasis: in situ and systemic immune response. Trans R Soc Trop Med Hyg 105: 438–444 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3157292&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Menezes-Souza D, Guerra-Sá R, Carneiro CM, Vitoriano-Souza J, Giunchetti RC, et al. (2012) Higher expression of CCL2, CCL4, CCL5, CCL21, and CXCL8 chemokines in the skin associated with parasite density in canine visceral leishmaniasis. PLoS Negl Trop Dis 6: e1566 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3323520&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katzman SD, Fowell DJ (2008) Pathogen-imposed skewing of mouse chemokine and cytokine expression at the infected tissue site. J Clin Invest 118: 801–811 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2176190&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bimal S, Sinha S, Singh SK, Narayan S, Kumar V, et al. (2012) Leishmania donovani: CD2 biased immune response skews the SAG mediated therapy for a predominant Th1 response in experimental infection. Exp Parasitol 131: 274–282 Available: http://www.ncbi.nlm.nih.gov/pubmed/22580024. [DOI] [PubMed] [Google Scholar]
- 60. Portillo J-AC, Feliciano LM, Okenka G, Heinzel F, Subauste MC, et al. (2012) CD40 and tumour necrosis factor-α co-operate to up-regulate inducuble nitric oxide synthase expression in macrophages. Immunology 135: 140–150 Available: http://www.ncbi.nlm.nih.gov/pubmed/22044243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kedzierski L, Curtis JM, Doherty PC, Handman E, Kedzierska K (2008) Decreased IL-10 and IL-13 production and increased CD44hi T cell recruitment contribute to Leishmania major immunity induced by non-persistent parasites. Eur J Immunol 38: 3090–3100 Available: http://www.ncbi.nlm.nih.gov/pubmed/18924210. [DOI] [PubMed] [Google Scholar]
- 62. Kamir D, Zierow S, Leng L, Cho Y, Diaz Y, et al. (2008) A Leishmania ortholog of macrophage migration inhibitory factor modulates host macrophage responses. J Immunol 180: 8250–8261 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2668862&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wiethe C, Debus A, Mohrs M, Steinkasserer A, Lutz M, et al. (2008) Dendritic cell differentiation state and their interaction with NKT cells determine Th1/Th2 differentiation in the murine model of Leishmania major infection. J Immunol 180: 4371–4381 Available: http://www.ncbi.nlm.nih.gov/pubmed/18354157. [DOI] [PubMed] [Google Scholar]
- 64. Pós Z, Müller K, Novalphak I, Buzás E, Solbach W, et al. (2004) Different patterns of the L-histidine decarboxylase (HDC) gene expression in mice resistant and susceptible to experimental cutaneous leishmaniasis. Inflamm Res 53: 38–43 Available: http://www.ncbi.nlm.nih.gov/pubmed/15021979. [DOI] [PubMed] [Google Scholar]
- 65. Xin L, Vargas-Inchaustegui DA, Raimer SS, Kelly BC, Hu J, et al. (2010) Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J Immunol 184: 7047–7056 Available: http://www.ncbi.nlm.nih.gov/pubmed/20483775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goto H, Gomes CM, Corbett CE, Monteiro HP, Gidlund M (1998) Insulin-like growth factor I is a growth-promoting factor for Leishmania promastigotes and amastigotes. Proc Natl Acad Sci USA 95: 13211–13216 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=23762&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Voronov E, Dotan S, Gayvoronsky L, White RM, Cohen I, et al. (2010) IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. Int Immunol 22: 245–257 Available: http://www.ncbi.nlm.nih.gov/pubmed/20181656. [DOI] [PubMed] [Google Scholar]
- 68. Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK (1993) Recombinant interleukin 12 cures mice infected with Leishmania major . J Exp Med 177: 1505–1509 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2191017&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Contreras I, Gómez MA, Nguyen O, Shio MT, McMaster RW, et al. (2010) Leishmania-induced inactivation of the macrophage transcription factor AP-1 is mediated by the parasite metalloprotease GP63. PLoS Pathog 6: e1001148 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2954837&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pelletier I, Hashidate T, Urashima T, Nishi N, Nakamura T, et al. (2003) Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J Biol Chem 278: 22223–22230 Available: http://www.ncbi.nlm.nih.gov/pubmed/12684513. [DOI] [PubMed] [Google Scholar]
- 71. Saraiva EM, Andrade AF, de Souza W (1987) Involvement of the macrophage mannose-6-phosphate receptor in the recognition of Leishmania mexicana amazonensis . Parasitol Res 73: 411–416 Available: http://www.ncbi.nlm.nih.gov/pubmed/2958844. [DOI] [PubMed] [Google Scholar]
- 72. Hutchins AS, Artis D, Hendrich BD, Bird AP, Scott P, et al. (2005) Cutting edge: a critical role for gene silencing in preventing excessive type 1 immunity. J Immunol 175: 5606–5610 Available: http://www.ncbi.nlm.nih.gov/pubmed/16237047. [DOI] [PubMed] [Google Scholar]
- 73. Jüttner S, Bernhagen J, Metz CN, Röllinghoff M, Bucala R, et al. (1998) Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J Immunol 161: 2383–2390. [PubMed] [Google Scholar]
- 74. Boaventura VS, Santos CS, Cardoso CR, de Andrade J, Dos Santos WLC, et al. (2010) Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur J Immunol 40: 2830–2836 Available: http://www.ncbi.nlm.nih.gov/pubmed/20812234. [DOI] [PubMed] [Google Scholar]
- 75. Kanaan SA, Saadé NE, Karam M, Khansa H, Jabbur SJ, et al. (2000) Hyperalgesia and upregulation of cytokines and nerve growth factor by cutaneous leishmaniasis in mice. Pain 85: 477–482 Available: http://www.ncbi.nlm.nih.gov/pubmed/10781922. [DOI] [PubMed] [Google Scholar]
- 76. Blos M, Schleicher U, Soares Rocha FJ, Meissner U, Röllinghoff M, et al. (2003) Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur J Immunol 33: 1224–1234 Available: http://www.ncbi.nlm.nih.gov/pubmed/12731047. [DOI] [PubMed] [Google Scholar]
- 77. Auderset F, Schuster S, Coutaz M, Koch U, Desgranges F, et al. (2012) Redundant Notch1 and Notch2 signaling is necessary for IFNγ secretion by T helper 1 cells during infection with Leishmania major . PLoS Pathog 8: e1002560 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3291656&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gomez MA, Contreras I, Hallé M, Tremblay ML, McMaster RW, et al. (2009) Leishmania GP63 alters host signaling through cleavage-activated protein tyrosine phosphatases. Sci Signal 2: ra58 Available: http://www.ncbi.nlm.nih.gov/pubmed/19797268. [DOI] [PubMed] [Google Scholar]
- 79. Canton J, Ndjamen B, Hatsuzawa K, Kima PE (2012) Disruption of the fusion of Leishmania parasitophorous vacuoles with ER vesicles results in the control of the infection. Cell Microbiol 14: 937–948 Available: http://www.ncbi.nlm.nih.gov/pubmed/22309219. [DOI] [PubMed] [Google Scholar]
- 80. Castellucci L, Jamieson SE, Almeida L, Oliveira J, Guimarães LH, et al. (2012) Wound healing genes and susceptibility to cutaneous leishmaniasis in Brazil. Inf Genet Evol 12: 1102–1110 Available: http://www.ncbi.nlm.nih.gov/pubmed/22554650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Osorio EY, Zhao W, Espitia C, Saldarriaga O, Hawel L, et al. (2012) Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression. PLoS Pathog 8: e1002417 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3261917&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stanley AC, Dalton JE, Rossotti SH, MacDonald KP, Zhou Y, et al. (2008) VCAM-1 and VLA-4 modulate dendritic cell IL-12p40 production in experimental visceral leishmaniasis. PLoS Pathog 4: e1000158 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2528938&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Suh W-K, Wang S, Duncan GS, Miyazaki Y, Cates E, et al. (2006) Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol 26: 6403–6411 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1592821&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Smith DF, Peacock CS, Cruz AK (2007) Comparative genomics: from genotype to disease phenotype in the leishmaniases. Int J Parasitol 37: 1173–1186 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2696322&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Raymond F, Boisvert S, Roy G, Ritt J-F, Légaré D, et al. (2012) Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucl Acids Res 40: 1131–1147 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3273817&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Carlborg O, Haley CS (2004) Epistasis: too often neglected in complex trait studies? Nat Rev Genet 5: 618–625 Available: http://www.ncbi.nlm.nih.gov/pubmed/15266344. [DOI] [PubMed] [Google Scholar]
- 87. Lipoldová M, Svobodová M, Krulová M, Havelková H, Badalová J, et al. (2000) Susceptibility to Leishmania major infection in mice: multiple loci and heterogeneity of immunopathological phenotypes. Genes Immun 1: 200–206 Available: http://www.ncbi.nlm.nih.gov/pubmed/11196712. [DOI] [PubMed] [Google Scholar]
- 88. Havelková H, Badalová J, Svobodová M, Vojtíšková J, Kurey I, et al. (2006) Genetics of susceptibility to leishmaniasis in mice: four novel loci and functional heterogeneity of gene effects. Genes Immun 7: 220–233 Available: http://www.ncbi.nlm.nih.gov/pubmed/16511555. [DOI] [PubMed] [Google Scholar]
- 89. Kurey I, Kobets T, Havelková H, Slapničková M, Quan L, et al. (2009) Distinct genetic control of parasite elimination, dissemination, and disease after Leishmania major infection. Immunogenetics 61: 619–633 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2744819&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Caron J, Loredo-Osti JC, Laroche L, Skamene E, Morgan K, et al. (2002) Identification of genetic loci controlling bacterial clearance in experimental Salmonella enteritidis infection: an unexpected role of Nramp1 (Slc11a1) in the persistence of infection in mice. Genes Immun 3: 196–204 Available: http://www.ncbi.nlm.nih.gov/pubmed/12058254. [DOI] [PubMed] [Google Scholar]
- 91. Atkinson A, Barbier M, Afridi S, Fumoux F, Rihet P (2011) Evidence for epistasis between hemoglobin C and immune genes in human P. falciparum malaria: a family study in Burkina Faso. Genes Immun 12: 481–489 Available: http://www.ncbi.nlm.nih.gov/pubmed/21451558. [DOI] [PubMed] [Google Scholar]
- 92. Zhang F, Liu H, Chen S, Low H, Sun L, et al. (2011) Identification of two new loci at IL23R and RAB32 that influence susceptibility to leprosy. Nat Genet 43: 1247–1251 Available: http://www.ncbi.nlm.nih.gov/pubmed/22019778. [DOI] [PubMed] [Google Scholar]
- 93. Serbina NV, Shi C, Pamer EG (2012) Monocyte-mediated immune defense against murine Listeria monocytogenes infection. Adv Immunol 113: 119–134 Available: http://www.ncbi.nlm.nih.gov/pubmed/22244581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van Wezel T, Stassen AP, Moen CJ, Hart AA, van der Valk MA, et al. (1996) Gene interaction and single gene effects in colon tumour susceptibility in mice. Nat Genet 14: 468–470 Available: http://www.ncbi.nlm.nih.gov/pubmed/8944029. [DOI] [PubMed] [Google Scholar]
- 95. Vladimirov V, Badalová J, Svobodová M, Havelková H, Hart AAM, et al. (2003) Different genetic control of cutaneous and visceral disease after Leishmania major infection in mice. Infect Immun 71: 2041–2046 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=152088&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krulová M, Havelková H, Kosařová M, Holáň V, Hart AA, et al. (1997) IL-2-induced proliferative response is controlled by loci Cinda1 and Cinda2 on mouse chromosomes 11 and 12: a distinct control of the response induced by different IL-2 concentrations. Genomics 42: 11–15 Available: http://www.ncbi.nlm.nih.gov/pubmed/9177770. [DOI] [PubMed] [Google Scholar]
- 97. Lipoldová M, Havelková H, Badalová J, Vojtíšková J, Quan L, et al. (2010) Loci controlling lymphocyte production of interferon γ after alloantigen stimulation in vitro and their co-localization with genes controlling lymphocyte infiltration of tumors and tumor susceptibility. Cancer Immunol Immunother 59: 203–213 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2776939&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kosařová M, Havelková H, Krulová M, Demant P, Lipoldová M (1999) The production of two Th2 cytokines, interleukin-4 and interleukin-10, is controlled independently by locus Cypr1 and by loci Cypr2 and Cypr3, respectively. Immunogenetics 49: 134–141 Available: http://www.ncbi.nlm.nih.gov/pubmed/9887350. [DOI] [PubMed] [Google Scholar]
- 99. Nadeau JH, Nelson VR (2010) Transgenerational genetic effects. Epigenomics 2: 797–806 Available: http://www.ncbi.nlm.nih.gov/pubmed/22122083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stenger S, Donhauser N, Thüring H, Röllinghoff M, Bogdan C (1996) Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med 183: 1501–1514 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2192515&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Badalová J, Svobodová M, Havelková H, Vladimirov V, Vojtíšková J, et al. (2002) Separation and mapping of multiple genes that control IgE level in Leishmania major infected mice. Genes Imun 3: 187–195 Available: http://www.ncbi.nlm.nih.gov/pubmed/12058253. [DOI] [PubMed] [Google Scholar]
- 102. Roper RJ, Weis JJ, McCracken BA, Green CB, Ma Y, et al. (2001) Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun 2: 388–397 Available: http://www.ncbi.nlm.nih.gov/pubmed/11704805. [DOI] [PubMed] [Google Scholar]
- 103. Kumar R, Bumb RA, Salotra P (2010) Evaluation of localized and systemic immune responses in cutaneous leishmaniasis caused by Leishmania tropica: interleukin-8, monocyte chemotactic protein-1 and nitric oxide are major regulatory factors. Immunology 130: 193–201 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2878464&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jandl RC, George JL, Dinarello CA, Schur PH (1987) The effect of interleukin 1 on IgG synthesis in systemic lupus erythematosus. Clin Immunol Immunopathol 45: 384–394 Available: http://www.ncbi.nlm.nih.gov/pubmed/3119264. [DOI] [PubMed] [Google Scholar]
- 105. Li R, Lyons MA, Wittenburg H, Paigen B, Churchill GA (2005) Combining data from multiple inbred line crosses improves the power and resolution of quantitative trait loci mapping. Genetics 169: 1699–1709 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1449540&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Moen CJ, Stoffers HJ, Hart AA, Westerhoff HV, Demant P (1997) Simulation of the distribution of parental strains' genomes in RC strains of mice. Mamm Genome 8: 884–889 Available: http://www.ncbi.nlm.nih.gov/pubmed/9383279. [DOI] [PubMed] [Google Scholar]
- 107. Gusareva ES, Havelková H, Blažková H, Kosařová M, Kučera P, et al. (2009) Mouse to human comparative genetics reveals a novel immunoglobulin E-controlling locus on Hsa8q12 . Immunogenetics 61: 15–25 Available: http://www.ncbi.nlm.nih.gov/pubmed/19015841. [DOI] [PubMed] [Google Scholar]
- 108. Johnson J, Suzuki Y, Mack D, Mui E, Estes R, et al. (2002) Genetic analysis of influences on survival following Toxoplasma gondii infection. Int J Parasitol 32: 179–185 Available: http://www.ncbi.nlm.nih.gov/pubmed/11812495. [DOI] [PubMed] [Google Scholar]
- 109. Rathkolb B, Noyes HA, Brass A, Dark P, Fuchs H, et al. (2009) Clinical chemistry of congenic mice with quantitative trait loci for predicted responses to Trypanosoma congolense infection. Infect Immun 77: 3948–3957 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2738039&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Miyairi I, Ziebarth J, Laxton JD, Wang X, van Rooijen N, et al. (2012) Host genetics and Chlamydia disease: prediction and validation of disease severity mechanisms. PLoS One 7: e33781 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3306297&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH (2010) Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 11: 446–450 Available: http://www.ncbi.nlm.nih.gov/pubmed/20479774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461: 747–753 Available: http://www.ncbi.nlm.nih.gov/pubmed/19812666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Numbers of mice analyzed in individual phenotypes.
(XLS)
Chromosomal positions of typed markers.
(XLS)
ID numbers of potential candidate genes localized in Ltr loci.
(XLS)