Abstract
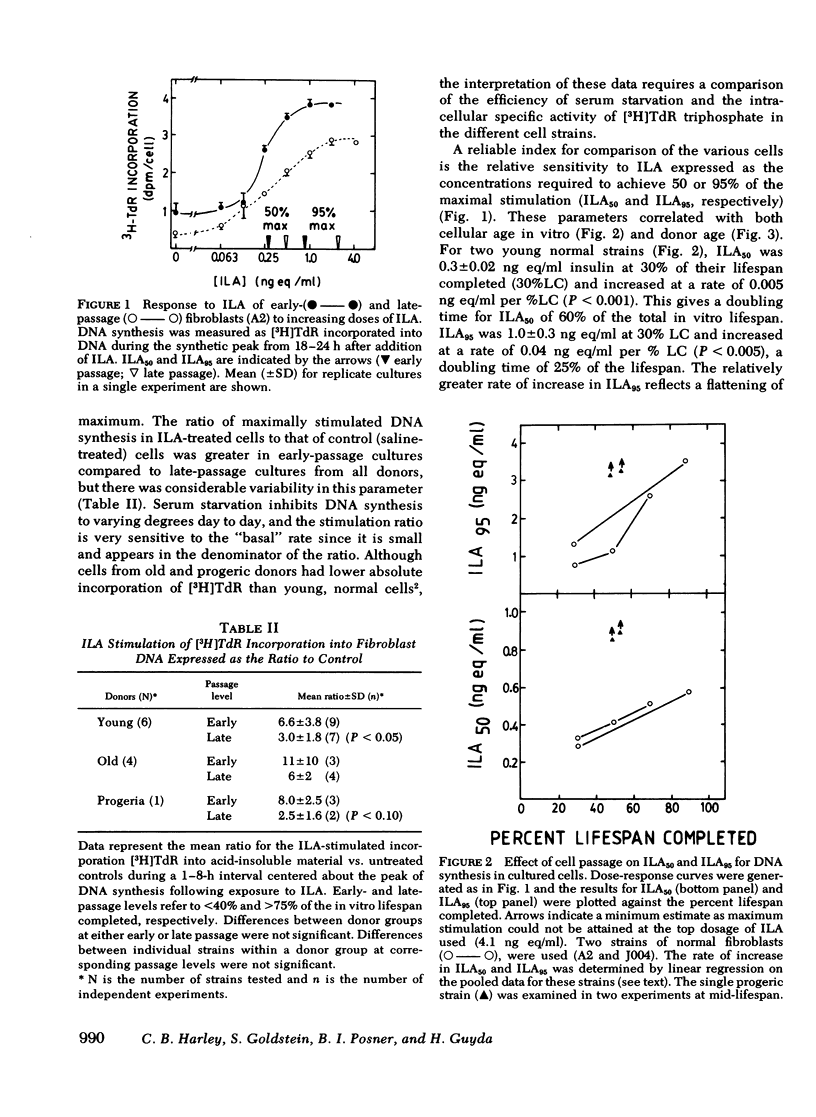
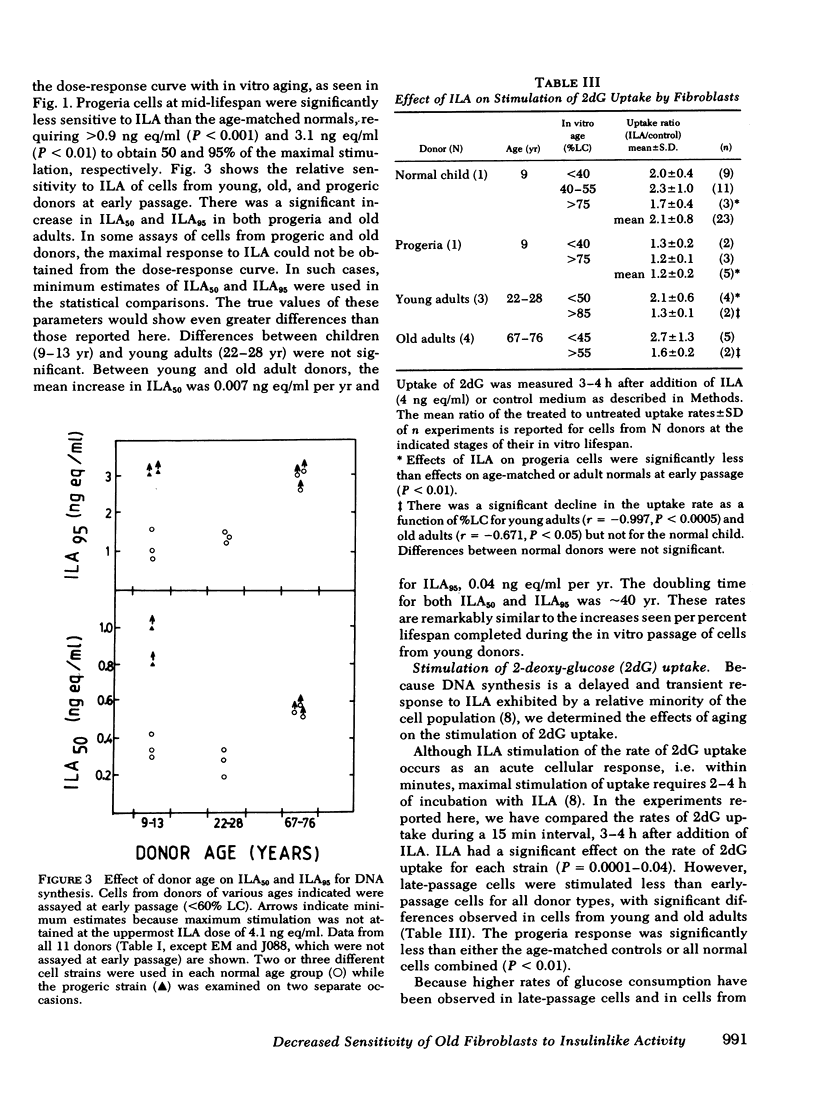
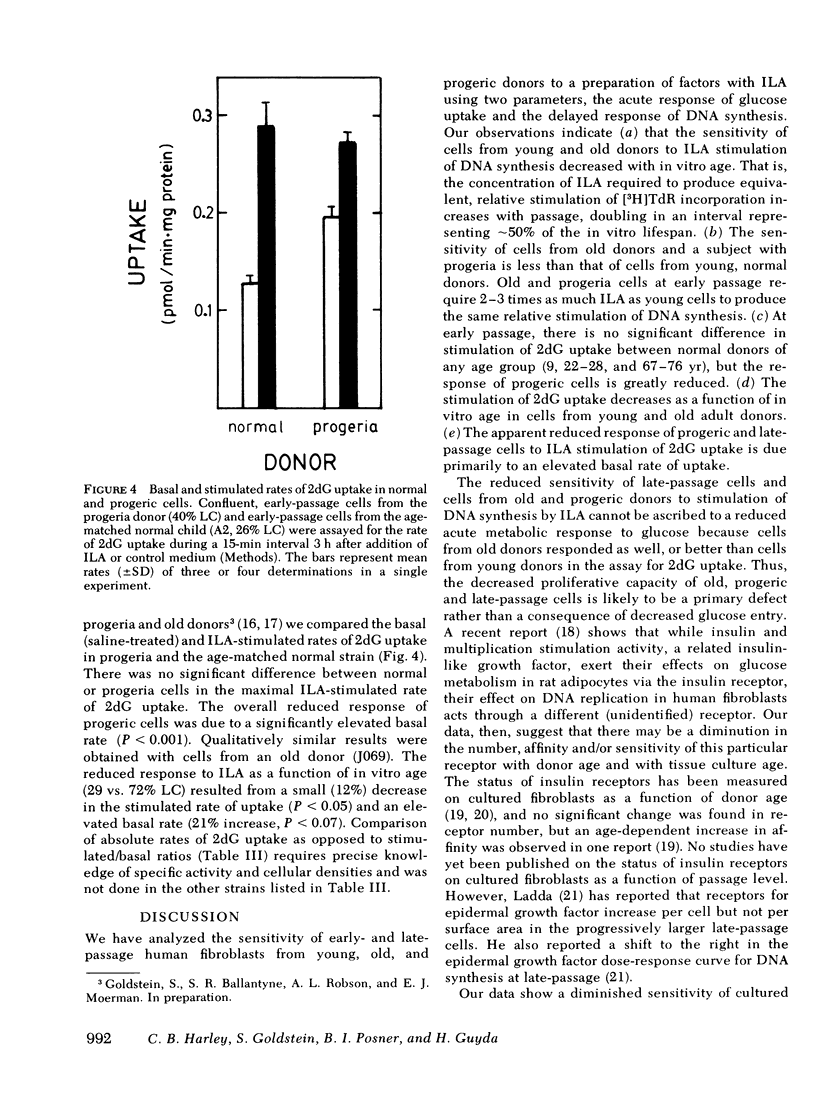
To determine whether old cells have a reduced response to a preparation of factors from human plasma with insulinlike activity (ILA), we analyzed the response to ILA of early and late passage human fibroblasts from young, old, and progeric donors in the acute stimulation of [3H]2-deoxy-D-glucose (2dG) uptake and the delayed stimulation of [3H]thymidine (TdR) incorporation into DNA. The ILA concentration required to produce equivalent, relative stimulation of TdR incorporation was increased two- to three-fold in late passage cells and cells from old and progeric donors (P less than 0.01). 50 and 95% of maximal stimulation (ILA50, ILA95) was achieved by 0.26 +/- 0.07 and 1.38 +/- 0.13 ng insulin equivalents/ml (mean +/- SD) respectively, in cells from young adults at early passage. Corresponding values were 0.54 +/- 0.05 and 2.90 +/- 0.25 in cells from old donors; greater than 0.9 +/- 0.1 and greater than 3.1 +/- 0.1 in cells from a 9-yr-old progeric donor; and 0.4 +/- 0.05 and 1.1 +/- 0.04 in cells from normal children (9-13 yr). For two cell strains from young adults, ILA50 and ILA95 were 0.30 +/- 0.02 and 1.0 +/- 0.3 ng eq/ml at 30% of their in vitro lifespan completed (%LC) and these values increased at rates of 0.005 ng eq/ml per %LC and 0.04 ng eq/ml per %LC, respectively. The mean stimulation of 2dG uptake ratio (ILA/control) decreased from early to late passage from 2.1 +/- 0.6 to 1.3 +/- 0.1 in young adult donors (P less than 0.05), but there were no significant differences between young and old donors at either early or late passage. The mean stimulation ratio in progeric cells (1.2 +/- 0.2) did not change with in vitro passage, but was significantly lower than that of age-matched normal cells (2.1 +/- 0.8, P less than 0.001). In progeria cells, the reduced stimulation of 2dG uptake upon addition of ILA was due to an increased basal rate of uptake (0.19 +/- 0.01 pmol [3H]2dG/min per mg protein vs. 0.13 +/- 0.01 in age-matched normal cells), and not to a decline in the maximal rate of uptake (0.26 +/- 0.01 vs. 0.27 +/- 0.02, respectively). Similar results were found for in vitro aging in cells from an old donor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres R. Aging and diabetes. Med Clin North Am. 1971 Jul;55(4):835–846. doi: 10.1016/s0025-7125(16)32479-8. [DOI] [PubMed] [Google Scholar]
- Bell E., Marek L. F., Levinstone D. S., Merrill C., Sher S., Young I. T., Eden M. Loss of division potential in vitro: aging or differentiation? Science. 1978 Dec 15;202(4373):1158–1163. doi: 10.1126/science.725592. [DOI] [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Littlefield J. W. Effect of insulin on the conversion of glucose-C-14 to C-14-O2 by normal and diabetic fibroblasts in culture. Diabetes. 1969 Aug;18(8):545–549. doi: 10.2337/diab.18.8.545. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Littlefield J. W., Soeldner J. S. Diabetes mellitus and aging: diminished planting efficiency of cultured human fibroblasts. Proc Natl Acad Sci U S A. 1969 Sep;64(1):155–160. doi: 10.1073/pnas.64.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. J., Soeldner J. S., Gleason R. E., Barnett D. M. Diabetes mellitus and genetic prediabetes. Decreased replicative capacity of cultured skin fibroblasts. J Clin Invest. 1979 Mar;63(3):358–370. doi: 10.1172/JCI109311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. Heat-labile enzymes in skin fibroblasts from subjects with progeria. N Engl J Med. 1975 Jun 19;292(25):1305–1309. doi: 10.1056/NEJM197506192922501. [DOI] [PubMed] [Google Scholar]
- Goldstein S. The biology of aging. N Engl J Med. 1971 Nov;285(20):1120–1129. doi: 10.1056/NEJM197111112852005. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Trieman G. Glucose consumption by early and late-passage diploid human fibroblasts during growth and stationary phase. Experientia. 1975 Feb 15;31(2):177–180. doi: 10.1007/BF01990690. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Goldstein S., Posner B. I., Guyda H. J. Insulin-like peptides stimulate metabolism but not proliferation of human fibroblasts. Am J Physiol. 1980 Aug;239(2):E125–E131. doi: 10.1152/ajpendo.1980.239.2.E125. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Schneider E. L. Receptors for insulin and epidermal growth factor-urogastrone in adult human fibroblasts do not change with donor age. Mech Ageing Dev. 1979 Aug;11(1):37–43. doi: 10.1016/0047-6374(79)90062-9. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Norwood T. H., Pendergrass W. R. Clonal selection, attenuation and differentiation in an in vitro model of hyperplasia. Am J Pathol. 1974 Jan;74(1):137–154. [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Guyda H. J., Corvol M. T., Rappaport R., Harley C., Goldstein S. Partial purification, characterization, and assay of a slightly acidic insulin-like peptide (ILAs) from human plasma. J Clin Endocrinol Metab. 1978 Dec;47(6):1240–1250. doi: 10.1210/jcem-47-6-1240. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Eisen H. J., Higa O. Z., Nissley P., Moses A. C., Schilling E. E., Fennoy I., Bruni C. B., Phillips L. S., Baird K. L. Characterization of a somatomedin (insulin-like growth factor) synthesized by fetal rat liver organ cultures. J Biol Chem. 1979 Aug 25;254(16):7942–7950. [PubMed] [Google Scholar]
- Rosenbloom A. L., Goldstein S., Yip C. C. Insulin binding to cultured human fibroblasts increases with normal and precocious aging. Science. 1976 Jul 30;193(4251):412–415. doi: 10.1126/science.180604. [DOI] [PubMed] [Google Scholar]
- Singal D. P., Goldstein S. Absence of detectable HL-A antigens on cultured fibroblasts in progeria. J Clin Invest. 1973 Sep;52(9):2259–2263. doi: 10.1172/JCI107412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyk J. J., Svoboda M. E., Underwood L. E. Evidence from radioligand assays that somatomedin-C and insulin-like growth factor-I are similar to each other and different from other somatomedins. J Clin Endocrinol Metab. 1980 Jan;50(1):206–208. doi: 10.1210/jcem-50-1-206. [DOI] [PubMed] [Google Scholar]



