Inducible extracytoplasmic stress responses (ESRs) help to maintain the integrity and function of the bacterial cell envelope in unfavorable conditions. ESRs can also have highly specialized functions linked to virulence-associated systems directly. One of the most intriguing and yet enigmatic examples is the widely conserved phage shock protein (Psp) response [1]–[4]. This article outlines the significance of envelope stress and the roles of the Psp response in supporting bacterial virulence. This particular ESR might be critical for different reasons in different bacteria, with implications for both extracellular and intracellular pathogenesis, as well as processes that include antibiotic resistance and biofilm formation.
Envelope Stress Responses Are Important for Bacterial Pathogens
Interaction with a mammalian host confronts bacteria with changes in temperature, osmolarity, and pH, which can cause cell envelope proteins to misfold and mislocalize, alter membrane properties, and even breach the membrane permeability barrier. The host can also attack the bacterial cell envelope with substances including bile salts, other surfactants, and antimicrobial peptides. To survive, bacteria can use their inducible ESRs to prevent lethal cell envelope defects. These responses have been studied most in Gram-negatives, where their importance during host interaction has been realized in recent years (reviewed in [5], [6]). The best-characterized ESRs are the widely conserved two-component system CpxAR and the RpoE extracytoplasmic function sigma factor system. These systems coordinate broad responses to aberrant cell envelope proteins [7]. However, both have also been linked specifically to virulence functions. For example, CpxAR regulates pili and type III secretion in pathogenic E. coli, type III secretion in Shigella sonnei [8]–[10], and type IV secretion in Legionella pneumophila [11]. The Pseudomonas aeruginosa RpoE system (known as AlgU/T) controls production of the exopolysaccharide alginate, a virulence factor in chronic lung infections [12].
Many bacteria remodel their envelope upon encountering the host. Often these changes involve the synthesis of complex envelope structures that are important virulence factors, such as T3SSs and pili. Improper assembly of these envelope structures might compromise the bacterial cell envelope and induce ESRs. One way to counter the potential envelope stress is for ESRs to downregulate these virulence factors, as proposed for the CpxAR systems of enteropathogenic E. coli and Yersinia pseudotuberculosis [13], [14]. However, another possibility is for an ESR to mitigate the stress while leaving virulence factor production unaffected. The Psp ESR does that in at least one of its roles to support bacterial virulence.
The Phage Shock Protein (Psp) Response
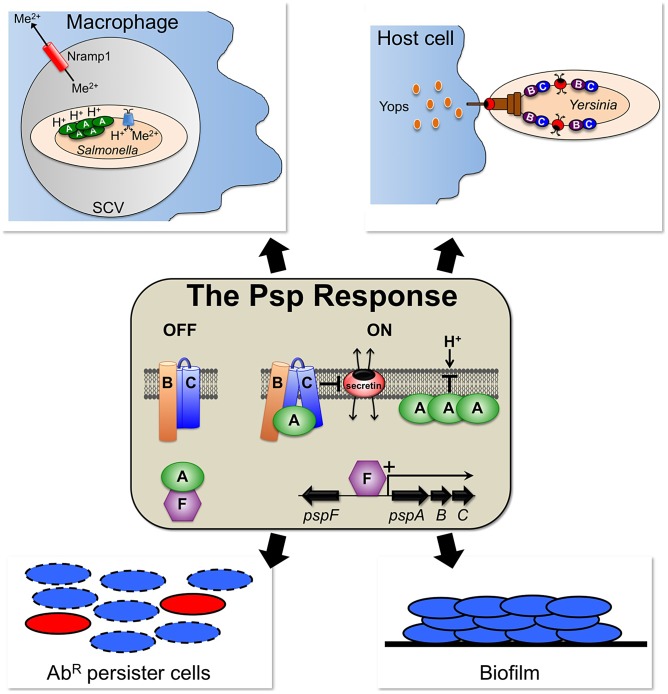
The “phage shock” name comes from the founding discovery that filamentous phage f1 infection massively induces the synthesis of an E. coli protein, which was named phage shock protein A (PspA) [15]. In E. coli, PspA is encoded by the pspABCDE operon, which is positively controlled by the transcription factor PspF (Figure 1). Filamentous phage infection induces pspA operon expression because a phage-encoded outer membrane pore-forming “secretin” protein (pIV) has a tendency to mislocalize within the E. coli envelope [3]. The Psp response is also induced by environmental shocks that can negatively affect the cell envelope. This, together with various other observations, has led to a hypothesis that the Psp system responds to, and helps mitigate, some aspect of cytoplasmic membrane perturbation [1]–[4].
Figure 1. The Psp response and its involvement in various virulence-assocaied processes.
In uninduced cells, PspA forms an inhibitory complex with the transcription factor PspF in the cytoplasm. An inducing trigger, such as the mislocalization of a pore-forming secretin protein, causes PspA to relocate to the cytoplasmic membrane, perhaps both in complex with PspBC and by making direct membrane contact. PspF induces pspA operon expression, leading to increased concentrations of PspA, -B, and -C that play roles in stress mitigation. PspA is believed to prevent proton (H+) leakage across the cytoplasmic membrane and maintain the proton motive force. This is thought to support S. Typhimurium virulence in Nramp1-positive mice by ensurng an energy supply for metal ion (Me2+) importers. PspB and -C prevent secretins from causing lethal cytoplasmic membrane permeability, which supports the T3SS-dependent virulence of Y. enterocolitica. The Psp response has also been shown to be required for the formation of biofilms and antibiotic-resistant (AbR) persister cells in E. coli, although the Psp protein(s) involved has not yet been reported.
Psp protein homologues, especially PspA, are present in Gram-negative and Gram-positive bacteria, as well as archaea and plants (reviewed in [16]). However, the Psp response has been studied most in the Gram-negative Enterobacteriaceae E. coli, Yersinia enterocolitica, and Salmonella enterica serovar Typhimurium (S. Typhimurium), where only PspF, -A, -B, and -C have been associated with robust phenotypes. One of their well-characterized functions is to autoregulate psp gene expression. This is achieved by PspA changing from an inhibitory interaction with PspF in the cytoplasm of uninduced cells, to a complex with the cytoplasmic membrane proteins PspB and -C of induced cells (Figure 1) [17], [18]. However, once pspA operon expression is induced, PspA, -B, and -C have additional roles in mitigating envelope stress. PspA has been focused upon due to its abundance, as well as experiments suggesting that it maintains the proton motive force (PMF) by preventing proton leakage across a damaged cytoplasmic membrane [19], [20].
The Psp Response Supports Virulence by Preventing T3SS-Induced Envelope Stress in the Extracellular Pathogen Yersinia enterocolitica
The Y. enterocolitica Psp system is essential for virulence because of a specific connection to the Ysc-Yop T3SS. A pspC null mutant is avirulent in a mouse model of infection, and grows slower than the wild type in conditions that induce production of the Ysc-Yop system [21], [22]. This growth inhibition is caused by the outer membrane pore-forming component of the T3SS, YscC [22]. Like the phage pIV protein that induces the E. coli Psp system, YscC is a secretin. Indeed, when the Y. enterocolitica Psp system is intact, production of the native Ysc-Yop T3SS, or of only YscC, induces pspA operon expression [22]. Therefore, the Psp system is induced by production of the T3SS, and is then essential to mitigate a growth-inhibiting stress that its YscC secretin component can cause. The consequence is that a psp null mutant essentially kills itself during host infection by producing the Ysc-Yop T3SS to evade the immune response, but being unable to survive the stress that T3SS production causes.
Secretins have the unique ability to insert into either membrane of Gram-negative bacteria [23]. It is their mislocalization into the cytoplasmic membrane that induces psp gene expression and kills a psp null strain. Therefore, the toxicity of native T3SS production to a psp null strain suggests that endogenously produced YscC can mislocalize [22]. The cell death results from severe cytoplasmic membrane permeability, thought to be caused by the multimeric secretin channel (Figure 1) [24]. Remarkably, only the small membrane proteins PspB and PspC are required to prevent this toxicity from occurring, although it is not yet known how they do it [24]. Whatever the mechanism, the relationship between the Psp system and secretin mislocalization is highly specific. Secretin production induces psp gene expression without having much effect on any other genes in Y. enterocolitica, E. coli, or S. Typhimurium [25], [26]. It is surprising that PspA, the most abundant Psp protein, linked with maintenance of the PMF in E. coli, is not required to prevent secretin toxicity [24]. However, this does not mean that PspA is without an important role to play, or that it is always dispensable for virulence, as explained below.
The Psp Response Supports Virulence by Maintaining the Proton Motive Force in the Intracellular Pathogen S. Typhimurium
Attention was drawn to the S. Typhimurium Psp system when it was found to be induced upon inactivation of RpoE [27]. Experiments suggested that PspA was compensating for the absence of the RpoE ESR by preventing a large drop in the PMF. The Psp system is also essential for S. Typhimurium virulence, but the explanation suggests a very different role from that in Y. enterocolitica [28]. S. Typhimurium is an intracellular pathogen that survives inside macrophages within a modified phagosome or Salmonella-containing vacuole (SCV; Figure 1). As a defense mechanism, the host uses the Nramp1 transporter to deplete phagosomes of divalent cations needed by bacteria. However, S. Typhimurium has energy-dependent metal ion importers to counter Nramp1. In Nramp1-positive mice an S. Typhimurium ΔpspA in-frame deletion mutant is severely attenuated [28]. The proposed explanation lies in the role of PspA in maintaining the PMF and so ensuring the energy to drive the metal ion importers. Consistent with this, in Nramp1-negative mice the ΔpspA mutation does not affect virulence [28]. Similarly, a ΔpspA in-frame deletion mutation has only a small effect on virulence of the extracellular pathogen Y. enterocolitica [22].
If PspA maintains the PMF of S. Typhimurium inside macrophages it could also be important for reasons beyond supplying energy to cation pumps. Lee and Groisman showed recently that the acidic pH inside the SCV drives increased ATP synthesis and elevates cytosolic ATP levels in S. Typhimurium [29]. The leader mRNA of the mgtCBR operon senses this elevated ATP concentration, inducing its expression and the production of proteins required for survival inside macrophages. If the absence of PspA decreases S. Typhimurium PMF inside macrophages it might also reduce PMF-driven ATP synthesis. This raises the intriguing but untested possibility that PspA might be important to ensure the induction of virulence genes such as mgtCBR.
Other Links between the Psp Response and Virulence-Associated Processes
Observations suggest additional medically relevant roles for the Psp response (Figure 1). First, the Shigella flexneri pspA operon is highly induced during macrophage infection [30]. This suggests the possibility of a role for the Psp system in other intracellular pathogens, even though S. flexneri behaves differently from S. Typhimurium by escaping into the host cell cytoplasm. Second, the Psp system is important for biofilm formation by E. coli K-12 [31]. If this extends to pathogens there could be a connection between the Psp system and biofilm-mediated disease. Third, the Psp system has been linked to persisters in E. coli [32]. Persisters are dormant antibiotic-resistant cells implicated in chronic and recurrent infections. E. coli persisters can be induced by indole, a molecule produced upon entry into stationary phase. Indole also induces pspA operon expression, and a ΔpspBC mutant is defective for indole-induced persister formation [32]. The mechanism has not yet been investigated. Nevertheless, this exciting finding reveals a role for the Psp system in a phenomenon thought to have broad clinical significance.
It is becoming apparent that the Psp response has various fingers in various pies related to bacterial virulence. It seems likely that this extends beyond the examples highlighted here. Thus, the Psp system might represent an Achilles' heel for many pathogens. The challenge is to elucidate the molecular mechanisms underlying Psp protein functions and so understand how they affect the function and regulation of so many diverse virulence-associated phenomena.
Funding Statement
Research in the Darwin laboratory is supported by the National Institutes of Health grant R01-AI052148. AJD holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Darwin AJ (2005) The phage-shock-protein response. Mol Microbiol 57: 621–628. [DOI] [PubMed] [Google Scholar]
- 2. Joly N, Engl C, Jovanovic G, Huvet M, Toni T, et al. (2010) Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiol Rev 34: 797–827. [DOI] [PubMed] [Google Scholar]
- 3. Model P, Jovanovic G, Dworkin J (1997) The Escherichia coli phage-shock-protein (psp) operon. Mol Microbiol 24: 255–261. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi S, Darwin AJ (2012) Recent findings about the Yersinia enterocolitica phage shock protein response. J Microbiol 50: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raivio TL (2005) Envelope stress responses and Gram-negative bacterial pathogenesis. Mol Microbiol 56: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 6. Rowley G, Spector M, Kormanec J, Roberts M (2006) Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat Rev Microbiol 4: 383–394. [DOI] [PubMed] [Google Scholar]
- 7. MacRitchie DM, Buelow DR, Price NL, Raivio TL (2008) Two-component signaling and Gram negative envelope stress response systems. Adv Exp Med Biol 631: 80–110. [DOI] [PubMed] [Google Scholar]
- 8. MacRitchie DM, Acosta N, Raivio TL (2012) DegP is involved in Cpx-mediated posttranscriptional regulation of the type III secretion apparatus in enteropathogenic Escherichia coli . Infect Immun 80: 1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitobe J, Arakawa E, Watanabe H (2005) A sensor of the two-component system CpxA affects expression of the type III secretion system through posttranscriptional processing of InvE. J Bacteriol 187: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakayama S, Watanabe H (1995) Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol 177: 5062–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gal-Mor O, Segal G (2003) Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila . J Bacteriol 185: 4908–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin DW, Schurr MJ, Mudd MH, Govan JR, Holloway BW, et al. (1993) Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci U S A 90: 8377–8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, Thanikkal EJ, Obi IR, Francis MS (2012) Elevated CpxR∼P levels repress the Ysc-Yop type III secretion system of Yersinia pseudotuberculosis . Res Microbiol 163: 518–530. [DOI] [PubMed] [Google Scholar]
- 14. Vogt SL, Raivio TL (2012) Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol Lett 326: 2–11. [DOI] [PubMed] [Google Scholar]
- 15. Brissette JL, Russel M, Weiner L, Model P (1990) Phage shock protein, a stress protein of Escherichia coli . Proc Natl Acad Sci U S A 87: 862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huvet M, Toni T, Sheng X, Thorne T, Jovanovic G, et al. (2011) The evolution of the phage shock protein response system: interplay between protein function, genomic organization, and system function. Mol Biol Evol 28: 1141–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamaguchi S, Gueguen E, Horstman NK, Darwin AJ (2010) Membrane association of PspA depends on activation of the phage-shock-protein response in Yersinia enterocolitica . Mol Microbiol 78: 429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamaguchi S, Reid DA, Rothenberg E, Darwin AJ (2013) Changes in Psp protein binding partners, localization and behaviour upon activation of the Yersinia enterocolitica phage shock protein response. Mol Microbiol 87: 656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleerebezem M, Crielaard W, Tommassen J (1996) Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the protonmotive force under stress conditions. EMBO J 15: 162–171. [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi R, Suzuki T, Yoshida M (2007) Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol 66: 100–109. [DOI] [PubMed] [Google Scholar]
- 21. Darwin AJ, Miller VL (1999) Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol 32: 51–62. [DOI] [PubMed] [Google Scholar]
- 22. Darwin AJ, Miller VL (2001) The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol Microbiol 39: 429–444. [DOI] [PubMed] [Google Scholar]
- 23. Guilvout I, Chami M, Engel A, Pugsley AP, Bayan N (2006) Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. EMBO J 25: 5241–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horstman NK, Darwin AJ (2012) Phage shock proteins B and C prevent lethal cytoplasmic membrane permeability in Yersinia enterocolitica . Mol Microbiol 85: 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lloyd LJ, Jones SE, Jovanovic G, Gyaneshwar P, Rolfe MD, et al. (2004) Identification of a new member of the phage shock protein response in Escherichia coli, the phage shock protein G (PspG). J Biol Chem 279: 55707–55714. [DOI] [PubMed] [Google Scholar]
- 26. Seo J, Savitzky DC, Ford E, Darwin AJ (2007) Global analysis of tolerance to secretin-induced stress in Yersinia enterocolitica suggests that the phage-shock-protein system may be a remarkably self-contained stress response. Mol Microbiol 65: 714–727. [DOI] [PubMed] [Google Scholar]
- 27. Becker LA, Bang I, Crouch M, Fang FC (2005) Compensatory role of PspA, a member of the phage shock protein operon, in rpoE mutant Salmonella enterica serovar Typhimurium. Mol Microbiol 56: 1004–1016. [DOI] [PubMed] [Google Scholar]
- 28. Karlinsey JE, Maguire ME, Becker LA, Crouch ML, Fang FC (2010) The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium. Mol Microbiol 78: 669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee EJ, Groisman EA (2012) Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature 486: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lucchini S, Liu H, Jin Q, Hinton JC, Yu J (2005) Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect Immun 73: 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beloin C, Valle J, Latour-Lambert P, Faure P, Kzreminski M, et al. (2004) Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol Microbiol 51: 659–674. [DOI] [PubMed] [Google Scholar]
- 32. Vega NM, Allison KR, Khalil AS, Collins JJ (2012) Signaling-mediated bacterial persister formation. Nat Chem Biol 8: 431–433. [DOI] [PMC free article] [PubMed] [Google Scholar]