Abstract
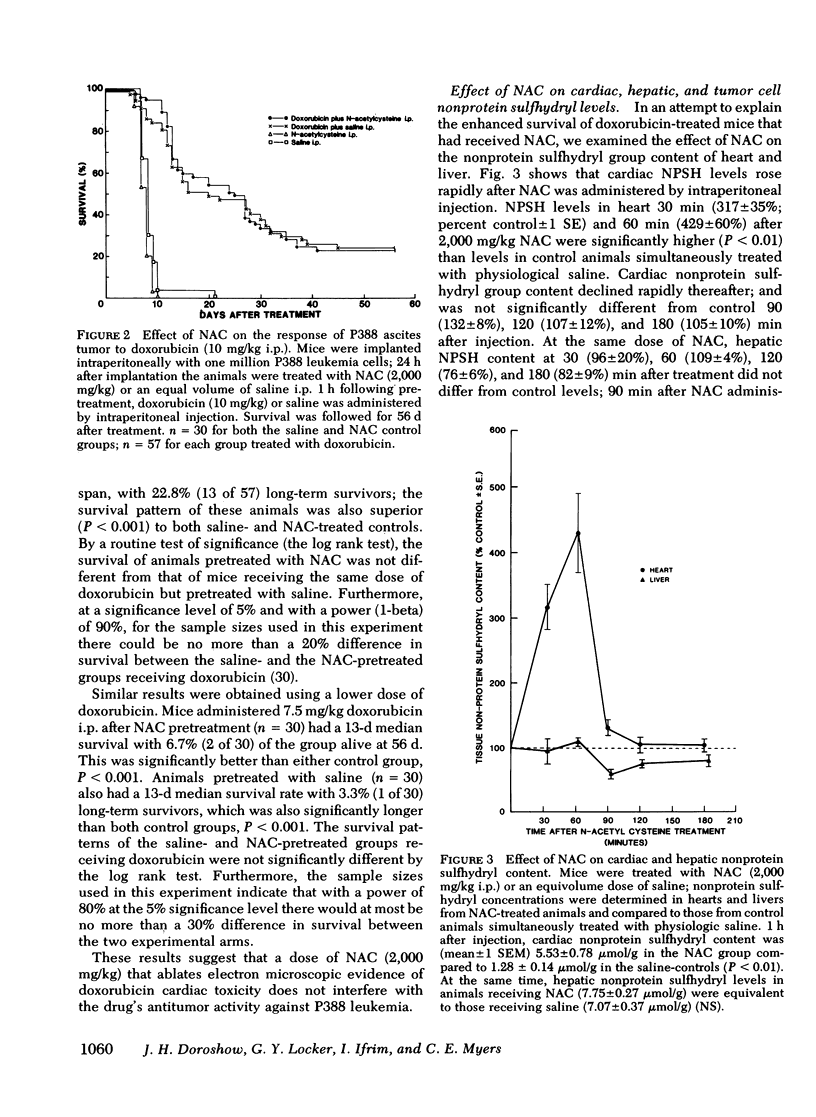
This study was undertaken to investigate the effect of exogenous sulfhydryl compound administration on the toxicity of doxorubicin in mice. Pretreatment of CDF1 mice with a pharmacologic dose (2,000 mg/kg) of n-acetyl-l-cysteine 1 h before doxorubicin (20 mg/kg, i.p.) decreased lethality from 100% (n = 44) to 37.7% (n = 53), P less than 0.001. Variation in the timing and dose of n-acetylcysteine significantly diminished its protective activity. Pretreatment with n-acetylcysteine also significantly reduced long-term mortality in animals receiving multiple doses of doxorubicin; 10 wk after the third of three doxorubicin doses (5 mg/kg, i.p.) administered at 2-wk intervals, survival in the n-acetylcysteine pretreated group was 51.4% (n = 35) compared with 16.7% (n = 30) for animals receiving saline before doxorubicin, P less than 0.01. In this experiment, n-acetylcysteine pretreatment also diminished doxorubicin-related losses in total body weight and heart wet weight by 55.2% (P less than 0.05), and 60.9% (P less than 0.02), respectively, compared with animals pretreated with saline. N-acetylcysteine pretreatment also ablated electron microscopic evidence of doxorubicin cardiomyopathy without alleviating morphological features of its toxic effects on the liver or small intestinal mucosa. The cardioprotective action of n-acetylcysteine may be partially explained by the 429 +/- 60% increase in cardiac nonprotein sulfhydryl content (P less than 0.01) that was measured one hour after n-acetylcysteine administration; nonprotein sulfhydryl concentration in the liver at the same time was insignificantly different from control levels. Treatment with n-acetylcysteine also increased the nonprotein sulfhydryl content of P388 leukemia cells nearly threefold; however, it did not after the chemotherapeutic activity of doxorubicin against this murine tumor. Whereas n-acetylcysteine blocked doxorubicin cardiac toxicity, it did not affect the uptake or metabolism of doxorubicin in the heart or liver. These results suggest that the concentration of free sulfhydryl groups in the heart may play a role in the development of doxorubicin cardiac toxicity and that augmenting cardiac nonprotein sulfhydryl group content with n-acetylcysteine may provide a means to enhance the chemotherapeutic index of doxorubicin.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. A., Buchanan J. D. Reactions of O-.2, H2O2 and other oxidants with sulfhydryl enzymes. Photochem Photobiol. 1978 Oct-Nov;28(4-5):743–755. doi: 10.1111/j.1751-1097.1978.tb07011.x. [DOI] [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- Ball C. R. Estimation and identification of thiols in rat spleen after cysteine or glutathione treatment: relevance to protection against nitrogen mustards. Biochem Pharmacol. 1966 Jul;15(7):809–816. doi: 10.1016/0006-2952(66)90157-2. [DOI] [PubMed] [Google Scholar]
- Benjamin R. S., Riggs C. E., Jr, Bachur N. R. Plasma pharmacokinetics of adriamycin and its metabolites in humans with normal hepatic and renal function. Cancer Res. 1977 May;37(5):1416–1420. [PubMed] [Google Scholar]
- Bertazzoli C., Bellini O., Magrini U., Tosana M. G. Quantitative experimental evaluation of adriamycin cardiotoxicity in the mouse. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):1877–1883. [PubMed] [Google Scholar]
- Bradner W. T., Schurig J. E., Huftalen J. B., Doyle G. J. Evaluation of antitumor drug side effects in small animals. Cancer Chemother Pharmacol. 1980;4(2):95–101. doi: 10.1007/BF00254029. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Mason J. W., Billingham M. E., Daniels J. R. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978 Feb;88(2):168–175. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Thompson P. D., Martin R. P., Mason J. W., Billingham M. E., Harrison D. C. Early anthracycline cardiotoxicity. Am J Med. 1978 Nov;65(5):823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- Burton G. M., Henderson C. A., Balcerzak S. P., Sagone A. L., Jr Effect of adriamycin on the metabolism of heart slices. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1287–1289. doi: 10.1016/0360-3016(79)90655-2. [DOI] [PubMed] [Google Scholar]
- Carter S. K. Adriamycin-a review. J Natl Cancer Inst. 1975 Dec;55(6):1265–1274. doi: 10.1093/jnci/55.6.1265. [DOI] [PubMed] [Google Scholar]
- Casagrande J. T., Pike M. C. An improved approximate formula for calculating sample sizes for comparing two binomial distributions. Biometrics. 1978 Sep;34(3):483–486. [PubMed] [Google Scholar]
- Chan K. K., Wong C. D. Quantitative thin-layer chromatography: thin-film fluorescence scanning analysis of adriamycin and metabolites in tissue. J Chromatogr. 1979 Apr 21;172:343–349. doi: 10.1016/s0021-9673(00)90971-3. [DOI] [PubMed] [Google Scholar]
- Chou T., Hill E. J., Bartle E., Woolley K., LeQuire V., Olson W., Roelofs R., Park J. H. Beneficial effects of penicillamine treatment on hereditary avian muscular dystrophy. J Clin Invest. 1975 Oct;56(4):842–849. doi: 10.1172/JCI108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Baldinger J., Myers C. E. The effect of doxorubicin on hepatic and cardiac glutathione. Res Commun Chem Pathol Pharmacol. 1979 Nov;26(2):285–295. [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Myers C. E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980 Jan;65(1):128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V. J. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treat Rep. 1978 Jun;62(6):955–961. [PubMed] [Google Scholar]
- Freeman R. W., MacDonald J. S., Olson R. D., Boerth R. C., Oates J. A., Harbison R. D. Effect of sulfhydryl-containing compounds on the antitumor effects of adriamycin. Toxicol Appl Pharmacol. 1980 Jun 15;54(1):168–175. doi: 10.1016/0041-008x(80)90018-6. [DOI] [PubMed] [Google Scholar]
- Freiman J. A., Chalmers T. C., Smith H., Jr, Kuebler R. R. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. N Engl J Med. 1978 Sep 28;299(13):690–694. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- Galvin M. J., Lefer A. M. Salutary effects of cysteine on cardiogenic shock in cats. Am J Physiol. 1978 Dec;235(6):H657–H663. doi: 10.1152/ajpheart.1978.235.6.H657. [DOI] [PubMed] [Google Scholar]
- Gardner H. W., Kleiman R., Weisleder D., Inglett G. E. Cysteine adds to liquid hydroperoxide. Lipids. 1977 Aug;12(8):655–660. doi: 10.1007/BF02533760. [DOI] [PubMed] [Google Scholar]
- Gardner H. W., Weisleder D. Addition of N-acetylcysteine to linoleic acid hydroperoxide. Lipids. 1976 Feb;11(2):127–134. doi: 10.1007/BF02532662. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Oki T., Inui T. A proposed reaction mechanism for the enzymatic reductive cleavage of glycosidic bond in anthracycline antibiotics. J Antibiot (Tokyo) 1979 Nov;32(11):1219–1222. doi: 10.7164/antibiotics.32.1219. [DOI] [PubMed] [Google Scholar]
- Kosower E. M. A role for glutathione in muscle contraction. Experientia. 1970;26(7):760–761. doi: 10.1007/BF02232532. [DOI] [PubMed] [Google Scholar]
- Lorber A., Chang C. C., Masuoka D., Meacham I. Effect of thiols in biological systems on protein sulfhydryl content. Biochem Pharmacol. 1970 May;19(5):1551–1560. doi: 10.1016/0006-2952(70)90143-7. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Mimnaugh E. G., Siddik Z. H., Drew R., Sikic B. I., Gram T. E. The effects of alpha-tocopherol on the toxicity, disposition, and metabolism of adriamycin in mice. Toxicol Appl Pharmacol. 1979 Jun 15;49(1):119–126. doi: 10.1016/0041-008x(79)90284-9. [DOI] [PubMed] [Google Scholar]
- Myers C. E., McGuire W. P., Liss R. H., Ifrim I., Grotzinger K., Young R. C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977 Jul 8;197(4299):165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Patnode R., Bartle E., Hill E. J., LeQuire V., Park J. H. Enzymological studies on hereditary avian muscular dystrophy. J Biol Chem. 1976 Jul 25;251(14):4468–4475. [PubMed] [Google Scholar]
- Prothero J. Heart weight as a function of body weight in mammals. Growth. 1979 Sep;43(3):139–150. [PubMed] [Google Scholar]
- Rosenoff S. H., Brooks E., Bostick F., Young R. C. Alterations in DNA synthesis in cardiac tissue induced by adriamycin in vivo-relationship to fatal toxicity. Biochem Pharmacol. 1975 Oct 15;24(20):1898–1901. doi: 10.1016/0006-2952(75)90413-x. [DOI] [PubMed] [Google Scholar]
- Schwartz H. S. A fluorometric assay for daunomycin and adriamycin in animal tissues. Biochem Med. 1973 Jun;7(3):396–404. doi: 10.1016/0006-2944(73)90060-4. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Sheffner A. L., Medler E. M., Bailey K. R., Gallo D. G., Mueller A. J., Sarett H. P. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966 Oct;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- Sonneveld P. Effect of alpha-tocopherol on the cardiotoxicity of adriamycin in the rat. Cancer Treat Rep. 1978 Jul;62(7):1033–1036. [PubMed] [Google Scholar]
- Thayer W. S. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem Biol Interact. 1977 Dec;19(3):265–278. doi: 10.1016/0009-2797(77)90050-3. [DOI] [PubMed] [Google Scholar]
- Van Vleet J. F., Greenwood L. A., Ferrans V. J. Pathologic features of adriamycin toxicosis in young pigs: nonskeletal lesions. Am J Vet Res. 1979 Nov;40(11):1537–1552. [PubMed] [Google Scholar]
- Wang Y. M., Madanat F. F., Kimball J. C., Gleiser C. A., Ali M. K., Kaufman M. W., van Eys J. Effect of vitamin E against adriamycin-induced toxicity in rabbits. Cancer Res. 1980 Apr;40(4):1022–1027. [PubMed] [Google Scholar]



