Abstract
OBJECTIVE
Periventricular white matter injury (PWMI), a precursor of cerebral palsy, is traditionally not diagnosed until 6 weeks of life by head ultrasound. We sought to determine if early neonatal glial fibrillary acidic protein (GFAP) levels could identify PWMI in low birth weight (< 2500 grams) infants.
STUDY DESIGN
Each case with PWMI on head ultrasound at 6 weeks from 4/09-4/11 was matched by gestational age and mode of delivery to 2 subsequent neonates with a normal head ultrasound. GFAP was measured in cord blood at birth, at neonatal intensive care unit (NICU) admission, and daily on days 1–4 of life.
RESULTS
During this 2 year period, 21 cases with PWMI with gestational age 27.4±3.3 weeks were compared to 42 controls. The incidence of cesarean delivery was 61.9% in both groups. GFAP was not significantly different in cord blood or at NICU admission, but was significantly elevated on day 1 (median, 5%–95%; 0, 0–0.98 ng/mL cases; 0,0–0.06 ng/mL controls, P=0.03), day 2 (0, 0–1.21 ng/mL; 0, 0–0.05 ng/mL; P=0.02), day 3 (0.05, 0–0.33 ng/mL; 0, 0–0.04 ng/mL, P=0.004) and day 4 (0.02, 0–1.03 ng/mL; 0, 0–0.05 ng/mL, P<0.001). The odds of developing PWMI significantly increased with increasing levels of GFAP from day 1 to day 4 of life adjusting for preeclampsia, antenatal steroid administration and neonatal chronic lung disease.
CONCLUSION
The ability to predict PWMI with a blood test for GFAP shortly after birth opens the possibility for rapid identification of infants for early intervention and provides a benchmark for qualifying new therapies to improve neurodevelopmental outcomes.
Keywords: Periventricular white matter injury, Glial fibrillary acidic protein, Cerebral palsy
Introduction
As a consequence of preterm birth, low birth weight infants (LBW, <2500 grams) are at increased risk for a spectrum of cerebral white matter abnormalities termed periventricular white matter injury (PWMI). Head ultrasound imaging is routinely performed in premature neonates in the first week of life to rule out intraventricular hemorrhage (IVH) and at 6 weeks for identification of PWMI. The incidence of PWMI is higher in infants who sustain IVH possibly due to the hemorrhage providing a rich source of iron for the generation of reactive oxygen species leading to oligodendrocyte death.(1) When PWMI is present it is associated with cerebral palsy in 60–100% of survivors.(2) Cerebral palsy affects 6–9% of infants born at less than 32 weeks and as many as 28% of infants born at less than 26 weeks.(3)
The fetal transition at birth is known to be a particularly vulnerable time for the preterm infant brain; however we lack the means to objectively identify infants at risk for long term neurologic injury in the early neonatal period. Currently available tools to identify the neonate at risk for encephalopathy shortly after birth include fetal heart rate tracings, Apgar scores, umbilical cord gases and physical exam of the newborn, all of which lack precision.
In adult brain injury, such as traumatic brain injury and stroke, measurement of circulating brain proteins have demonstrated significant diagnostic and prognostic potential.(4–7) A number of proteins, including the highly brain-specific glial fibrillary acidic protein (GFAP), have been used in adults and children to identify patients with stroke or traumatic brain injury in an effort to provide prognostic data on survival or density of residual deficits.(5) GFAP is a brain specific, cytoskeletal intermediate filament protein found in the astroglia of the central nervous system and is a specific marker of differentiated astrocytes. Serum GFAP is entirely derived from brain and not routinely secreted in blood, but is only released after cell injury or death. In adult patients who had sustained mild traumatic brain injury a relation was found between GFAP measured directly after admission, imaging studies and outcome determined by return to work.(8)
Our group has found that elevated serum GFAP levels in neonates undergoing extracorporeal membrane oxygenation (ECMO) were associated with acute brain injury and death.(9) We have also found that in term and near-term neonates with hypoxic-ischemic encephalopathy treated with whole body cooling, elevated GFAP levels upon admission to the neonatal intensive care unit (NICU) and through day 4 of life were predictive of brain MRI abnormalities at 7 days of life and abnormal neurodevelopmental outcomes.(10) Our aim in this study was to determine if GFAP levels measured within the first 4 days of life could be used to identify LBW neonates at risk for developing PWMI.
Materials and Methods
This was an institutional review board-approved case control study of all liveborn, non-anomalous LBW (< 2500 grams) neonates born at a single university hospital admitted to the neonatal intensive care unit (NICU). Neonates with major congenital malformations, chromosomal abnormalities or genetic syndromes were excluded. For GFAP studies cord blood and the residual unused portion of serum from daily routine clinical laboratory tests were used. We collected cord blood at delivery and the remaining portion of serum from daily neonatal blood draws during the 1st 4 days of life for all neonates admitted to our NICU weighing < 1500 grams for those with birth weights 1500–2500 grams with suspected neurologic morbidity at birth including prolonged hypotonia or seizures. The majority of these samples were obtained via heel stick, but some were obtained via umbilical arterial lines. Residual serum was aliquoted and stored at −80°C until assayed. After neonatal discharge we reviewed the results of head ultrasounds performed at 6 weeks of life to identify infants with PWMI who were then compared to the subsequent 2 infants with normal head ultrasounds at the same gestational age within 1 week and delivered by the same mode, matching in a 2:1 fashion. In addition, from this group we compared those that developed both IVH and PWMI to those with IVH only.
Our GFAP assay has previously been described.(9, 10) Case and control samples blinded to the neonatal head ultrasound results were assayed at the same time. Samples and standards were assayed in duplicate. Assays were analyzed on a Sector Imager 2400 (MesoScale Discovery) according to the manufacturer’s protocol. Values were reported out as the mean concentration of a single sample assayed in duplicate. GFAP concentrations are a continual range from the lower to the upper limit of quantification for the assay (0.04 ng/ml to 40 ng/ml).(9) GFAP values lower than 0.04 ng/ml, the lower limit of quantitation, were reported as 0. The GFAP assay as described has an inter-assay coefficient of variability of <2.5%. The intra-assay coefficient of variability ranged from 0.18–3.07%. GFAP values obtained via plasma and serum were equivalent.
All neonates had a head ultrasound within 1 week and at 2 weeks to rule out IVH, and a 3rd ultrasound at 6 weeks to rule out PWMI as per our standard clinical practice. Head ultrasounds were all performed in the NICU. Transfontanellar head ultrasonography was performed using state-of-the-art ultrasound equipment (Zonare, Medical Systems, Mountain View, CA). Standardized optimized sets of coronal and sagittal images were obtained through the anterior fontanel using curved and linear array transducers (8 to 17 MHz). Head ultrasounds were evaluated by 2 pediatric neuroradiologists (AT, TH) for overall white matter echointensity, gray-white matter differentiation, echointensity of the central gray matter, ventricular size (based on the largest ventricle size), germinal matrix hemorrhage (based on Papile’s classification from I–III)(11), periventricular hemorrhagic infarction (previously known as grade IV hemorrhage), and cystic periventricular leukomalacia. Diffuse white matter injury was defined as generalized white matter volume loss, with or without echointensity alterations. Focal white matter injury was defined as focal areas of decreased echointensity representing remote ischemia or hemorrhage.
Neonates with PWMI were scheduled for a comprehensive neonatal neurodevelopmental examination after discharge from the NICU as per our standard of care. This exam draws from the work of many researchers in the area and has been previously reported.(12) This exam is corrected for gestational age at birth and assessed the emergence of extremity flexor tone, axial (neck, trunk, shoulder and hip) tone, deep tendon reflexes, pathologic reflexes, primitive reflexes, postural head control and sensory responses. These exams were conducted by a single examiner (MCA) certified in pediatrics, neonatology, and neurodevelopmental disabilities. The diagnosis of cerebral palsy was based on a persistently abnormal neurological examination (e.g. spasticity and/or variable tone and/or persistent primitive and pathologic reflexes) and functional impairment (including abnormal quality of movement).(13) In the case group with abnormal head ultrasounds, GFAP levels were compared between infants that developed cerebral palsy and those that did not.
Infant and maternal medical records were reviewed to identify relevant clinical data. Intrauterine growth restriction was defined as an estimated fetal weight less than the 10th percentile.(14) Oligohydramnios was defined as an amniotic fluid index (AFI) < 5.0 cm at the time of the admission at which delivery occurred. Preeclampsia was defined as proteinuria, edema, and the presence of new onset hypertension. The clinical diagnosis of chorioamnionitis was made in the presence of maternal fever, with the presence of at least one other finding of fetal tachycardia, uterine tenderness, or purulent vaginal discharge. Patients diagnosed with clinical chorioamnionitis were immediately started on intravenous ampicillin and gentamicin if not allergic. All the placentas in the study were examined by an attending pathologist at our institution. Histologic chorioamnionitis was diagnosed when any polymorphonuclear leukocytes were seen in either the chorion or amnion, or in significant amounts in the subchorionic space. Histologic funisitis was diagnosed when polymorphonuclear leukocytes were seen in the umbilical cord. The diagnosis of nonreassuring fetal heart rate (FHR) tracing was made by the physician attending delivery prior to performing a cesarean.
All statistical analyses were conducted using STATA (STATA Intercooled, version 12.0; STATA Corp., College Station, TX). Comparisons of cases of PWMI and matched controls on demographic and clinical characteristics were conducted to ensure the success of the matching and to identify differences between the two groups. Linear regression was used to determine the association between gestational age and GFAP levels in the control population. Multivariable conditional logistic regression was conducted to determine whether GFAP levels in the first four days of life predicted PWMI at six weeks adjusting for differences between cases and controls. Cases with both IVH and PWMI were compared to neonates with IVH only using independent samples t-tests, chi square and Wilcoxon rank-sum as appropriate. GFAP was compared within the PWMI cases based on the later diagnosis of cerebral palsy using Wilcoxon rank-sum. Statistical significance was determined by a confidence interval that did not include 1.0 and a P value less than 0.05.
Results
During the two year period from April 2009 to April 2011 there were 177 inborn very low birth weight (VLBW) neonates without major congenital malformations admitted to our NICU of which 18 cases of PWMI were identified, for an incidence of 10.2%. There were 221 inborn, nonanomalous LBW neonates with birth weight 1500–2500 grams admitted to the NICU during this period of which 3 (1.4%) developed PWMI. These 21 cases with PWMI were matched to 42 controls with normal head ultrasounds. The mean gestational age and standard deviation for cases and controls were 27.4 ± 3.3 weeks, and 27.4 ± 3.2 weeks respectively. The incidence of cesarean delivery was 61.9% in both groups. Comparisons between cases and matched controls on maternal demographic and clinical characteristics identified a number of similarities and differences.(Table 1) Similarities (i.e. non-statistical differences) between cases and matched controls included the incidence of twin gestation, IUGR and clinical and histologic chorioamnionitis. Cases were significantly less likely to have preeclampsia and to have received antenatal steroids prior to delivery as compared to matched controls.
Table 1.
Univariate analysis comparing maternal variables between cases with periventricular white matter injury on head ultrasound at 6 weeks of life and controls matched by gestational age with normal head ultrasounds
| Cases N=21 |
Controls N=42 |
P value | |
|---|---|---|---|
| Maternal Age (years) | 27.8±7.2 | 28.1±7.4 | 0.86 |
| Parity (median) | 1 | 1 | 0.65 |
| Race | 0.83 | ||
| White | 6 (28.6%) | 9 (21.4%) | |
| Black | 13 (61.9%) | 30 (71.4%) | |
| Other | 2 (9.5%) | 3 (7.1%) | |
| Cesarean | 13 (61.9%) | 26 (61.9%) | 1.0 |
| Twins | 4 (19.0%) | 5 (11.9%) | 0.49 |
| Preterm labor | 14 (66.7%) | 19 (45.2%) | 0.16 |
| PPROMa | 5 (23.8%) | 14 (33.3%) | 0.49 |
| Preeclampsia | 2 (9.5%) | 15 (35.7%) | 0.03* |
| Antenatal Magnesium | 8 (38.1%) | 22 (52.4%) | 0.29 |
| IUGRb | 0 | 7 (16.7%) | 0.09 |
| Oligohydramnios | 1 (4.8%) | 5 (11.9%) | 0.24 |
| Steroid administration | 12 (57.1%) | 35 (83.3%) | 0.03* |
| Abruption | 5 (23.8%) | 4 (9.5%) | 0.15 |
| Nonreassuring fetal heart rate tracing | 6 (28.6%) | 5 (11.9%) | 0.11 |
| Clinical chorioamnionitis | 1 (4.8%) | 11 (26.2%) | 0.08 |
| Histologic chorioamnionitis | 3 (14.3%) | 16 (38.1%) | 0.053 |
| Histologic funisitis | 2 (9.5%) | 8 (19.0%) | 0.21 |
| Histologic placental infarct | 1 (4.8%) | 5 (11.9%) | 0.73 |
indicates p<0.05
PPROM = preterm premature rupture of membranes
IUGR = intrauterine growth restriction
Neonatal characteristics often associated with prematurity were also compared between cases and matched controls.(Table 2) The cases and controls were similar with regard to birth weight, Apgar scores, umbilical arterial cord gases, and culture positive sepsis. During the first 4 days of life there was no difference in hypocarbia or hyperoxia. During their neonatal hospitalization there was no difference in indocin exposure or need for high frequency ventilation. Cases with abnormal head ultrasounds were significantly more likely to have IVH and seizures. Cases had a higher incidence of chronic lung disease as compared to matched controls.
Table 2.
Univariate analysis comparing neonatal variables between cases with periventricular white matter injury on head ultrasound at 6 weeks of life and controls matched by gestational age with normal head ultrasounds
| Cases N=21 |
Controls N=42 |
P value | |
|---|---|---|---|
| Birth Weight (grams) | 1077±544 | 1015±458 | 0.41 |
| 1 minute Apgar < 7 | 20 (95.2%) | 40 (95.2%) | 1.0 |
| 5 minute Apgar < 7 | 14 (66.7%) | 17 (40.5%) | 0.06 |
| Cord pH | 7.22±0.19 | 7.27±0.12 | 0.36 |
| Cord Base Deficit (mM) | 4.4±6.7 | 2.4±2.1 | 0.19 |
| Cord pH < 7.0 & Base deficit > 12 mM | 3/17 (11.8%) | 2/32 (6.3%) | 0.41 |
| Lowest arterial pCO2 (mm Hg) | 32±7 | 32±7 | 0.91 |
| Highest arterial pO2 (mm Hg) | 105±31 | 100±58 | 0.58 |
| Indocin exposure | 6 (28.6%) | 14 (33.3%) | 0.33 |
| High frequency ventilation | 14 (66.7%) | 22 (52.4%) | 0.11 |
| Neonatal length of stay (days) | 72.0±39.1 | 58.3±42.4 | 0.22 |
| Respiratory distress syndrome | 19 (90.5%) | 41 (97.6%) | 0.26 |
| Chronic lung disease | 15 (71.4%) | 20 (47.6%) | 0.04* |
| Necrotizing enterocolitis | 2 (9.5%) | 6 (14.3%) | 0.56 |
| Death | 0 | 4 (9.5%) | 0.29 |
| Intraventricular hemorrhage | 19 (90.5%) | 11 (26.2%) | <0.001* |
| Seizures | 9 (42.9%) | 0 | <0.001* |
| + Blood culture | 2 (9.5%) | 6 (14.3%) | 0.58 |
| + Cerebrospinal fluid culture | 2 (9.5%) | 0 | 0.11 |
| + Urine culture | 2 (9.5%) | 3 (7.1%) | 0.76 |
indicates p<0.05
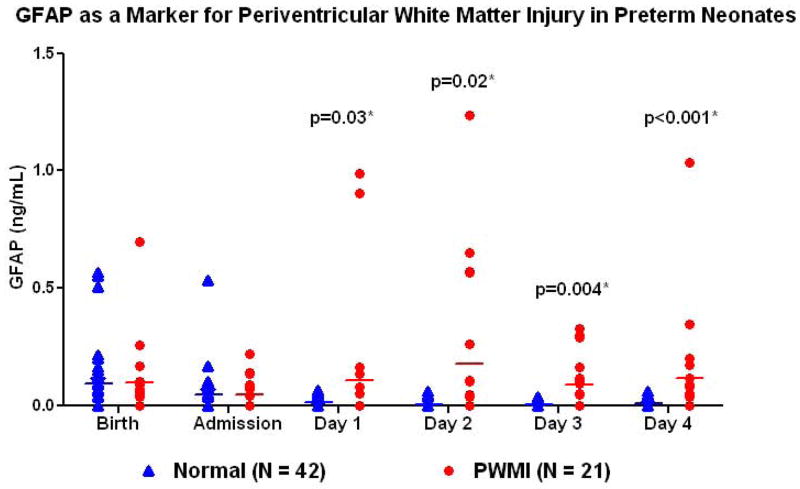
Serum GFAP levels were available for comparison at 111/126 (88.1%) and 212/252 (84.2%) of the desired time points for cases and controls respectively. Missing data were due to inability to obtain cord blood at delivery, insufficient serum after clinically indicated tests had been performed, or absence of blood being drawn on some days. GFAP was not significantly different in cord blood at birth (median, 5%–95%; 0.06, 0–0.7 ng/mL cases; 0.04, 0–0.56 ng/mL controls; p=0.97) or at NICU admission (0, 0–0.22 ng/mL; 0, 0–0.37 ng/mL; p=0.98), but was significantly increased on day 1 (0, 0–0.98 ng/mL; 0,0–0.06 ng/mL, p=0.03), day 2 (0, 0–1.21 ng/mL; 0, 0–0.05 ng/mL; p=0.02), day 3 (0.05, 0–0.33 ng/mL; 0, 0–0.04 ng/mL, p=0.004) and day 4 (0.02, 0–1.03 ng/mL; 0, 0–0.05 ng/mL, p<0.001).(Figure 1) Multivariable conditional logistic regression showed that the odds of developing PWMI increased with increasing levels of GFAP from day 1 to day 4 of life adjusting for preeclampsia, antenatal steroid administration and neonatal chronic lung disease.(Table 3) GFAP levels did not significantly change with gestational age in the control infants on day 1 (R=0.31, P=0.08), day 2 (R=0.2, P=0.24), day 3 (R = 0.1, P=0.48) or day 4 (R=0.19, P=0.92). There was a significant increase in cases with detectable GFAP levels > 0.04 ng/mL on day 2 (8/20 (40%) cases; 2/37 (5.4%) controls, p=0.001), day 3 (9/17 (52.9%) cases; 0/36 controls, p<0.0001) and day of life 4 (8/18 (44.4%) cases; 3/30 (10%) controls, p=0.006). The sensitivity of detectable GFAP levels (> 0.04 ng/mL) in identifying PWMI on a head ultrasound at 6 weeks of life was 52.4% on day 1, 55.0% on day 2, 64.7% on day 3 and 66.7% on day 4; specificity was 91.2% on day 1, 91.9% on day 2, 94.4% on day 3 and 86.7% on day 4.
Figure 1.
GFAP as a biomarker for periventricular white matter injury (PWMI) in low birth weight neonates comparing cases with PWMI to controls with normal head ultrasounds at 6 weeks.
Table 3.
Multivariable conditional logistic regression adjusted for preeclampsia, antenatal steroid administration and chronic lung disease to determine if GFAP levels from birth, neonatal intensive care unit (NICU) admission and during the 1st 4 days of life predict periventricular white matter injury on head ultrasound at 6 weeks of life.
| Time | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Birth | 3.58 | −2.96 – 10.1 | 0.28 |
| NICU Admission | 14.5 | −3.0 – 31.9 | 0.10 |
| Day 1 | 32.2 | 2.97 – 61.4 | 0.03* |
| Day 2 | 27.8 | 4.74 – 50.8 | 0.02* |
| Day 3 | 46.6 | 14.5 – 78.7 | 0.004* |
| Day 4 | 424 | 324 – 524 | <0.001* |
indicates P < 0.05
This cases and controls were all preterm neonates <35 weeks gestation, none of which were candidates for treatment with whole body cooling, which is limited to those with suspected hypoxic-ischemic encephalopathy that are > 35 weeks gestation. Only 3/21 (14.3%) of the preterm cases in this study had an umbilical arterial gas at birth consistent with metabolic acidosis from intrapartum hypoxia-ischemia (pH < 7.0 and base deficit > 12 mM). The PWMI seen in these preterm neonates on a head ultrasound at 6 weeks was not related to intrapartum hypoxia-ischemia in the other 85.7% of cases since their cord gas was normal. We did not find a difference in GFAP levels within the case group when the 9 cases with seizures were compared to the 11 cases without seizures at any time point from birth to day 4 of life.(data not shown)
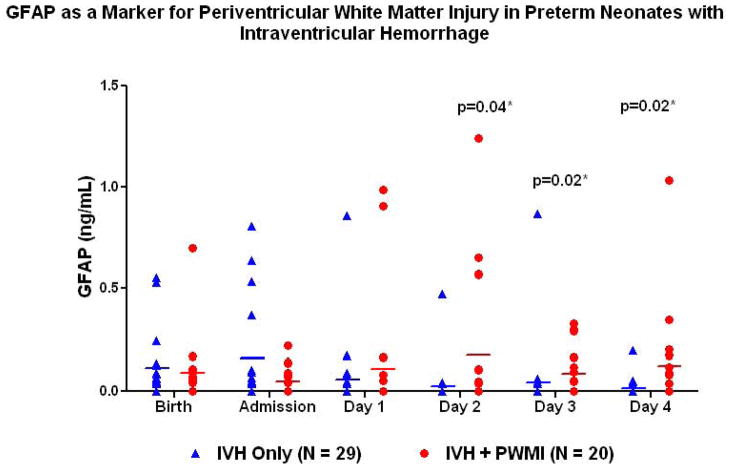
Among all neonates with IVH, those that later developed PWMI (n=20) were compared to those with IVH only (n=29). Neonates with IVH and PWMI did not differ from those with IVH only in gestational age (26.6±2.7, 27.1±2.8 weeks, P=0.52), birth weight (960±444, 952±366 grams, P=0.94) or incidence of cesarean delivery (60%, 48.3%, P=0.42). There was no difference in preeclampsia, steroid administration or chronic lung disease between the groups. Those with IVH and PWMI had a significantly higher incidence of seizures (45%, 6.9%, p=0.004) and severe (III or IV) grade (85%, 17.2%, p<0.0001). GFAP was significantly increased in those with IVH and PWMI on day of life 2–4 (P=0.04, 0.02, 0.02).(Figure 2)
Figure 2.
GFAP as a biomarker for periventricular white matter injury (PWMI) in low birth weight neonates with intraventricular hemorrhage (IVH) comparing cases with both PWMI and IVH to controls with IVH only.
We were able to obtain neurodevelopmental follow-up for 15/21 (71.4%) of the cases with PWMI. These neurodevelopmental exams were conducted at 14.4±6.6 months postnatally (range 7–27 months) and were corrected for gestational age at birth. Cerebral palsy was diagnosed in 12/15 (80%) of these infants. GFAP for the 3 cases without CP was 0 on day of life 1–4. The mean, 5% and 95% for GFAP levels for the 12 cases with CP was 0, 0–0.99 ng/mL on day 1 (P=0.27); 0, 0–0.57 ng/mL on day 2 (P=0.65); 0, 0–0.33 ng/mL on day 3 (P=0.26) and 0, 0–0.13 ng/mL on day 4 (P=0.31).
Comment
Although we chose to study LBW infants because of the high incidence of abnormal neurodevelopment in this population, the majority of the neonates we identified as having PWMI were VLBW, a population in which PWMI is the predominant form of structural neonatal brain damage and who have a 25–50% occurrence of neurodevelopmental deficits and a 5–10% occurrence of major motor deficits such as cerebral palsy.(15) Presently head ultrasound imaging at six weeks of age is the gold standard for the diagnosis of PWMI. MRI at term equivalent in very preterm infants has been shown to identify moderate to severe cerebral white matter abnormalities which strongly predict adverse neurodevelopmental outcome at 2 years of age;(16) however, at this time MRI is not standard for these infants. In the present study we demonstrate that levels of circulating GFAP on days 1–4 of life are significantly elevated in preterm neonates at risk for developing PWMI, and that even among those neonates with IVH, GFAP can identify which are at high risk for the later development of PWMI. This allows for the identification of these infants much sooner than by either head ultrasound or MRI.
Our controls were significantly more likely to have received antenatal steroids prior to delivery, which agrees with prior research showing a decreased risk for PWMI in preterm neonates that received antenatal betamethasone,(17) and emphasizes the importance of steroid administration in preterm neonates at risk for delivery. Neonates that receive antenatal steroids may have improved pulmonary function and be at lower risk for hypoxia which could decrease their risk for brain injury. Cases with PWMI were also significantly more likely to have chronic lung disease, a known risk factor for neurologic impairment in VLBW infants.(18) Neonatal chronic lung disease could be a source of hypoxia which could lead to brain injury. Controls were significantly more likely to have been delivered from mothers with preeclampsia, and there is evidence showing maternal preeclampsia may provide neonatal neuroprotection.(18) Intravenous magnesium sulfate is routinely given to mothers with preeclampsia to prevent seizures, and 3 large randomized placebo controlled trials have found that antenatal magnesium sulfate therapy reduced the risk of cerebral palsy in children that survived very early preterm birth.(19–21) There was no difference in magnesium exposure between the groups in our study because it has become standard practice in our center to administer intravenous magnesium sulfate for neuroprotection to any mother at risk for delivering at less than 32 weeks gestation.
Many new therapies for PWMI in the premature neonate are currently being investigated, providing the impetus to identify neonates at risk in the early postnatal period. An example is erythropoietin and its derivative carbamylated erythropoietin which have been shown to decrease the risk of cerebral white matter injury in mouse models of PWMI.(22) Stem cell treatments have been studied in animal models of cerebral palsy, and shown to produce beneficial effects.(23) Specific brain biomarkers such as GFAP could play an important role as early surrogate outcome measures and benchmarks for the efficacy of these interventions.
GFAP was not significantly elevated in the cord blood at birth or upon NICU admission in these preterm neonates later diagnosed with PWMI suggesting that brain injury was postnatal. Prenatally the fetus could be protected by having adequate cerebral blood flow in utero due to placental perfusion. Not until after delivery when the neonate must maintain their own cerebral autoregulation do hypotensive episodes occur which lead to astrocyte injury, and manifestations of this are not detectable by serum GFAP until the 1st day after birth. The 1st few days after birth are a critical period during which there may also be differences in ventilator support, maximal mean airway pressures, hyperoxia, hypocarbia and differences in drugs administered which could contribute to these injuries. No such differences were found between cases and controls in this study.
Because of very weak cerebrovascular autoregulation, cerebral perfusion in preterm infants is dependent on systemic blood pressure,(24) and pressure passivity increases with increasing degrees of prematurity in critically ill preterm neonates.(25) Blood pressure treatment in preterm neonates is empiric, and there are no guidelines for appropriate cerebral blood pressure management. Some studies have reported an association between hypotension due to neonatal sepsis and PWMI,(26) and others have reported neonatal sepsis alone to be associated with PWMI.(27) In our study we did not find clinical chorioamnionitis, histologic chorioamnionitis/funisitis or positive blood, cerebrospinal or urine cultures to be significantly more common in neonates with PWMI; however, our sample size may not be large enough to detect these possible differences. Although our sample size was large enough to find significant differences in GFAP levels based on head ultrasound abnormalities, we lacked adequate power to determine differences in GFAP based on the later diagnosis of cerebral palsy. Although there was a trend towards those neonates with cerebral palsy having higher GFAP levels, a larger sample size may have allowed us to detect statistically significant differences.
This study addresses one of the major problems in the care of preterm infants, that many now survive, but with neurological deficits. Although a head ultrasound performed at 6 weeks of life is the traditional tool used to identify infants with PWMI, a brain specific biomarker such as GFAP that could identify these infants shortly after birth would allow their identification and triage for therapy much earlier. A rapid perinatal test for neonatal brain injury would have great clinical utility in identifying infants who could benefit from investigational treatments for PWMI, quantifying therapeutic efficacy, and providing early prognostic information.
Clinical Implications.
Preterm neonates that develop periventricular white matter injury have elevated serum levels of glial fibrillary acidic protein on days 1–4 of life which may allow for the identification of these infants soon after birth rather than at the time of a head ultrasound performed at 6 weeks of life.
A rapid perinatal test that could identify neonates at risk for brain injury shortly after birth would allow these infants to be identified and triaged for therapy much earlier than is currently possible and may help quantify therapeutic efficacy and improve prognostic information.
Acknowledgments
This research was presented in abstract form and as a poster presentation at the 32nd Annual Meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, Feb. 9–11, 2012.
Footnotes
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–62. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Leviton A, Paneth N. White matter damage in preterm newborns--an epidemiologic perspective. Early Hum Dev. 1990;24:1–22. doi: 10.1016/0378-3782(90)90002-z. [DOI] [PubMed] [Google Scholar]
- 3.Milligan DW. Outcomes of children born very preterm in Europe. Arch Dis Child Fetal Neonatal Ed. 2010;95:F234–40. doi: 10.1136/adc.2008.143685. [DOI] [PubMed] [Google Scholar]
- 4.Wunderlich MT, Wallesch CW, Goertler M. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol. 2006;13:1118–23. doi: 10.1111/j.1468-1331.2006.01435.x. [DOI] [PubMed] [Google Scholar]
- 5.Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–10. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 6.Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65:778,82. doi: 10.1097/TA.0b013e318185db2d. discussion 782–4. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko T, Kasaoka S, Miyauchi T, Fujita M, Oda Y, Tsuruta R, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80:790–4. doi: 10.1016/j.resuscitation.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78:1428–33. doi: 10.1212/WNL.0b013e318253d5c7. [DOI] [PubMed] [Google Scholar]
- 9.Bembea MM, Savage WJ, Strouse JJ, Schwartz J, Graham EM, Thompson CEAD. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:572–9. doi: 10.1097/PCC.0b013e3181fe3ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ennen CS, Huisman TA, Savage WJ, Northington FJ, Jennings JM, Everett AD, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol. 2011;205:251, e1–7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 12.Allen MC, Aucott S, Cristofalo EA, Alexander GR, Donohue PK. Extrauterine neuromaturation of low risk preterm infants. Pediatr Res. 2009;65:542–7. doi: 10.1203/PDR.0b013e3181998b86. [DOI] [PubMed] [Google Scholar]
- 13.Allen MC, Alexander GR. Using motor milestones as a multistep process to screen preterm infants for cerebral palsy. Dev Med Child Neurol. 1997;39:12–6. doi: 10.1111/j.1469-8749.1997.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 14.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 15.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 17.Baud O, Foix-L’Helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341:1190–6. doi: 10.1056/NEJM199910143411604. [DOI] [PubMed] [Google Scholar]
- 18.Wilson-Costello D. Risk factors for neurologic impairment among very low-birth-weight infants. Semin Pediatr Neurol. 2001;8:120–6. doi: 10.1053/spen.2001.25228. [DOI] [PubMed] [Google Scholar]
- 19.Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–76. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 20.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marret S, Marpeau L, Follet-Bouhamed C, Cambonie G, Astruc D, Delaporte B, et al. Effect of magnesium sulphate on mortality and neurologic morbidity of the very-preterm newborn (of less than 33 weeks) with two-year neurological outcome: results of the prospective PREMAG trial. Gynecol Obstet Fertil. 2008;36:278–88. doi: 10.1016/j.gyobfe.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Shen Y, Plane JM, Pleasure DE, Deng W. Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia. Exp Neurol. 2011;230:227–39. doi: 10.1016/j.expneurol.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, et al. Stem cell therapy for neonatal brain injury: perspectives and challenges. Ann Neurol. 2011;70:698–712. doi: 10.1002/ana.22518. [DOI] [PubMed] [Google Scholar]
- 24.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011;31:722–9. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- 26.Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–64. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 27.Silveira RC, Procianoy RS, Dill JC, da Costa CS. Periventricular leukomalacia in very low birth weight preterm neonates with high risk for neonatal sepsis. J Pediatr (Rio J) 2008;84:211–6. doi: 10.2223/JPED.1777. [DOI] [PubMed] [Google Scholar]