Abstract
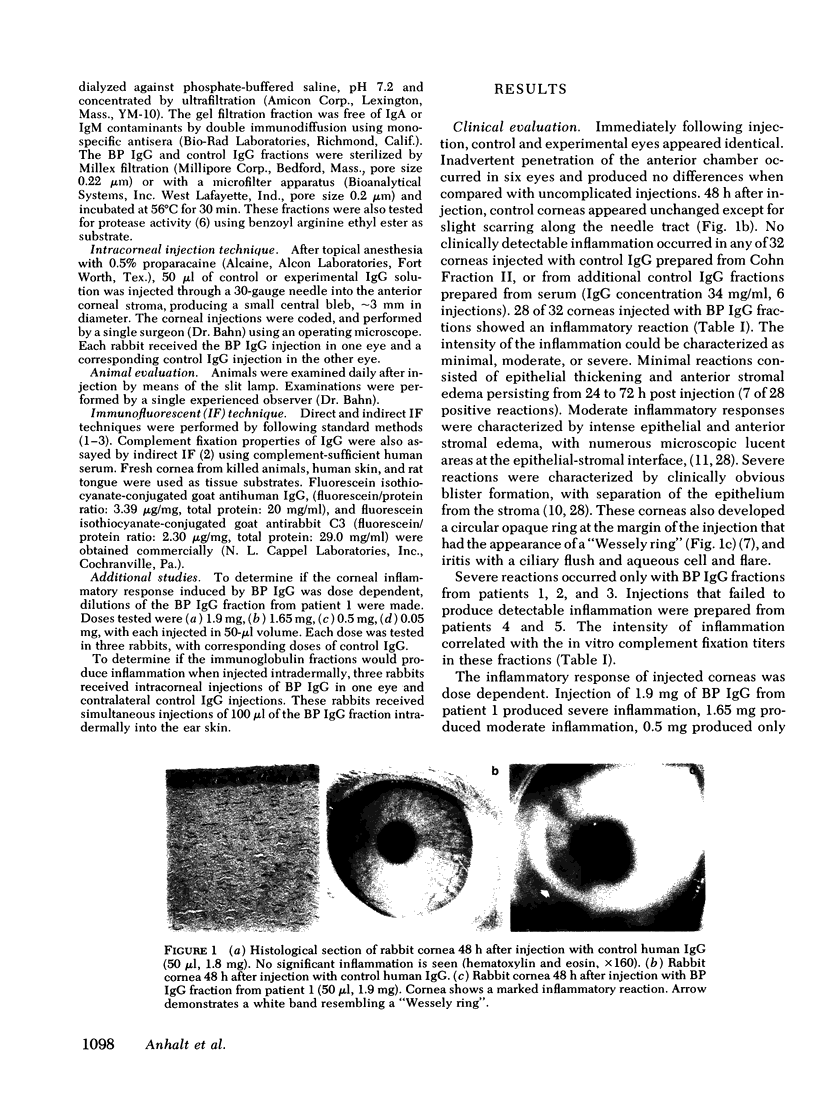
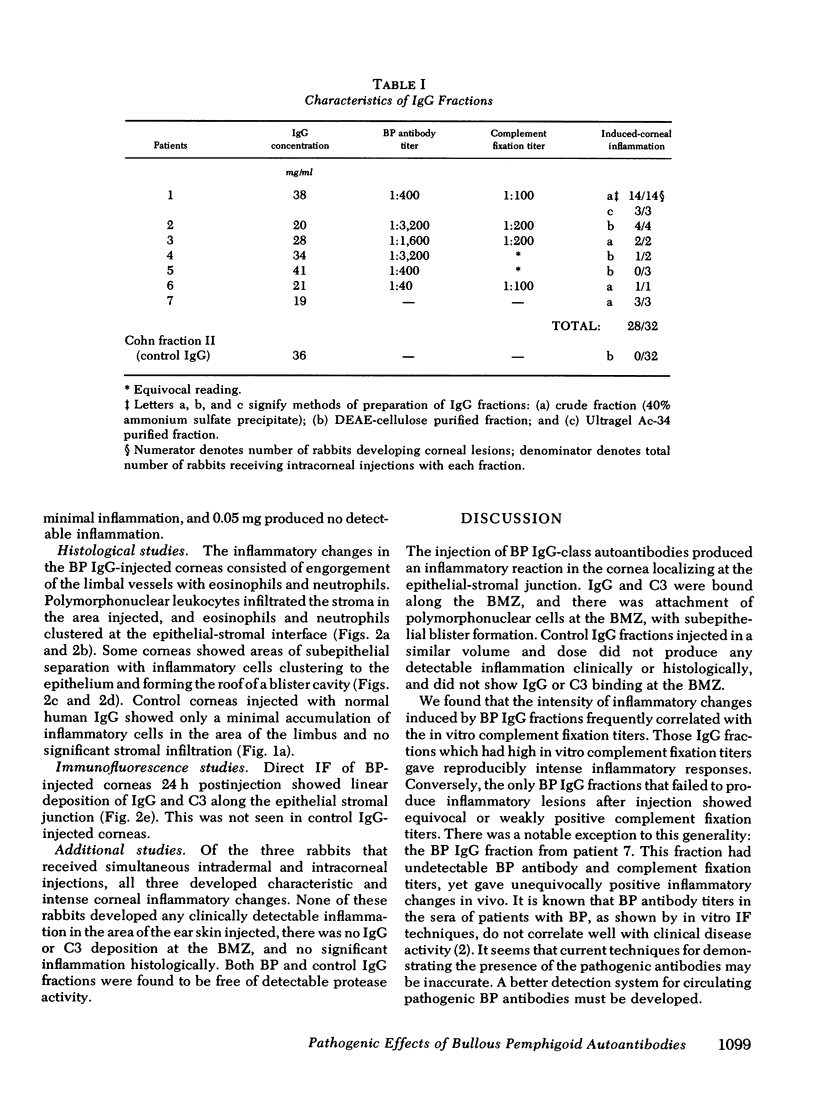
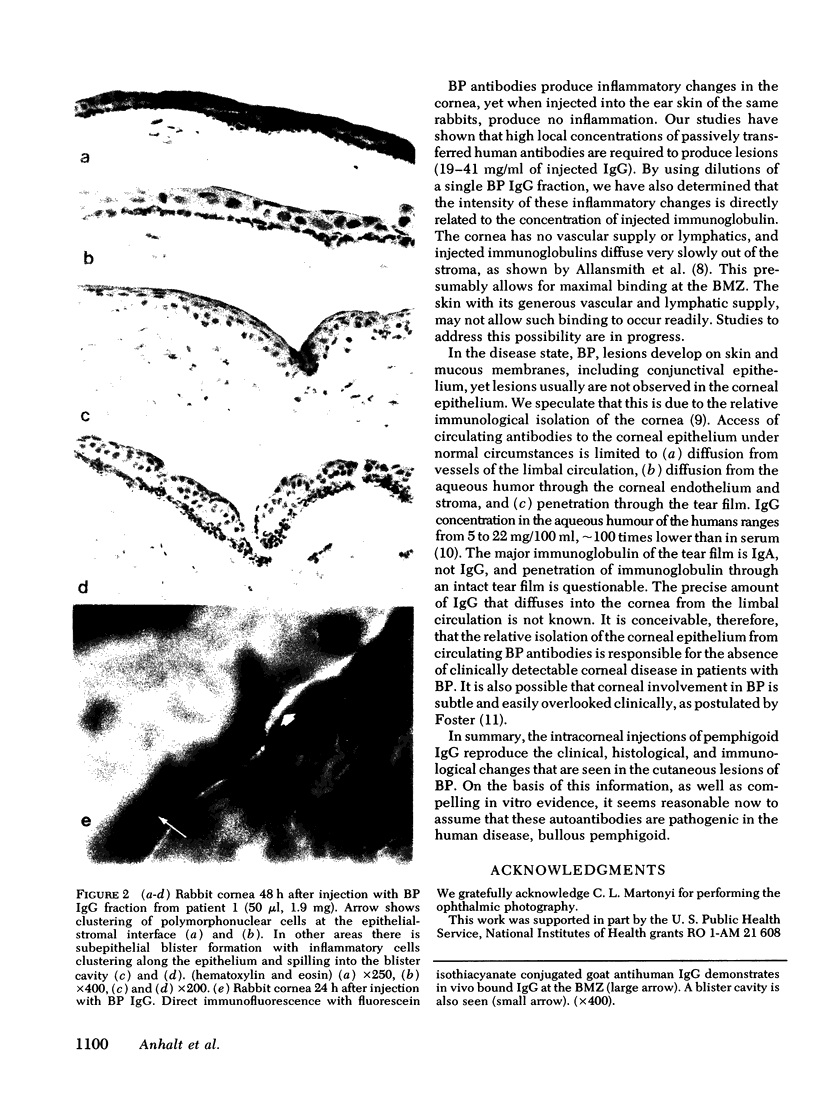
Bullous pemphigoid (BP) is associated with circulating autoantibodies reactive with an antigen(s) of the basement membrane zone (BMZ) of skin and mucosae. The pathogenicity of these autoantibodies, although suspected, is unconfirmed. We have investigated the effects of BP autoantibodies on a closely related tissue, the corneal epithelium of the rabbit. IgG fractions from the sera of seven patients with BP were purified by (a) ammonium sulfate precipitation, (b) ion exchange chromatography, or (c) gel filtration. Control IgG was prepared by ion exchange chromatography of pooled normal human gamma globulins. 32 rabbits received corneal intrastromal injections of BP IgG fractions (50 microliter, 0.95-2.05 mg total dose) in one eye, and control IgG (50 microliter, 1.8 mg) in the contralateral cornea. 28 of 32 BP IgG injections produced corneal inflammatory lesions, 10 of which developed visible blisters. Histologically, lesions showed polymorphonuclear cells clustering along the BMZ, and subepithelial blister formation. Immunofluorescence showed in vivo bound IgG and C3 at the BMZ. The intensity of inflammation was dose dependent and correlated often with in vitro complement fixation titers of the fractions. None of 32 corneas injected with control IgG became inflamed. BP IgG fractions injected intradermally into the ear skin of rabbits failed to produce inflammation. This may be due to slow clearance of IgG in the cornea, and optimal binding by the corneal epithelium. The intracorneal injections of BP IgG reproduce the clinical, histological, and immunological features of BP. This study provides evidence that BP autoantibodies are pathogenic.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M., de Ramus A., Maurice D. The dynamics of IgG in the cornea. Invest Ophthalmol Vis Sci. 1979 Sep;18(9):947–955. [PubMed] [Google Scholar]
- Barker C. F., Billingham R. E. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Foster C. S. Ocular surface manifestations of neurological and systemic disease. Int Ophthalmol Clin. 1979 Summer;19(2):207–242. [PubMed] [Google Scholar]
- Gammon W. R., Lewis D. M., Carlo J. R., Sams W. M., Jr, Wheeler C. E., Jr Pemphigoid antibody mediated attachment of peripheral blood leukocytes at the dermal-epidermal junction of human skin. J Invest Dermatol. 1980 Oct;75(4):334–339. doi: 10.1111/1523-1747.ep12531082. [DOI] [PubMed] [Google Scholar]
- Jordon R. E., Beutner E. H., Witebsky E., Blumental G., Hale W. L., Lever W. F. Basement zone antibodies in bullous pemphigoid. JAMA. 1967 May 29;200(9):751–756. [PubMed] [Google Scholar]
- Jordon R. E. Complement activation in pemphigus and bullous pemphigoid. J Invest Dermatol. 1976 Sep;67(3):366–371. doi: 10.1111/1523-1747.ep12514708. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Gleich G. J. Failure to transfer bullous pemphigoid with serum from patients. Proc Soc Exp Biol Med. 1971 Apr;136(4):1027–1031. doi: 10.3181/00379727-136-35421. [DOI] [PubMed] [Google Scholar]
- Zirm M. Proteins in aqueous humor. Adv Ophthalmol. 1980;40:100–172. [PubMed] [Google Scholar]





