Abstract
Statement of Problem
Porous tantalum trabecular metal has recently been incorporated in titanium dental implants as a new form of implant surface enhancement. However, there is little information on the applications of this material in implant dentistry.
Methods
We, therefore review the current literature on the basic science and clinical uses of this material.
Results
Porous tantalum metal is used to improve the contact between osseous structure and dental implants; and therefore presumably facilitate osseointegration. Success of porous tantalum metal in orthopedic implants led to the incorporation of porous tantalum metal in the design of root-from endosseous titanium implants. The porous tantalum three-dimensional enhancement of titanium dental implant surface allows for combining bone ongrowth together with bone ingrowth, or osseoincorporation. While little is known about the biological aspect of the porous tantalum in the oral cavity, there seems to be several possible advantages of this implant design. This article reviews the biological aspects of porous tantalum enhanced titanium dental implants, in particular the effects of anatomical consideration and oral environment to implant designs.
Conclusions
We propose here possible clinical situations and applications for this type of dental implant. Advantages and disadvantages of the implants as well as needed future clinical studies are discussed.
It is estimated that over 26% of people, ages 65–74 in the US are edentulous.1 The number of edentulous people and people with significant number of missing teeth is even worse in the developing world. It is known that edentulism is a comorbidity to several systemic and oral diseases such as osteoporosis, hypertension, atherosclerosis, diabetes, cancer, etc. 2–7 However, the underlying molecular mechanism that may lead an edentulous individual to be at risk for these diseases is not known. Several biological changes occur after loss of natural teeth. These include reduction on masticatory efficiency, altered neuronal/physiolocal sensation, psycological effects, alveolar bone remodeling, and changes on microflora composition. Complete and partial edentulism clearly reduces mechanical chewing function and esthetics. Edentulism and its comobidities have a bidirectional relationship, in other words, each condition worsens the other. While current treatment modalities for edentulism, such as dental implant therapy, are aimed at improving function and esthetics for patients, the systemic and oral co-morbidities of edentulism, including diabetes, osteoporosis, as well as a lack of sufficient remaining alveolar bone, challenge the immediate and long-term success of dental implant therapy. Recently there has been an incorporation of porous tantalum metal into titanium dental implants. This new type of dental implant may improve dental implant therapy in certain populations. This article therefore aims to review the basic science development, advantages and cautions, as well as possible clinical applications of the new tantalum metal implants.
Tantalum
Tantalum (Ta) is a rare, highly corrosion resistant transitional metal element with atomic number 73. The word tantalum was coined from Tantalus, a Greek mythology figure who was eternally punished to stand in a pool of water under a tree with low hanging fruit. When Tantalus reached to get the water, the water would recede. And when he reached for the fruit the tree branch would move higher.8,9 This “tantalizing” property of Ta was seen by the early chemists when Ta was immersed in acids.10 Tantalum, they found, was highly unreactive in almost all acids, except hydrofluoric acid and acids containing fluoride and sulfur trioxide. Tantalum is a member of the refractory metals group, which are widely used as components in alloys. The Swedish chemist, Anders Gustav Ekebereg, discovered Tantalum in 1802.11 Tantalum, in the early years of discovery, was found in its oxide form—as columbium, which is a combination of columbite and tantalite.12 William Hyde Wollaston, an English chemist, showed that both columbite and tantalite are derivatives of the same element and kept the name, tantalum.13
Industrial mining and purification of tantalum
Tantalum is often extracted from the mineral, tantalite. It is primarily mined in western Australia, and produced as a by-product of tin mining in Thailand and Malaysia and ore mining in China, Ethiopia, and Mozambique.14 Extraction of tantalum from naturally occurring tantalite is accomplished by gravity separation, which separates components of the mixture based on the differences of their specific weights. This is followed by chemical separation using hydrofluoric and sulfuric acid solutions and heat. The process will extract the oxides of tantalum from its natural cohabitant element, Niobium.15 From there, the compound is further purified using liquid extraction of the fluorides. Tantalum fluorides can then be extracted by organic solvents and further precipitated with potassium fluoride. Then, molten sodium is used to create tantalum powder.14
Because tantalum is a rare element, only making up 1–2 ppm of the Earth’s crust by weight, recycling of tantalum oxide is important to maintain supply.15 The main source of material for recycling tantalum is waste from capacitors. In 2005, Mineta and Okabe investigated a recycling method for tantalum. In the study, they found that sintered tantalum electrodes inside capacitor scraps could be collected after oxidation, and high purity Ta2O5 powder could be recovered following chemical treatment.16 This process yielded tantalum powder with 99 mass% purity.16 Tantalum is extremely inert and resistant to acid corrosion. Only hydrofluoric acid and acid solutions containing fluoride and sulfur trioxide can dissolve tantalum.14 This inertness is ideal for fabrication of orthopedic implants.17 The inertness and biocompatibility of tantalum is a result of tantalum oxides forming on the surface of tantalum—similar to titanium and its oxides. Tantalum has two forms of oxide, +5 (Ta2O5) and +4 (TaO2). The +5 (Ta2O5) or tantalum pentoxide form is the most stable oxide.18 Similar to titanium, tantalum is very reactive to oxygen and the oxide layer of tantalum can form on the metal surface immediately after the surface is exposed to oxygen. Annealed tantalum has great ductility. However, grinding annealed tantalum is very difficult. Because tantalum is reactive to oxygen, it cannot be soldered and welding can only be done under inert gas environment.14 Tantalum is used in electrodes for pacemakers, devices for nerve repair, radiographic markers, and cranioplasty plates.17,19
Production of porous tantalum trabecular metal (PTTM)
Cobalt chromium, titanium alloys, and stainless steel have been the conventional materials used for orthopedic implants. With alterations and enhancements including surface coatings and porous designs, these materials have shown a high clinical effectiveness. Nevertheless, they still have several limitations including low volumetric porosity, relatively high modulus of elasticity and low frictional characteristics.17 The development of porous tantalum metal has allowed for stronger, more biocompatible orthopedic, craniofacial, and dental implants. The structure of porous tantalum metal affords a high volumetric porosity, a low modulus of elasticity, and relatively high frictional characteristics.17
Even though tantalum is highly biocompatible, inert and extremely resistant to corrosion, its use in orthopedic implant devices was limited because of the difficulty in manipulating solid tantalum. Replacing osseous structure traditionally uses solid materials such as titanium or porous materials such as hydroxyapatite (HA) or tricalcium phosphate (TCP). Attempts had been made to coat some alloys, such as chrome-cobalt or titanium alloy with HA or TCP. However, these attempts in the past failed to mimic the structure of osseous cancellous bone. Additionally, there was mechanical failure, resulting from lack of yield strength and ductility of the coating materials. It is not until the early 1990s that porous tantalum trabecular metal (PTTM) was introduced.20
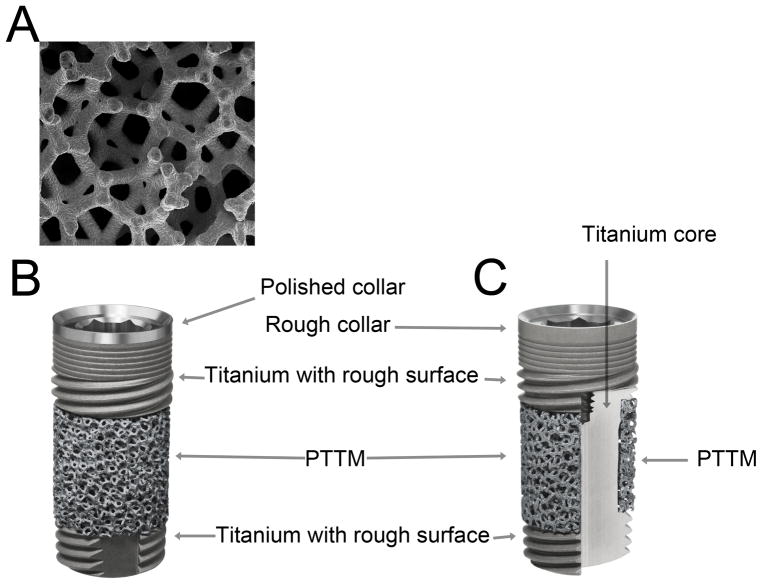
PTTM, known commercially as Trabecular Metal Material (Zimmer, Trabecular Metal Technology, Inc., Parsippany, NJ) is an open-cell porous biomaterial with a structure similar to trabecular bone by having three-dimensional dodecahedron repeats (Figure 1a). The open-cell dodecahedron repeats are fabricated by a foam-like vitreous carbon scaffold,20,21 which forms an initial general scaffold and eventually becomes the internal skeleton of the PTTM implant device. The vitreous carbon scaffold is then placed in an air sealed chamber. Note that unlike the extraction of tantalum from nature, recycled tantalum metal, commonly used in industry such as capacitors in computers and cellular phones, is used here in the PTTM fabrication process. Tantalum coating of the scaffold is done by chemical vapor diffusion process using hydrogen and chlorine gases. The tantalum is evaporated as TaCl2 and the tantalum molecules are then deposited onto the scaffold.20–22 PTTM is, therefore, superior to other metal implant technologies such as titanium because it has a high degree of porosity.21 The vitreous carbon skeleton comprising the trabecular framework of the implant can be altered.17 This means that a variety of designs of PTTM may be created, which is utilized especially in creating orthopedic implants. Review of the literature on PTTM as an orthopedic implant material suggests that this material has great biocompatibility, osteoconductivity, bone ingrowth and vascularization in both in vitro/in vivo experiments and in human studies.17,23–27 The PTTM allows an implant surface enhancement not only for bone ongrowth but also bone ingrowth. PTTM structure allows neovascularization and new bone formation directly into the implant. This concept is known as “osseoincorporation”.21,23
Figure 1. Porous Tantalum Trabecular Metal (PTTM) Enhanced Titanium Dental Implants.
A) PTTM structure, B) The overall structure of a PTTM-enhanced titanium dental implant with cervical smooth titanium metal tissue collar, and C) the structure of a PTTM-enhanced titanium dental implant with total rough titanium surface demonstrating the cross-sectional of the middle-third of the implant that has outer layer of PTTM and titanium core
PTTM-enhanced titanium dental implants
While the PTTM technology has had great success in the orthopedic field for almost two decades, the technology has not been applied to dental implants until recently (Figure 1b). The designs of modern root-form endosseous implants date back even before the discovery of osseointegration by P. I. Branemark in the early 1970s to 1980s.28,29 Branemark revolutionized the surgical protocol by controlling the heat generated during implant site preparation, using root-form implants, and allowing a certain period of unloading of implants for healing. Branemark recognized that heat generated from surgical preparation of the implant site needs to be controlled. He experimented with varieties of implants but ended up with a root-form titanium implant design, which allows simplicity of implant placement. In addition, failure of root-form implants only leave a small defect that can be easily repaired in most cases. Root-form implants also allow a close proximity of the implant site and the implant, which enhances bone healing. While a large part of the original concept of Branemark’s osseointegration remains valid, the unloading period for implant healing after surgery has been challenged many times.
Since the introduction of the osseointegration concept, there have been two distinct changes from the implants Branemark used: first, the internal connection and second, the implant surface designs. The implants that were introduced by Branemark had an external hex feature. Implant screw loosening and screw fracture demonstrated to be a major problem with this design.30 Nowadays almost all implants in the current market have some features of internal connection. More importantly, the titanium implants originally used by Branemark have a machine-finished surface. Similar to the external hex design, this relatively smooth surface design has also disappeared from the market. The smooth machine finished surface is often blamed for the peri-implant bone loss originally thought of by Branemark’s group as a physiological condition.31 All of the implant surface designs in the current market have some features that increase surface roughness in order to obtain a larger and more stable osseointegrated bone-implant contact area. Improving the roughness of the implant surface has shown to reduce the peri-implant bone loss seen in the original Branemark-designed implants. This roughness or coating of implants can be done using blasting with various types of grit particles, acid etching, plasma spraying or a combination of these techniques.32 Improvement in hydrophilicity or lowering the liquid surface contact angle of implant surfaces with roughness or surface coating has also been used.33 In any case, these surface roughness technologies often improve the surface contact in the micro- and nano-level by reducing the free-energy of the surface and thereby facilitating the adsorption of platelets, monocytes and clotting proteins.34 Reducing the free-energy of the surface promotes the adherence of platelets that when adsorbed release PDGFs (platelet-derived growth factors) which are chemotactic and mitogenic for mesenchymal cells and osteoblast progenitor cells. This serves to draw cells toward the implant surface. Monocytic cells are also among the first to adsorb and the hydrophilic nature of the surface stimulates the differentiation into a “M2” cellular phenotype which secretes growth factors facilitating would healing and tissue regeneration, rather than the “M1” phenotype which secretes pro-inflammatory molecules such as TNFa and IL-1b which are catabolic to connective tissue metabolism. The M1 phenotype secretes molecules such as FGFs (fibroblast growth factors), IGFs (Insulin-like growth factors) and transforming growth factors including bone morphogenetic proteins (BMPs) that further attract osteoprogenitor cells, and facilitate rapid angioneogenesis and osseous regeneration. While it seems that current implant surface treatment improves osteoblastic activity at the implant surface and therefore enhances implant-bone contact resulting in lower peri-implant bone loss, it does little to improve the surface area and bone ingrowth into the implant.
Recently, PTTM technology was introduced to create a three-dimensional bone ingrowth scaffold around dental implants (Figure 1b–c). The PTTM material was added to the middle section of the Titanium multi-threaded self-tapping endosseous dental implant (Tapered Screw-Vent® Implant. Zimmer Dental Inc.). The apical and cervical sections of this PTTM-enhanced titanium dental implant retain the screw-type design with a rough surface created by grit-blasting with hydroxyapatite or HA particles (MTX surface, Zimmer Dental Inc). The titanium alloy (Ti-6Al-4V grade 5) and the PTTM components of the implant are manufactured separately. The cervical portion and the middle core of the titanium alloys are milled in one piece. Similarly the apical portion is milled separately. The PTTM sleeve, ~ 2 mm cylinder, is composed of 2% vitreous carbon core scaffold and 98% tantalum coating. The PTTM sleeve is then placed into the middle titanium alloy core and the core is then laser welded to the apical portion.22
Advantages of PTTM-enhanced titanium dental implants
The PTTM-enhanced titanium dental implants theoretically provide several advantages over other implant designs. The PTTM portion of the implant has an open-cell dodecahedron repeat structure which allows for rapid endothelial budding and ingrowth through the expanded structure in response to the angiogenic and anabolic growth factor gradient produced within the scaffold by the metal surface interacting with the initial blood clot. The dimensions of the open structure are designed to accommodate rapid endothelial neovascularization which is critical to permit subsequent osteoblast precursor recruitment and osteoblastic cell differentiation, growth and matrix secretion.35 This healing granulation tissue promotes new osseous tissue formation and bone ingrowth shown to be suitable for withstanding immediate and early loading in orthopedic implants.25 Similar to titanium, the tantalum layer of PTTM, when oxidized, is highly unreactive, and therefore, biocompatible in the body. Tantalum does not exhibit toxicity to surrounding cells, nor does it inhibit local cell growth, i.e. osseous ingrowth of surrounding bone. An in vitro study, comparing titanium, tanlalum and chromium in osteoblastic differentiation using human mesenchymal stem cells, suggests that tatalum has similar biocompatibity to titanium. While titanium allows faster cell proliferation, tantalum enhances osteoblastic differentiation process.36 The trabecular structure of the porous tantalum metal in PTTM-enhanced titanium dental implants can also improve osseointegration simply by increasing bone-implant interface area in the three-dimensional manner promoting angiogenesis and mimicking natural osseous structure.25 The porous metal structure exhibited by the implants is exceedingly similar to that of natural spongy bone and appears to be one the reasons why osseous ingrowth occurs so readily. The open cell structure of PTTM is superior to other surface treatment methods that attempt to produce porosity of the implant, but does not achieve complete porosity. Pore size can also be altered with PTTM to match the surrounding bone.24 However, the commercially available PTTM has a standardized pore size thought to be an optimal dimension for promoting osseoincorporation37 (Figure 1a). In addition to the advantage of trabecular makeup, PTTM exhibits an elastic modulus similar to bone and is mechanically superior to other alloys used in dental implants.25 PTTM allows for elastic deformation and load distribution. This means it is able to avoid placing local stress on the surface of articular cartilage in orthopedic artificial joint. Also, bone resorption is less likely since the stress is distributed throughout the structure to the surrounding bone. Harrison et al38 showed that the use PTTM in a knee implant prevents resorption of adjacent tibia that contributes to the common failure of artificial joints. They further suggested that the similarity in the mechanical properties of PTTM and surrounding bone prevents stress shielding and, therefore, long-term bone loss. Finally, the PTTM-enhanced titanium dental implants retain most if not all of the advantages of the root-form endosseous self-tapping implants in terms of primary stability, ability to be removed and grafted when it fails, and prosthetic/restorative simplicity. The advantages of high biocompatibility, similar porous structure to natural bone, and excellent mechanical properties of PTTM-enhanced titanium dental implants may give them an advantage over other dental implants, particularly for patients through enhancement of osseointegration, or osseoincorporation.
Potential clinical applications for PTTM-enhanced titanium dental implants
Modern root-form endosseous titanium dental implants are commonly used because of their high success rate. However, there are certain case scenarios that show current dental implant therapy can be enhanced. First, in the case of poor tissue healing, for instance patients with diabetes39,40, osteoporosis41, irradiated bone42,43, and heavy use of tobacco44,45, may benefit from this type of implant. Second, when there is insufficient remaining bone structure that requires simultaneous bone augmentation or in the newly grafted bone, PTTM in orthopedic cases have shown adequate healing of the grafted tissues.20 In the oral cavity, PTTM may help in cases that need simultaneous implant placement and horizontal/vertical bone augmentation or cases with simultaneous implant placement with sinus augmentation or cases with newly grafted sinuses or sockets (Figure 2). Third, PTTM-enhanced titanium dental implants may therefore serve as a valuable alternative for patients with bone quality type 3 and 4. In subjects with Type 3 or 4 bone or with impaired wound healing due to systemic complications, the improved and enlarged surface area that is provided by the PTTM collar may result in faster and more robust osseointegration. Fourth, in the normal dental implant cases that require immediate provisionalization and loading or sooner insertion of permanent prostheses, due to patient’s demand, faster healing time may be needed (Figure 3 and 4). PTTM-enhanced titanium dental implants may give patients and clinicians another treatment option for immediate placement and loading of implants. While we may assume that immediate and early loading of PTTM-enhanced titanium dental implants is recommended based on orthopedic literature, there is currently no study on such topics. Prospective clinical trials will be needed to examine if this type of implants is really superior to conventional titanium dental implants.
Figure 2. Placement of the implant with simultaneous osteotome sinus augmentation.
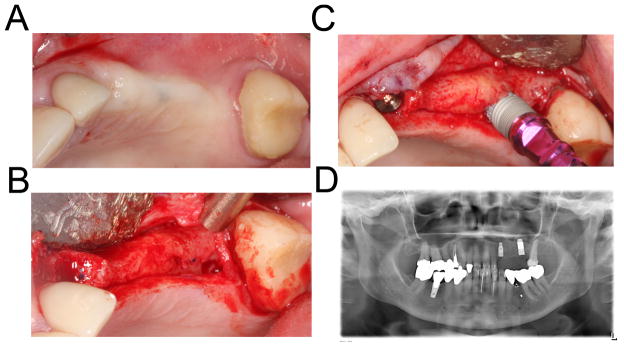
A 78-year-old female presented with missing teeth from her right canine to first molar (2a). To minimize healing time and number of surgical visits, we performed osteotome sinus augmentation and placed a 4.7 mm × 13 mm PTTM-enhanced titanium dental implants (Trabecular Metal Dental Implants, Zimmer Dental Inc) in the first molar site. Conventional titanium implant, 4.1 mm × 11.5 mm tapered threaded implant (Tapered Screw-Vent, Zimmer Dental Inc) was placed in the canine site (2b–d).
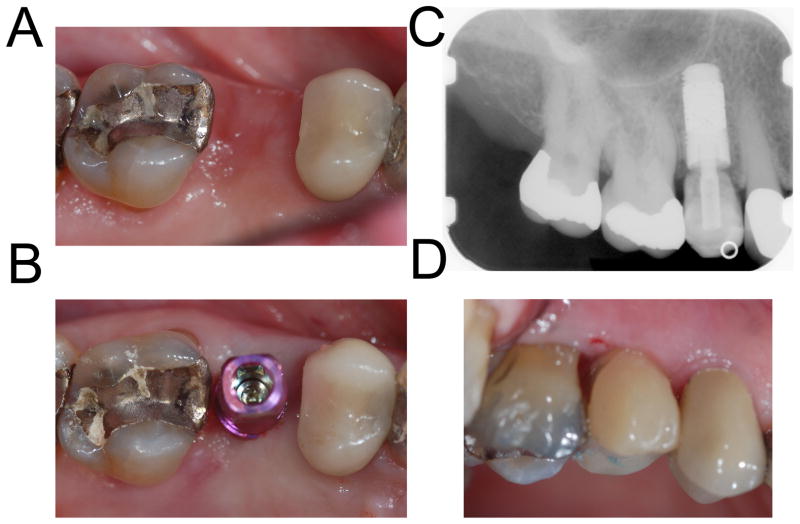
Figure 3. Immediate loading of a single tooth implant.
A 79-year-old female presented with a fractured maxillary right second premolar. The tooth was extracted and the socket was grafted with demineralized freeze-dried allograft and demineralized bone matrix (Puros Demineralized Bone Matrix, Zimmer Dental Inc). Four months after the extraction, a 4.7 mm × 11.5 mm PTTM-enhanced titanium dental implant (Trabecular Metal Dental Implant, Zimmer Dental Inc) was placed using flapless procedure. The implant was immediately restored using a screw-retained provisional abutment and bisacryl composite resin material (Integrity, Dentsply).
Figure 4. Early loading of an implant-retained overdenture.
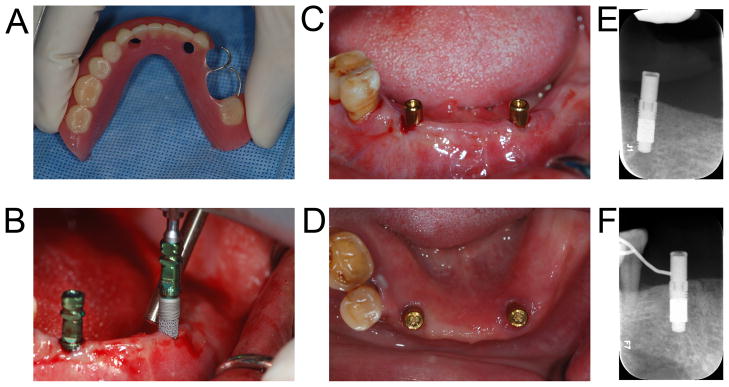
A 49-year-old female presented with chief complaint that her mandibular acrylic partial denture was not stable and she had hard time chewing. Her existing denture was used as a radiographic and later as a surgical guide (4a). In the mandibular canine areas bilaterally, a 4.1 × 11.5 mm PTTM-enhanced titanium dental implants (Trabecular Metal Dental Implants, Zimmer Dental Inc) was placed using flapless procedure (4b). Overdenture abutments (Locator, Zest Anchors) were immediately placed (4c). A denture tissue conditioner (Coe Comfort, GC America) was used to reline the denture in the area directly coronal to the implants. Due to a family issue, the patient had to leave the country three weeks after the surgery. Locator attachments were then added to the denture three weeks after the implants were placed (4d). The patient in her home country will have her remaining teeth extracted due to limited periodontal support and receive a mandibular implant-supported overdenture.
Potential problems with PTTM-enhanced titanium dental implants
While PTTM implants have been used widely and successfully as orthopedic implants, the application of PTTM in the oral cavity remains limited until now. The major concern for this type of implant is perhaps the uniqueness of the oral environment. Unlike most orthopedic surgical sites, the oral cavity is a complex unsterile field, which can harbor over 500 different bacterial species. Various microorganisms live in the oral cavity. The interactions of the host tissue, saliva, and microorganisms can make it difficult to predict how PTTM implants would react to this complex environment. Titanium implants can be susceptible to infection because of: (1) surface biofilm and (2) compromised immune ability at implant tissue interface.46 While tantalum itself is similar to titanium in that it is highly biocompatible and corrosion resistant, the interactions with oral fluid, oral microbes and biofilm of the PTTM portion are not known. The concern can extend into the case of peri-implantitis and how we may treat it in the case of PTTM-enhanced titanium dental implant. This concern will remain unanswered until we see the results of long-term studies of these implants. It is our anecdotal opinion that if the peri-implant bone loss extends into the PTTM portion of the implant, removal of the implant will be needed. Moreover, implant removal, grafting and subsequently a new implant placement may be indicated as a treatment of choice for peri-implantitis for this type of implants. Finally, a mechanical concern has been raised around PTTM-enhanced titanium dental implants. Due to implant manufacturing, the connection between the relatively small titanium core/PTTM in the middle-third portion and the apical titanium portion (Figure 1c) may be prone to fracture especially if they are placed in hard bone (type 1) with in appropriate high torque. These implants are however recommended for bone that are relatively soft (type 3 or 4). The fracture of implants during insertion may therefore not be a major issue in soft bone.
Conclusion
Development of PTTM-enhanced titanium dental implants combined conventional titanium implant design and instrumentation with PTTM technology. This theoretically allows for true enhancement of osseointegration in a three-dimensional manner, which may be a major breakthrough compared with the current focus on titanium implant surface technologies. Based on assumptions from orthopedic clinical studies, this type of implant may be indicated in poor healing situations, immediate/early loading of implants, and missing osseous structure requiring simultaneous implant placement and bone grafting. The uniqueness of the oral cavity, in particular the host-oral microbial interaction, is a concern. The extent and aggression of peri-implantitis in this type of implant is not known. Prospective longitudinal studies are clearly needed, as well as a focus on biomaterial research in humans in the oral cavity. In the meantime, clinicians will need to use their own judgment with careful case selection criteria until the research proves otherwise.
Acknowledgments
Zimmer Dental Inc has provided the figures of porous tantalum metal and PTTM-enhanced dental implants. The authors thank the members of the University of North Carolina at Chapel Hill Dental Faculty Practice, Graduate Prosthodontic Clinic, and the General-Oral Health Clinic where clinical work was done. The work of author SB was partly supported by the American Academy of Implant Dentistry Research Grant, the American College of Prosthodontists Educational Fund, and the the National Institutes of Health (NIH) grant HL092338.
Footnotes
Ethical Statement
Written consent forms were obtained from all patients for the use of their intra-oral photographs for educational use and publication purposes.
Statement of conflicts of Interests
There was no direct compensation for this review and Zimmer Dental Inc has no role in the writing and publication of this manuscript. However, authors SB and GR are Zimmer Institute lecturers. In addition, Zimmer Dental Inc does not directly support salary for any authors; however, Zimmer Dental Inc was supporting the work of authors SB and SO through unrestricted research and educational grants.
References
- 1.Healthy People 2010, Volume ii, Section 21. Oral Health. :21-18–21-19. Available at http://www.healthypeople.gov/Publications.
- 2.Felton DA. Edentulism and comorbid factors. J Prosthodont. 2009 Feb;18(2):88–96. doi: 10.1111/j.1532-849X.2009.00437.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirai T, Ishijima T, Hashikawa Y, Yajima T. Osteoporosis and reduction of residual ridge in edentulous patients. J Prosthet Dent. 1993 Jan;69(1):49–56. doi: 10.1016/0022-3913(93)90240-o. [DOI] [PubMed] [Google Scholar]
- 4.Loesche WJ, Schork A, Terpenning MS, Chen YM, Dominguez BL, Grossman N. Assessing the relationship between dental disease and coronary heart disease in elderly U.S. veterans. J Am Dent Assoc. 1998 Mar;129(3):301–311. doi: 10.14219/jada.archive.1998.0204. [DOI] [PubMed] [Google Scholar]
- 5.Medina-Solis CE, Perez-Nunez R, Maupome G, Casanova-Rosado JF. Edentulism among Mexican adults aged 35 years and older and associated factors. Am J Public Health. 2006 Sep;96(9):1578–1581. doi: 10.2105/AJPH.2005.071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008 May;17(5):1222–1227. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 7.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003 Jan;34(1):47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- 8.Aycan S. Chemistry education and mythology. J Social Sciences. 2005;1(4):238–239. [Google Scholar]
- 9.Colakis M, Masello MJ. Classical Mythology and More: A Reader Workbook. Bolchazy-Carducci Publishers; 2007. p. 204. [Google Scholar]
- 10.Greenwood N, Earnshaw A. Chemistry of the Elements. 2. Oxford: Butterworth–Heinem; 1997. p. 1138. [Google Scholar]
- 11.Weeks ME. The discovery of the elements. VII. Columbium, tantalum, and vanadium. J Chem Educ. 1932 May;9(5):867–872. [Google Scholar]
- 12.Griffith WP, Morris PJT. Charles Hatchett FRS (1765–1847), chemist and discoverer of niobium. Notes Rec Roy Soc. 2003 Sep;57(3):299–316. [Google Scholar]
- 13.Wollaston WH. On the identity of columbium and tantalum. JSTOR. 1809(99):246–252. [Google Scholar]
- 14.Kock W, Paschen P. Tantalum - Processing, Properties and Applications. Jom-J Min Met Mat S. 1989 Oct;41(10):33–39. [Google Scholar]
- 15.Agulyanski A. The chemistry of tantalum and niobium fluoride compounds. 1. Amsterdam: Elsevier; 2004. pp. 1–4. [Google Scholar]
- 16.Mineta K, Okabe TH. Development of a recycling process for tantalum from capacitor scraps. J Phys Chem Solids. 2005 Feb-Apr;66(2–4):318–321. [Google Scholar]
- 17.Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006 Sep;27(27):4671–4681. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Fairbrother F. The chemistry of niobium and tantalum. Amsterdam, New York etc: Elsevier Pub. Co; 1967. pp. 20–36. [Google Scholar]
- 19.Black J. Biological performance of tantalum. Clin Mater. 1994;16(3):167–173. doi: 10.1016/0267-6605(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RB. Open cell tantalum structures for cancellous bone implants and cell and tissue structures. 5282861 US Patent No. 1994
- 21.Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop. 2002 Apr;31(4):216–217. [PubMed] [Google Scholar]
- 22.Zardiackas LD, Parsell DE, Dillon LD, Mitchell DW, Nunnery LA, Poggie R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J Biomed Mater Res. 2001;58(2):180–187. doi: 10.1002/1097-4636(2001)58:2<180::aid-jbm1005>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999 Sep;81(5):907–914. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 24.Heiner AD, Brown TD, Poggie RA. Structural efficacy of a novel porous tantalum implant for osteonecrosis grafting. Trans Orthop Res Soc. 2001;26:480. [Google Scholar]
- 25.Miyazaki T, Kim HM, Kokubo T, Ohtsuki C, Kato H, Nakamura T. Mechanism of bonelike apatite formation on bioactive tantalum metal in a simulated body fluid. Biomaterials. 2002;23(3):827–32. doi: 10.1016/s0142-9612(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 26.Bobyn JD, Poggie RA, Krygier JJ, et al. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86-A(Suppl 2):123–129. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 27.Christie MJ. Clinical applications of Trabecular Metal. Am J Orthop. 2002 Apr;31(4):219–220. [PubMed] [Google Scholar]
- 28.Bothe RT, Beaton LE, Davenport HA. Reaction of bone to multiple metallic implants. Surg Gynecol Obstet. 1940;7:1598–602. [Google Scholar]
- 29.Leventhal GS. Titanium, a metal for surgery. J Bone Joint Surg Am. 1951 Apr;33-A(2):473–474. [PubMed] [Google Scholar]
- 30.Henry PJ, Laney WR, Jemt T, et al. Osseointegrated implants for single-tooth replacement: a prospective 5-year multicenter study. Int J Oral Maxillofac Implants. 1996 Jul-Aug;11(4):450–455. [PubMed] [Google Scholar]
- 31.Adell R, Lekholm U, Rockler B, et al. Marginal tissue reactions at osseointegrated titanium fixtures (I). A 3-year longitudinal prospective study. Int J Oral Maxillofac Surg. 1986 Feb;15(1):39–52. doi: 10.1016/s0300-9785(86)80010-2. [DOI] [PubMed] [Google Scholar]
- 32.Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007 Jul;23(7):844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006 Feb;76(2):323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 34.Mendonca G, Mendonca DB, Aragao FJ, Cooper LF. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials. 2008 Oct;29(28):3822–3835. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Hacking SA, Bobyn JD, Toh K, Tanzer M, Krygier JJ. Fibrous tissue ingrowth and attachment to porous tantalum. J Biomed Mater Res. 2000 Dec;52(4):631–638. doi: 10.1002/1097-4636(20001215)52:4<631::aid-jbm7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Stiehler M, Lind M, Mygind T, Baatrup A, Dolatshahi-Pirouz A, Li H, Foss M, Besenbacher F, Kassem M, Bünger C. Morphology, proliferation, and osteogenic differentiation of mesenchymal stem cells cultured on titanium, tantalum, and chromium surfaces. J Biomed Mater Res A. 2008;86(2):448–58. doi: 10.1002/jbm.a.31602. [DOI] [PubMed] [Google Scholar]
- 37.Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop Relat Res. 1980 Jul-Aug;(150):263–270. [PubMed] [Google Scholar]
- 38.Harrison AK, Gioe TJ, Simonelli C, Tatman PJ, Schoeller MC. Do porous tantalum implants help preserve bone?: evaluation of tibial bone density surrounding tantalum tibial implants in TKA. Clin Orthop Relat Res. 2010;468(10):2739–2745. doi: 10.1007/s11999-009-1222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodontol. 2009 Nov;80(11):1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- 40.Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005 Jul-Aug;20(4):569–577. [PubMed] [Google Scholar]
- 41.Roberts WE, Simmons KE, Garetto LP, DeCastro RA. Bone physiology and metabolism in dental implantology: risk factors for osteoporosis and other metabolic bone diseases. Implant Dent. 1992;1(1):11–21. doi: 10.1097/00008505-199200110-00002. [DOI] [PubMed] [Google Scholar]
- 42.Taylor TD, Worthington P. Osseointegrated implant rehabilitation of the previously irradiated mandible: results of a limited trial at 3 to 7 years. J Prosthet Dent. 1993 Jan;69(1):60–69. doi: 10.1016/0022-3913(93)90242-g. [DOI] [PubMed] [Google Scholar]
- 43.Brogniez V, Lejuste P, Pecheur A, Reychler H. Dental prosthetic reconstruction of osseointegrated implants placed in irradiated bone. Int J Oral Maxillofac Implants. 1998 Jul-Aug;13(4):506–512. [PubMed] [Google Scholar]
- 44.Bain CA, Moy PK. The association between the failure of dental implants and cigarette smoking. Int J Oral Maxillofac Implants. 1993;8(6):609–615. [PubMed] [Google Scholar]
- 45.Schwartz-Arad D, Samet N, Mamlider A. Smoking and complications of endosseous dental implants. J Periodontol. 2002 Feb;73(2):153–157. doi: 10.1902/jop.2002.73.2.153. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009 Oct;91(1):470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]