Abstract
The dual-route interactive two-step model explains the variation in the error patterns of aphasic speakers in picture naming, and word and nonword repetition tasks. The model has three parameters that can vary across individuals: the efficiency of the connections between semantic and lexical representations (s-weight), between lexical and phonological representations (p-weight), and between representations of auditory input and phonological representations (nl-weight). We determined these parameter values in 103 participants with chronic aphasia from left hemisphere stroke whose lesion locations had been determined. Then, using voxel-based lesion-parameter mapping, we mapped the parameters onto the brain, thus determining the neural correlates of the model’s mechanisms. The maps and the behavioral findings supported the model’s central claim that word repetition is affected by both the p and nl parameters. We propose that these two parameters constitute the model’s analogue of the “dorsal stream” component of neurocognitive models of language processing.
Keywords: Aphasia, Language production, Computational models, Voxel-based Lesion Parameter Mapping
1. Introduction
Most computational models of cognition aim to simulate behavioral data. For example in the domain of language production, the topic of this article, models simulate speaker choices (e.g. Chang et al., 2006), the temporal dynamics of those choices (e.g. Levelt et al., 1999), and the characteristics of speech errors, including normal slips as well as production errors made by speakers with brain damage (e.g. Dell et al., 1997). To explain these data, the models postulate representations and processes, and parameters regarding how these vary across individuals and circumstances.
More recently, cognitive models have been used to guide cognitive neuroscience. The models identify cognitive functions whose brain correlates can be sought. Language production models, in particular, have been used to interpret functional imaging data obtained from a variety of methods (e.g. Costa et al., 2009; Graves, Grabowski, Mehta, & Gordon, 2007; Indefrey & Levelt, 2004; Price, 2000) and analyses of lesion locations in speakers with aphasia (e.g. DeLeon et al., 2007; Schwartz et al., 2009). In this article, we identify the neural correlates of a particular model of lexical access in production, the dual-route interactive two-step model (e.g. Dell et al., 2007; Hanley et al., 2004; Nozari et al., 2010; Schwartz et al., 2006). This and related models have been applied to several aspects of lexical processing in aphasic and unimpaired speakers.
Here, the focus is on the relationship between word production from meaning, for example, in the picture naming task, and production in the auditory repetition task, in which speakers repeat heard words or nonwords. Relating the model’s characteristics to the brain can, at the very least, provide a test of the model by determining whether its distinctions map onto the brain in an interpretable way. Perhaps more importantly, this test can also constrain other recent models that make specific claims about brain pathways that are relevant for production (e.g., Hickok, 2012; Hickok & Poeppel, 2004; Ueno, Saito, Rogers, & Lambon Ralph, 2011). That is, we hope to take a first step in linking a cognitive model of production that simulates speech errors made by normal and impaired speakers to neurocognitive models of language that have been developed from different data sources.
Our methods are based on voxel-based lesion symptom mapping (VLSM, Bates et al., 2003). VLSM is one of a family of fMRI-inspired techniques aimed at identifying voxels or anatomically defined regions in which the presence or extent of tissue dysfunction predicts a symptom at a statistically reliable level (e.g., Hillis et al., 2006; Kimberg, Coslett, & Schwartz, 2007; Rorden, Karnath, & Bonilha, 2007; Rudrauf et al., 2008). The typical VLSM study involves a large sample of individuals with chronic focal lesions who have been assessed on the symptom of interest and have undergone a structural brain scan to locate the lesion. The lesions are traced and registered to a common template, enabling a determination at each voxel of who had a lesion in that voxel and who did not. In each voxel, a statistic is computed measuring the association between lesion status and the presence or severity of the symptom. Using a threshold that corrects for the many thousands of tests performed, voxels are identified that exceed the threshold and thereby qualify as being related to the symptom in question.
In this article, we present results of a specific kind of VLSM, called voxel-based lesion parameter mapping (VLPM). VLPM is just like VLSM, except that voxel lesion status predicts the properties of the model’s characterization of patients, rather than patient symptoms directly. The dual-route interactive two-step model has three parameters on which aphasic individuals can differ, s (semantic) weight, p (phonological) weight, and nl (non-lexical) weight. Each patient is assigned a value for these parameters based on a set of procedures for fitting the model to the patient’s error patterns in a picture naming test and an auditory repetition test. For this article, we performed this model evaluation for 103 individuals with post-stroke aphasia and used VLPM to create brain maps that identify which voxels predict the variation in the parameters.
1.1. The Dual-Route Interactive Two-step Model
The model explains the errors that aphasic speakers make in picture naming (hereafter, naming) and auditory repetition. The details of its architecture, processing mechanisms, and parameter fitting procedures are described elsewhere (e.g. Dell et al., 2007; Schwartz et al., 2006), but we provide a short summary of these and some background. The earliest version of the model explained speech error patterns from normal speakers in spontaneous sentence production (Dell, 1986). Its key assumptions were that representations of the utterance to be spoken are constructed at semantic, syntactic, morphological, and phonological levels, and the items that participate in these representations are retrieved through spreading activation in a network of linguistic units. When the model was first applied to aphasia, a version that simulated single-word utterances was created (Martin et al., 1994). This model was initially set up so that it mimicked normal performance. Parameters values were chosen to make the model’s error patterns in retrieving words match that of normal controls in a picture naming task (Dell, et al., 1997). Then the model was “lesioned” in an attempt to simulate aphasia. In this respect, the model is, first and foremost, a model of production, and only secondarily a model of impaired production. Over the past 15 years, however, much of the work that has developed and tested the model has used data from aphasic speakers (e.g. Hanley & Nickels, 2009; Rapp & Goldrick, 2000; but see Budd, Hanley, & Nozari, 2011, for an application of the model to normally developing children). Moreover, although these applications have concerned single-word production, it is worth noting that the model’s lexical selection mechanism is constrained by the utterance’s syntactic-sequential structure. This mechanism has been tested with sentence production data (Dell, Oppenheim, & Kittredge, 2008).
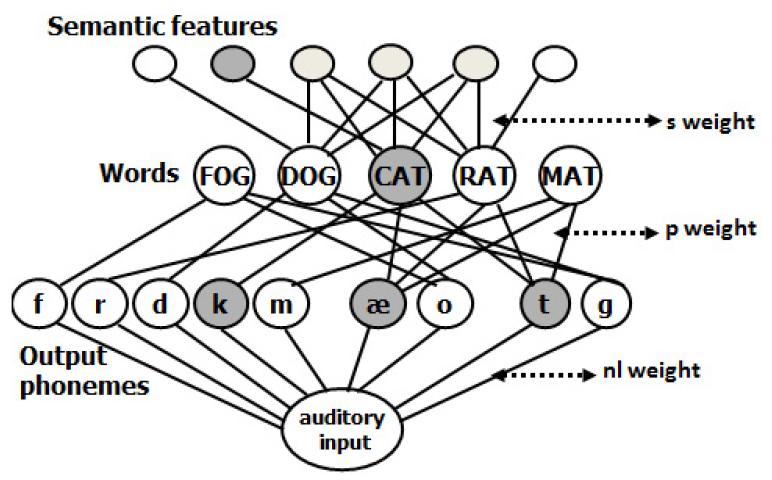
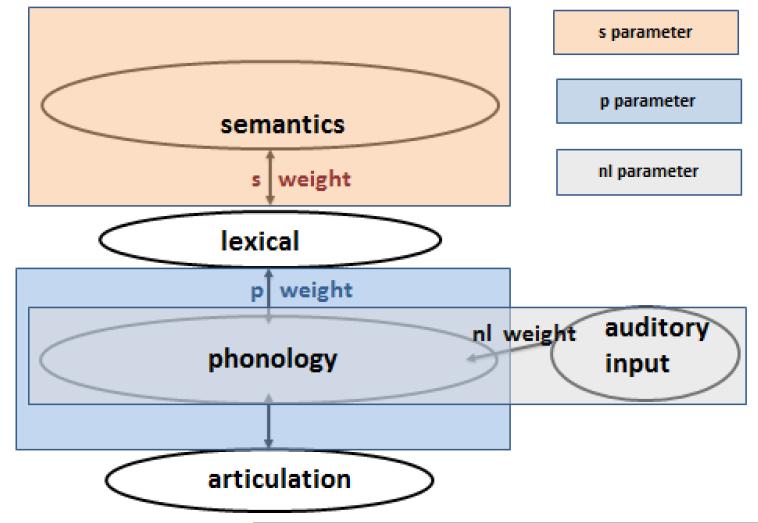
In its present form, the model consists of an interconnected network of semantic, lexical, and output phonological units, and a further set of connections between auditorily presented verbal input and the output phonological units, as shown in Figure 1. All connections are bidirectional, thus making the model’s flow of activation interactive. In naming, lexical access starts with a jolt of activation to the target word’s semantic features. This activation flows through the network and, after a fixed period of time, the most active word unit of the appropriate grammatical category is selected. This selection completes the first “step” of lexical access. Errors at this step are necessarily lexical in nature (e.g. semantic, CAT→DOG; unrelated, CAT→LOG; formal, CAT→MAT, or mixed semantic-formal, e g. CAT→RAT). The second step also begins with a jolt of activation, this time to the selected word unit. Activation once again spreads throughout the network, culminating in the selection of the most activated phonological units. Errors at this step are typically nonwords (e.g. CAT→ “cag”) but they can also be formally related words (e.g. CAT→“mat”). Errors, in general, are possible because the spreading activation process activates units other than the target units and, given the model’s activation function, which includes random noise, there is some chance that erroneous units’ activations will exceed those of the target units. The model has been successful at simulating the variety of error patterns in naming. For example, Schwartz et al. (2006) found that it explained 94.5% of the variance in naming error patterns in an unselected group of 94 chronic aphasic speakers. Nonetheless, a few patients’ error patterns cannot be well simulated by the model (e.g. Rapp & Goldrick, 2000; Ruml et al., 2005; Schwartz et al., 2006) and there are model features that cannot be effectively tested solely by the degree to which it fits naming error patterns (e.g. Goldrick, 2011).
Figure 1.
The dual-route interactive two step model and its three parameters. For the naming task, the semantic features are activated and selection occurs first at the word level, and then at the output phonemes. For nonword repetition, the auditory input node is activated and selection occurs at the output phonemes. For word repetition by the lexical route, the target word node is activated and selection occurs at the output phonemes. For dual-route word repetition, both the target word node and the auditory input node are activated, and selection occurs at the output phonemes.
The model’s account of auditory repetition depends on whether the target is a word or a nonword. To allow for nonword repetition, the model incorporates a non-lexical repetition route, a mechanism that allows for production of phonological sequences that are not already stored in the lexicon (e.g. Gupta, 2003; Gupta & Tisdale, 2009; Hanley, Kay, & Edwards, 2002; similar to analogous mechanisms in models of reading, e.g. Seidenberg & McClelland, 1989; Coltheart et al., 1993). The model’s non-lexical route is contained in the connections from auditory input directly to the output phonological units. Word repetition involves two routes: the non-lexical route as well as a lexical route that corresponds to the second step of lexical access from meaning. In this dual-route approach to word repetition, the activation generated over both routes converges on the output phonology (e.g. the summation hypothesis, Hillis & Caramazza, 1991). Specifically, to repeat a word, the model starts with a jolt of activation to the non-lexical route input unit, as well as one to the word unit. Activation flows from these units throughout the network and, after a fixed period of time, the most active phonological units are chosen for output. The model also allows for word repetition to be performed in an entirely lexical manner without the non-lexical contribution. Comparisons between this purely lexical model and the dual-route model have generally showed that many, but not all, aphasic individuals repeat words by combining activation across both routes (Abel et al., 2009; Hanley et al., 2004; Nozari et al., 2010; but see Baron et al., 2008). Given that the model’s characterization of word repetition allows for multiple influences, a major goal of this work is to see how model parameters that are critical for repetition relate to brain areas.
The model’s account of pathological naming and repetition is that brain damage decreases the network’s ability to transmit activation. Specifically, the model assumes three lesionable parameters, that is, parameters whose values differ among the patients: s, the strength or weight of the bidirectional connections between semantic and lexical units, p, the weight for the corresponding lexical-phonological connections and nl, the weight of the connections between the auditory input and the phonological units. In a naming task, both s and p weights contribute, with s weights contributing more to the first step of access, and p weights contributing more during the second step. For nonword repetition, the nl parameter is the most important factor, and in word repetition both p and nl contribute. Because of the interactive property of the model, the s weight also matters to a small extent in repetition, a claim that is supported by demonstrations of semantic influence on word and nonword repetition (e.g. Jeffries et al., 2005; Martin et al., 1996; Patterson et al., 1994).
Patients are assigned values of s, p, and nl by a model fitting process. The entire procedure is illustrated in Table 1 with data from a previously studied patient (Schwartz et al., 2006; Dell et al., 2007). First, the s and p parameters are set so that the model mimics the patient’s naming error pattern (steps 1-2). With the model set up with those s and p parameters, it is then fit to the patient’s nonword repetition performance by adjusting the nl weight (steps 3-4). Finally, the entire set of parameters is tested to see whether it can accurately predict word repetition (steps 5-6). In this final repetition test, both the dual-route and lexical-route approaches to word repetition can be evaluated. In Table 1, a test using the dual-route approach is illustrated.
Table 1.
Computation of model parameters for an example patient (Schwartz et al., 2006; Dell et al., 2007).
| 1. Obtain patient naming error pattern on the Philadelphia Naming Test (PNT). | |||||
| Patient naming response proportions | |||||
| Correct | Semantic Error | Formal Error | Mixed Error | Unrelated Word | Nonword Error |
| .20 | .07 | .15 | .02 | .29 | .26 |
| 2. Fit model to naming response proportions to obtain s and p parameters. | |||||
| Model naming proportions for s = .005, p = .018, rmsd = .045 | |||||
| Correct | Semantic Error | Formal Error | Mixed Error | Unrelated Word | Nonword Error |
| .21 | .12 | .19 | .03 | .20 | .26 |
| 3. Obtain patient response proportions in nonword repetition test. | |||||
| Patient nonword repetition response proportions | |||||
| Correct Response | Lexicalization Error | Nonword Error | |||
| .78 | .12 | .10 | |||
|
4. Using model, parameterized to the patient (e.g. s=.005 and p=.018), determine the best value of nl so as to match nonword repetition performance. | |||||
| Model nonword repetition proportions for nl = .046 | |||||
| Correct Response | Lexicalization Error | Nonword Error | |||
| .78 | .09 | .13 | |||
| 5. Using model parameters, predict word repetition performance. | |||||
| Prediction from Dual-Route Repetition model using s=.005, p=.018, nl=.046 | |||||
| Correct | Semantic Error | Formal Error | Mixed Error | Unrelated Word | Nonword Error |
| .98 | .00 | .01 | .00 | .00 | .01 |
|
6. Obtain word repetition performance (Philadelphia Repetition Test) for the patient to compare to model prediction. | |||||
| Patient word repetition response proportions | |||||
| Correct | Semantic Error | Formal Error | Mixed Error | Unrelated Word | Nonword Error |
| .97 | .00 | .02 | .00 | .00 | .02 |
It is important to emphasize that the model’s parameters each index specific and separate properties of lexical retrieval. The s parameter is most strongly associated with what is sometimes called lemma-access, L-access, or post-semantic lexical access. It is not supposed to be a measure of the semantic representation, but of the ability to map from semantics to abstract lexical units. Given this, it is further assumed that the errors that are associated with the s parameter are post-semantic errors, that is, they are not errors at the semantic level itself (see, Rapp & Goldrick, 2000 for discussion of this distinction as it relates to these kinds of models). By mapping s to lesion sites, we may be able to see whether s picks up multiple functions by examining whether it maps to multiple regions. For example, some aphasic individuals do have semantic-level damage, and this damage can create semantic errors in naming. Or some may have difficulty manipulating otherwise intact semantic representations also leading to error. In all of these cases, the model associates the errors with the s parameter. Thus, the s value assigned to a patient may absorb several functions, including those that are outside of the model’s characterization of the parameter. Similarly, the p and nl parameters, and the errors that are associated with them, index separate production-related retrieval processes in the model. We would thus expect them to associate with distinct brain areas. Our VLPM study may support or challenge these assumptions.
1.2. Relating Model Parameters to the Brain
The functional distinctness of the s, p, and nl parameters is easy to see. Each reflects a different set of connections in the model, and variation in all three was found to be required to account for the behavioral variation among patients and between patients and normal speakers. Given this, what can be expected about their mapping to the brain? First of all, as we just mentioned, we will ask whether each parameter maps to a different brain region or set of regions. The VLPM can provide a straightforward answer to this question. Secondly, what region or set of regions should associate with each parameter? In what follows, we describe some recent developments in the neuroscience of language that have relevance and can be used to guide preliminary expectations.
The 19th century Broca-Wernicke-Lichtheim model of word production and comprehension has garnered new interest with recent evidence that sensory and motor speech representations are tightly coupled in a functional-anatomical circuit loosely extending between Wernicke’s area and Broca’s area (Hickok & Poeppel, 2000; Pulvermüller, 2005). This classical circuit has been assimilated into accounts of a dorsal language pathway that translates between sensory (auditory and somatosensory) representations of heard speech and articulatory-motor codes (Gow, 2012; Hickok & Poeppel, 2004, 2007; Isenberg et al., 2012; Pulvermüller, Huss, Kherif, & Moscoso del Prado Martin, 2006; Saur et al., 2008; Warren, Wise, & Warren, 2005). In Hickok and Poeppel’s influential “dual stream” model (Hickok & Poeppel, 2004, 2007), the dorsal stream encompasses auditory-phonological representations in the superior temporal gyri and sulci of both hemispheres (including Wernicke’s area on the left); a sensori-motor interface located within the posterior aspect of the Sylvian fissure (posterior planum temporale) and extending into the parietal-temporal junction and parietal operculum (area Spt, shorthand for Sylvian parietal-temporal); and a frontal articulatory network (including Broca’s area). The evidence is strong that these dorsal stream regions underpin production processes in repetition and naming: The left posterior superior temporal gyrus has been linked to phonological form retrieval (Graves, et al., 2007; Graves, Grabowski, Mehta, & Gupta, 2008; Indefrey, 2007; Indefrey & Levelt, 2004; Wilson, Isenberg, & Hickok, 2009), the parietal-temporal and inferior parietal regions to phonological short-term memory (Baldo & Dronkers, 2006; Buchsbaum, et al., 2011); and parieto-frontal cortices and insula to articulatory-motor planning in speech and STM rehearsal (Baldo & Dronkers, 2006; Blank, Scott, Murphy, Warburton, & Wise, 2002; Dronkers, 1996; Hillis et al., 2004). The hypothesis that phonological errors in production stem from dorsal pathway lesions, possibly including the arcuate fasiculus fiber tract, dates back to Wernicke (Compston, 2006) and finds support in contemporary patient research (Buchsbaum, et al., 2011; Cloutman et al., 2009; Duffau, Gatignol, Mandonnet, Capelle, & Taillandier, 2008; Foundas, Daniels, & Vasterling, 1998; Schwartz, Faseyitan, Kim, & Coslett, 2012).
The dual pathway framework proposes a complementary, ventral temporal-frontal system in which auditory-phonological information is associated with lexical and semantic long-term memory representations. Several key sites have been identified: the left middle temporal gyrus and posterior inferior temporal gyrus for the retrieval of lexical-semantic information (lemmas) (Damasio, Grabowski, Tranel, Hichwa, & Damasio, 1996; Gow, 2012; Hickok & Poeppel, 2004; Indefrey & Levelt, 2004; Schwartz, et al., 2009); the anterior temporal lobe and parietal-temporal-occipital region (angular gyrus) for the amodal or multimodal representation of object and event concepts (Binder & Desai, 2011; Binder, Desai, Graves, & Conant, 2009; Patterson, Nestor, & Rogers, 2007); and portions of the inferior frontal gyrus (Broca’s area) for controlled selection from semantic and lexical long-term memory (Badre & Wagner, 2007; Schnur et al., 2009; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997). Damage to all these regions is causally linked to semantically-based word retrieval difficulties (Antonucci, Beeson, Labiner, & Rapcsak, 2007; Damasio, et al., 1996; DeLeon, et al., 2007; Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001; Noonan, Jefferies, Corbett, & Lambon Ralph, 2012; Schnur, et al., 2009) including semantic errors in naming (Cloutman, et al., 2009; Schwartz, et al., 2012; Schwartz et al., 2011; Schwartz, et al., 2009; Walker et al., 2011).
Very recently, researchers have begun to build models of spoken language production that integrate neuroanatomical and computational claims (e.g. Indefrey & Levelt, 2004; Guenther et al., 2006; Hickok, 2012; Ueno et al., 2011.) Later in the paper, we will consider our results in light of two recent computational instantiations of the dual-pathway framework (Ueno et al., 2011; Hickok, 2012). As a starting point, we draw upon the dual stream model tenet that the dorsal pathway is particularly important when there is high phonological load and low semantic constraint (Hickok & Poeppel, 2004; Saur, et al., 2008). We therefore predict that p and nl will both localize to the dorsal stream. However, as p is derived from naming, a task with high semantic constraint, it is possible that the lesion map for p might extend further towards or into ventral stream territory, e.g., in the middle temporal gyrus or posterior superior temporal sulcus. The lesion map for word repetition, which, according to the model, is sensitive to both p and nl, should overlap with both of those parameters’ maps. We expect the s parameter to associate with the ventral pathway, and particularly with components of this pathway that previous studies have found to be associated with semantic errors, such as the temporal lobe areas mentioned above. However, it should be noted that the relationship between s and semantic errors in the model is indirect, and moreover, not even monotonic (see, e.g. Schwartz & Dell, 2010). Strong, that is, near normal, and very weak s weights produce fewer semantic errors than do moderate values. Hence, the intuitive expectation that the VLPM of s weight should pattern as in the VLSM of semantic errors is far from certain.
In general, the model parameters are not, as a rule, simple transformations of a particular kind of error. The p parameter, for instance, is negatively associated with both nonword (e.g. “cag” for CAT) and formally-related word errors (“mat” for CAT) in naming. But, if the s parameter is low, formal errors will be caused more by the low s weight than a low p weight (see Schwartz et al., 2006, for an analysis of the parameter interactions that influence formal errors). These complexities in the mapping between the model’s parameters, and the proportions of particular error types motivate the study of the parameter-brain relations, aside from studies of the errors themselves. Ultimately, errors are just symptoms of derailed cognitive processes, and it is these processes that we seek to characterize and, here, to map to the brain. The parameters are direct indices of those processes as conceived in the model.
2. Methods
2.1. Participants
We analyzed data from 103 participants in an ongoing investigation of psycholinguistic deficits in aphasia (the Moss Aphasia Psycholinguistic Database; www.mappd.org; see Mirman et al., 2010) and their neural correlates. Participants authorized release of medical records and gave informed consent to participate in multiple sessions of language testing under protocols approved by the Institutional Review Board (IRB) at Einstein Medical Center. Those who participated in MRI or CT scan studies as part of this project did so under an approved protocol of the Perelman School of Medicine at the University of Pennsylvania. All participants were paid for their participation.
As criteria for inclusion, participants had to be (at time of testing) at least 1 month post onset of aphasia; living at home; medically stable; and without major psychiatric or neurologic co-morbidities or uncorrected visual or hearing impairment. All were premorbidly right handed and had English as their primary language. Potential participants were excluded if they failed an audiometric screening exam (adapted from Ventry & Weinstein, 1983), were unable to produce a single correct, intelligible response in confrontation naming, or had bilateral or solely subcortical lesions.
The 103 study participants were 43% female and 46% African-American, with a mean age of 58 and 14 years of education. Mean and median months post onset was 52 and 23, respectively; 87% were in the chronic phase (at least six months post). On the Western Aphasia Battery (Kertesz, 1984), the aphasia quotient, measuring aphasia severity, averaged 73 (mild-moderate). 71 participants had fluent aphasia (46 anomic, 17 conduction, 8 Wernicke’s), 32 had non-fluent aphasia (28 Broca’s, 3 transcortical motor, 1 global). According to a clinical evaluation of speech articulation, described below, 23 participants (17 with Broca’s aphasia) had apraxia of speech.
2.2. Language tests
All participants received a battery of tests (Mirman et al., 2010) to determine their aphasia status, their linguistic and cognitive skills, and to provide the data used in assigning model parameters. The tests that are referenced here include the following:
Philadelphia Naming Test (PNT, Roach et al., 1996)
The PNT tests basic-level object naming ability. Participants see line drawings of 175 familiar pictured objects from several semantic categories and must produce the single word name of each. The procedures for administration, transcription, and response categorization are described in other work (e.g. Schwartz et al., 2006). For our purposes, the responses are categorized as correct, semantic errors, form-related word errors, mixed semantic-formal errors, unrelated word errors, and nonword errors. These response proportions (relative to all responses) are used as described below to assign the s and p model parameters.
Philadelphia Repetition Test (PRT)
This is the word repetition counterpart to the PNT. The PNT words were presented auditorily for repetition, and responses were categorized exactly as they were for the PNT. See Dell et al. (2007) for further details and test administration procedures.
Nonword repetition test
60 nonwords were derived from one- and two- and three-syllable items of the PNT (mean = 1.57 syllables) by pseudo-randomly changing two phonemes to create a pronounceable nonword that was clearly different from the word. They were recorded on tape and presented one at a time for immediate repetition (single hearing only). Responses were categorized as correct, lexical error, non-lexical error, or omission. The responses were used to determine the nl parameter as described below.
Additional tests
Verbal comprehension was assessed using two tests: Peabody Picture Vocabulary Test-Third Edition (PPVT-III; Dunn & Dunn, 1997), and the Synonymy Triplets Test (N. Martin, Schwartz, & Kohen, 2005). In PPVT-III, the patient must select one of the four pictures that best matches a spoken word. The test consists of 204 items arranged in order of increasing difficulty, representing various parts of speech. In the Synonym Judgment Test, the patient must select two of three words that best go together (e.g., violin, fiddle, clarinet). The test comprises 30 trials (half nouns, half verbs), and all words are semantically related in any given trial, with two having a closer relationship. The scores on these tests were converted to percentages, and a composite score was calculated by averaging those percentages. This composite measure (Vcomp) was used in the behavioral regression analyses.
Similarly, a composite of two measures was used to assess nonverbal comprehension (NV comp) The 52-item Pyramids and Palm Trees test (Howard & Patterson, 1992) requires trial by trial matching of a pictured item to the closer of two pictured choices (e.g., item: pyramid, choices: palm tree, pine tree). The 64-item Camels and Cactus test (Bozeat et al., 2000) is similar but with four same-category items in the choice set (e.g., item: camel; choices: cactus, tree, sunflower, rose). The scores on these two tests were converted into percentages and averaged to create the NVcomp score used in the behavioral regression analyses.
To evaluate auditory phonological processing, we used the no-delay version of the Auditory Discrimination of Word and Non-word Pairs task (N. Martin & Saffran, 1997). The participant hears two items in immediate succession (20 word trials; 20 nonword trials) and must judge whether the two were the same or different. Non-identical pairs differ in either onset or the final phoneme.
Two measures of short-term memory were used, to determine the maximum capacity of semantic and phonological short-term memory (R. C. Martin & Freedman, 2001). In the Category Probe Span test, the patient listens to a string of n words, immediately followed by a probe word, and must determine if the probe is from the same semantic category as any of the preceding words by saying or pointing to ‘Yes/No’. The n gradually increases and the test is terminated when accuracy drops to 75%. The Rhyme Probe Span test follows the same pattern, but the judgment concerns whether the probe rhymes with any of preceding words.
Patients were diagnosed as having apraxia of speech if, on selected subtests of the Apraxia Battery for Adults (Dabul, 2000), their speech was found to contain multiple instances of segmental distortion/substitution, as well as abnormal prosody, slow rate (lengthened consonants and vowels), and/or sound, syllable or word prolongation often accompanied by intrusive schwa.
2.3. Model fitting
The proportions of response categories in the PNT and the Nonword repetition test were used to derive model parameters for each patient as illustrated in Table 1. Specifically, step 2 in the Table involves a search for the s and p parameters that make the model match the patient’s naming response proportions as well as possible; that is, the selected parameters maximize the likelihood of the data. The formal properties of the search are those described in Dell et al. (2004), with the exception that, for the present analysis, parameters were constrained to be either in the normal range (.04-.06) or less than normal (0-.04).1 For the new sample of 103 patients, the resulting fit quality to the naming data was good. The mean uncorrected root mean squared deviation (rmsd) provides an intuitive measure. In the present sample, the average rmsd was .023 (range .002-.145). This fit quality is similar to that obtained with the model for other patient groups (Foygel & Dell, 2000, rmsd = .029; Schwartz et al., 2006, rmsd = .024).To get a feel for how close a fit an rmsd of .023 is, consider that the rmsd of the fit illustrated in Table 1 is considerably worse (.045).
The determination of the nl parameter involved adding the performance on the nonword repetition test to the mix. For each patient, the model was set up with the s and p parameters that were determined from the patient’s naming. Then the nl parameter was varied through its range so that the model’s nonword repetition performance matched the patient’s. As there is only one parameter to vary, the best fits were easy to find.
2.4. Lesion analysis
Structural brain images were acquired using MRI (n = 57) or CT (n = 46). Details of imaging, segmentation, and registration procedures have been published previously (Schwartz et al., 2009). For patients with MRI scans, lesions were first drawn manually on a 1×1×1 mm T1-weighted structural image. Lesions were masked, and then the scans and lesion maps were registered to a common template (MNI “Colin 27”) using an automated registration algorithm (Avants, Schoenemann, & Gee, 2006). The final lesion map was quantized to produce a 1/0 map using 0.5 as the cutoff. All lesions were drawn by a trained technician and checked by co-author H.B.C., an experienced behavioral neurologist; both were blind to the behavioral data. For patients with CT scans, H.B.C. drew lesion maps directly onto the Colin27 volume.
For stability in the comparison of lesioned/nonlesioned performance, only voxels with 10 or more lesions (10% of the sample) were included in the analysis. In each of these voxels, a t statistic was computed comparing patients with and without lesions on the dependent variable. The resulting t map was thresholded to control for false discovery rate (FDR; Genovese, Lazar, & Nichols, 2002) at q = 0.05, where q is the expected proportion of false positives among suprathreshold voxels. There is no standard for setting FDR thresholds in voxel-wise lesion analysis. The separate VLPM analyses of p, nl, and s reached significance at q-values between .01 and .05, depending on the parameter. We report effects at q = .05 in the interest of uniformity.
Lesion analyses were carried out using the VoxBo brain imaging package (http://www.nitrc.org/projects/voxbo/). The anatomical locations of voxels found to exceed threshold were determined by the judgment of H.B.C. in consultation with the Brodmann map and automated anatomical labeling (AAL) atlas within MRIcro (www.mccauslandcenter.sc.edu/mricro/). The AAL atlas was used to locate and quantify the size of supra-threshold voxel clusters.
3. Results and preliminary discussion
First, we consider some of the properties of the model parameter distributions independently of their mapping onto the brain. Then we present the results of the VLPM analysis and conclude with a critical test of a prediction from the model regarding the relationship between word repetition and model parameters.
3.1. Analysis of cross-parameter relationships
As this is the largest patient sample that has been modeled, it is useful first to identify some of the properties of the parameter relationships. Because of the mechanisms whereby parameter values affect the model’s performance, differences between smaller parameter values have more impact than differences between larger values. For example, when comparing a model with s=p=.010 to one with s=p=.015, the increase in weights of .005 decreases error probability by .23; the same weight increase from s=p=.030 to s=p=.035 decreases the error rate by only .04. Consequently, all of the analyses of parameters in this paper use the square root transform of the values, rather than the values directly. This makes the impact of parameter variation in the lower and higher end more comparable and also leads to reasonably normal distributions, suitable for parametric regressions.
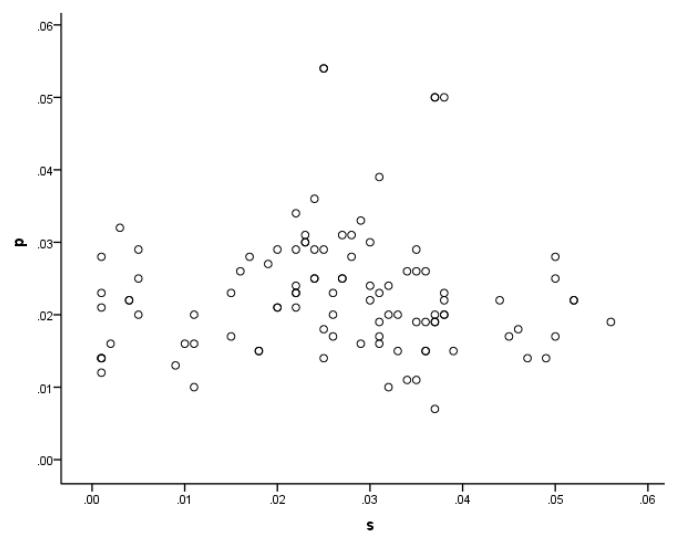
According to the model, each parameter represents the strength of different sets of weights. Moreover, it is assumed that each set can be damaged independently of the others, that is, that the abilities linked to the parameters can dissociate. Previous work had demonstrated that the two parameters derived solely from naming data, s and p, were largely independent in samples of aphasic individuals (e.g. Schwartz et al., 2006). Figure 2 shows that this clearly was the case for the current sample as well. The values were uncorrelated, with r = .08. There were patients with a high value of one parameter, but a low value of the other, and the reverse. In short, there was a double dissociation between s and p.
Figure 2.
Scatterplot for untransformed values of s and p for the sample. (Note that the associated correlation coefficient reported in the text, and all other statistical analyses of parameter values, were calculated using the square-root transformed values.)
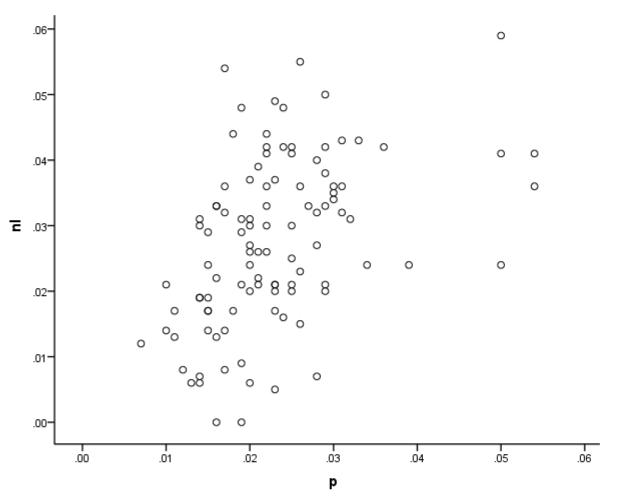
Was this true for the two parameters that are more associated with the repetition task, p and nl? It was not (Figure 3). p and nl were moderately positively correlated, r = .46, p < .001, and there was a notable lack of patients with low values of nl and high values of p. Thus, the behavioral data that determines the model parameters suggested that p and nl may not be as separate as the model implies. To further explore the association between nl and p, we carried out regressions in which each parameter was predicted from the other parameter, also including measures of speech apraxia, auditory discrimination, working memory, and semantic comprehension ability referred to in the methods sections. The predictors in these and subsequently reported regressions were entered simultaneously, except where noted.
Figure 3.
Scatterplot for untransformed values of p and nl for the sample.
The results showed that nl was predicted significantly by only two factors, both of which contributed positively to the nl value, parameter p (t = 3.39, p =.001) and auditory discrimination (t = 4.98, p <.001). Thus, the non-lexical repetition route, whose strength is indexed by nl, reflected both the ability to process the auditory input and a production ability, shared with parameter p. The absence of a sizable effect of the verbal and nonverbal semantic comprehension measures was expected from the model, as the effectiveness of the model’s non-lexical route is known to be only weakly affected by supra-lexical information (see Nozari et al., 2010). The lack of an effect of the speech apraxia measure was also noteworthy because it dissociated nl (and its shared variance with p) from articulatory-motor effects.
Parameter p was predicted, as expected, by nl (t = 3.39, p=.001). The only other significant predictor of p was the presence of speech apraxia which was negatively associated with high values of the parameter (t = −2.46, p = .016). It appears that parameter p, with its association to apraxia, absorbs some motoric processes, whereas nl, which was significantly linked to auditory discrimination, picks up auditory abilities.
3.2. Predicting word repetition from nl and p
The central claim of the dual-route model is that word repetition is carried out by summing activation from the lexical route (largely determined by parameter p), and the non-lexical route (parameter nl). Thus, patient word repetition (the score on the PRT) should be predicted primarily by nl and p. Because of the interactive property of the model, we also expected that semantic ability would make a small contribution to word repetition. Input from semantics interactively promotes the strength of the repeated word’s phonology (e.g. Dell et al, 2007; Jeffries et al., 2005, 2006). We carried out this regression on the sample of 103 patients, using as independent variables, p, nl, and the secondary cognitive measures used in the regressions predicting p and nl; as the dependent variable, we used accuracy on the PRT. As expected, there were positive contributions from both model parameters, with a sizable adjusted R2 of .61. nl (t = 6.05, p <.001) and p (t = 3.75, p<.001) were potent contributors, and the composite measure of verbal semantic comprehension (VCOMP) was a weaker but significant factor as well (t = 2.15, p = .034). If nl is left out of the prediction equation, the R2 drops by .141, and if p is left out, it drops by .053. For each of these two parameters, the additional variance explained by it is reliable, F(1,94) = 36.6, for nl, and F(1.94) = 13.9 for p. No other independent variables contributed significantly. This result provides excellent behavioral support for the interactive dual-route approach to word repetition (e.g. Hanley et al., 2004; Nozari et al., 2010).
The analyses of cross-parameter relationships and the ability of the parameters to predict word repetition set the stage for mapping the parameters to the brain. For example, the correlation between p and nl leads to an expectation of some degree of similarity in their lesion maps. Also, we can examine the neural analogue to the regression in which word repetition is predicted from the parameter values. The expectation is that the voxels that predict word repetition should correspond to those that predict nl and p.
3.3. Anatomical findings
The statistical power to detect brain-behavior relationships at a given voxel depends on the number of patients with and without a lesion in the voxel (Kimberg, et al., 2007). Since the strokes that cause aphasia generally are those that compromise the anterior cortical circulation (left middle and anterior cerebral arteries), it was predictable that, in this large sample of aphasia stroke patients, coverage would be excellent in and around the peri-Sylvian region. For example, in the inferior frontal gyrus (IFG), supramarginal gyrus (SMG), superior temporal gyrus (STG) and middle temporal gyrus (MTG), maximal lesion counts were in the 40-50 range, which is around the 50%-50% lesion/nonlesioned breakdown that leads to the greatest power. Coverage was predictably poor in the territory of the posterior circulation, e.g., counts <10 in some inferior temporal/fusiform gyrus voxels that have been implicated in lexical and lexical-semantic processing (e.g., Cloutman et al., 2009). The present data cannot speak to the possible contributions of these areas of low coverage.
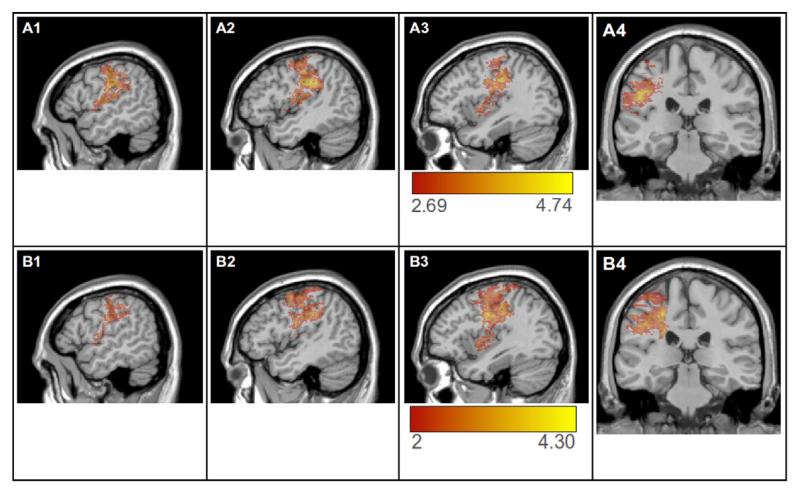
3.3.1. Map for parameter p
In the VLPM analysis for p, with the FDR-thresholded critical t = 2.69, a total of 30,437 significant voxels were identified. These were largely concentrated in the anterior part of the dorsal stream, including the SMG, postcentral gyrus, precentral gyrus, and insula (See Figure 4, A1-A4). Voxels with maximal t-values (>4.50) were found primarily in the SMG and postcentral gyrus. There were few if any voxels identified in posterior temporal or parietal-temporal dorsal steam cortices, or in the semantically-important ventral stream regions (e.g., MTG, angular gyrus, anterior temporal lobe). These results are similar to what Schwartz et al., (2012) found in their VLSM analysis of phonological errors, a major factor in determining the value of the p parameter.
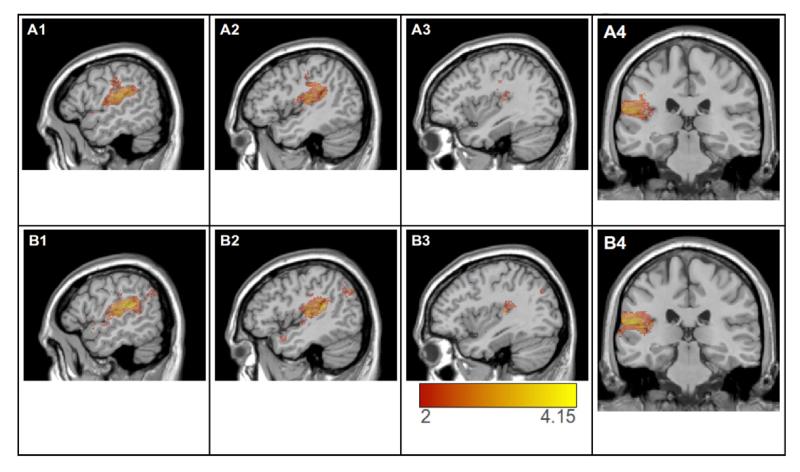
Figure 4.
Top panels (A1-A4) show results of the VLSM analysis of p, thresholded at a false discovery rate correction, q = .05 (critical t = 2.69). Lower panels (B1-B4) shows the VLSM of the component of p that is orthogonal to nl, thresholded at t = 2.0 (uncorrected). Both maps are rendered on the MNI-space Colin27 template. Panels 1-3 show sagittal slices at MNI coordinate x = −54, x=−46, and x=−38; panel 4 shows coronal slice at MNI coordinate y=−28.
The link between p and the anterior parietal cortices (postcentral and supramarginal gyri) suggests that some of the processes leading to phonological errors in production from meaning are performed by somatosensory cortical areas. Also of importance was the finding that there were very few significant voxels in the posterior superior and middle temporal gyri (Wernicke’s area), a region sometimes associated with lexical-phonological word forms (Graves, et al., 2008; Indefrey & Levelt, 2004; Wernicke, 1874/1969). An interpretation of this finding, which we expand on later, is that phonological errors are generated at a later production stage than that of the retrieval of word forms.
3.3.2. Map for parameter nl
The VLPM analysis of nl (critical t = 2.97) identified 14,544 significant voxels (Figure 5, A1-A4). The nl-associated areas included substantial portions of the STG, the posterior third of the planum temporale and cortex at the juncture of the parietal and temporal lobes (area Spt) as well as the SMG and postcentral gyrus.
Figure 5.
Top panels (A1-A4) show the VLSM analysis of nl, thresholded at a false discovery rate correction, q = .05 (critical t = 2.97). Lower panels (B1-B4) shows the VLSM of the component of nl that is orthogonal to p, thresholded at t = 2.0 (uncorrected). Both maps are rendered on the MNI-space Colin27 template. Panels 1-3 show sagittal slices at MNI coordinate x = −54, x=−46, and x=−38; panel 4 shows coronal slice at MNI coordinate y=−28.
To quantify the extent of the overlap between the p and nl maps, we used the Phi statistic, which is essentially a correlation coefficient for two binary variables. For this correlation, each observation comes from a single voxel, and consists of a pair of numbers, a 1 (significant) or 0 (not significant) for p, and then the same for nl. Across the more than 300,000 voxels examined in the study, 8,025 voxels were significant for both nl and p, and approximately 29,000 were significant for either p or q but not both. The Phi coefficient equals .34, indicating both considerable overlap and nonoverlap, much as a correlation coefficient of this value would indicate an imperfect association. Exact statistical inference, however, is not possible given the inherent smoothness of lesion data, thus leading to very large, but unpredictable, dependencies among close voxels.
As Figures 4 and 5 illustrate, the area common to the p and nl maps, identifying voxels what were significant in both analyses, occupies the SMG and postcentral gyrus, with minimal extension into the precentral gyrus. Outside this common region, the map for p extends into the insula and more superior regions of the parietal lobe, while the nl map extends into the superior temporal auditory areas.
In VLSM studies, one can create maps for two or more residualized variables to enhance the contrast of their sets of significant voxels (e.g. Schwartz et al., 2011). We used this method to more closely visualize the differences in which voxels were significantly activated for p and nl. In addition to the main maps for p and nl, the lower panels of Figures 4 and 5 also show residualized maps of each parameter. Figure 4 maps the residuals from a regression in which nl predicts p, that is, a new variable reflecting the component of p that is orthogonal to nl. Figure 5 contains a map for the reverse, a set of residuals for the component of nl that is orthogonal to p. These maps are shown with an arbitrary threshold of t=2.0 in order to make large clusters more apparent. The locations of those clusters on the residualized maps support the characterization of the differences between p and nl presented in the previous paragraph.
The similarities and differences between maps for p and nl corresponded well to the behavioral data. Recall that p and nl were positively correlated, but that p, and not nl, was associated with speech apraxia, while nl, and not p, was linked to auditory discrimination abilities. We return to this parallel in the general discussion.
3.3.3. Word repetition predicted from p and nl
The dual-route model predicts that word repetition is carried out by summing the lexical and non-lexical route. In the behavioral data this prediction was supported by showing that word repetition accuracy was strongly predicted by the nl and p parameters. It is important to note that the successful test of this prediction constituted a cross-task and cross-material verification of the model. The p parameter is derived from word naming, and the nl parameter from nonword repetition, and what was predicted was word repetition. With respect to anatomy, we make three specific predictions. First, the voxels associated with p should overlap with the voxels associated with word repetition. Second, the same should be true for nl. Finally, because the model’s word repetition performance is a function of activation summed over sources strongly associated with p (the lexical route) and nl (the nonlexical route), the summed value of the two parameters would be expected to overlap particularly extensively with word repetition.
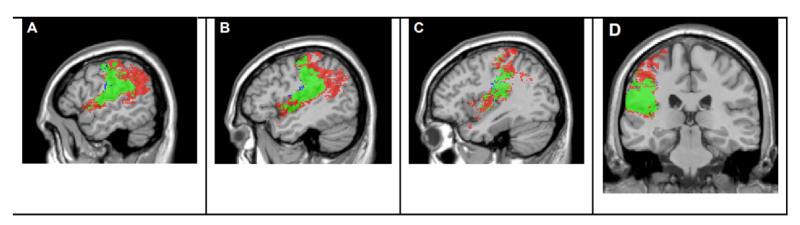
Figure 6 shows the map for word repetition (PRT score). With the critical t = 2.42, there were a total of 63,753 voxels that significantly predicted word repetition There is a moderate degree of overlap between word repetition voxels and p voxels (Phi = .45), and also for word repetition and nl (Phi = .44). The overlap is much greater, though, when word repetition voxels are compared to the significant voxels for the sum nl+p (critical t=2.69). The value of Phi is .63. Figure 6 superimposes the maps for the nl+p sum and word repetition, and the high degree of similarity of the maps is apparent. These findings provide neuroanatomic support for the dual-route property of the model.
Figure 6.
Lesion masks derived from the VLSM analyses of repetition accuracy, in red, and the VLSM analysis of sum nl+p, in blue, with the overlap in green. Statistical maps used to create masks were thresholded at a false discovery rate correction (q = .05) and rendered on the MNI-space Colin27 template. The critical t-value for PRT was 2.42 and for sum nl+p, 2.69. Panel show sagittal slices at MNI coordinate x = −54, x= −46, x=−38 and coronal slices at MNI coordinate y = −28 respectively.
A notable feature of the lesion map for repetition is the extension of the lesion map posteriorly into the angular gyrus and the posterior temporal lobe, including most of the STG and small portions of the MTG. As we noted earlier, the angular gyrus and MTG are known to be important for semantic processing, so one interpretation of this finding is that in addition to the processes captured by p and nl, successful word repetition also is promoted by good semantic comprehension of the target word.
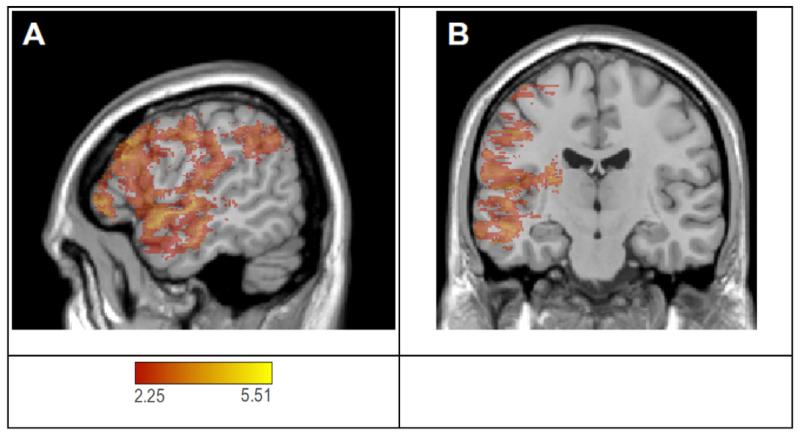
3.3.4. Map for parameter s
Figure 7 shows the VLPM map for s (critical t-value = 2.25). A very large number of voxels (110,395) exceeded this threshold and, different from the p and nl parameters, these were widely distributed in the left hemisphere. Anteriorly, there were large concentrations of voxels in the anterior temporal lobe (anterior superior and middle temporal gyri and temporal pole; maximal t-value 4.94), and prefrontal cortex (middle and inferior frontal gyri; maximal t-value 5.0). Posteriorly, there was a sizeable cluster at the juncture of parietal-temporal and parietal-temporal-occipital lobes, including the angular gyrus (2613 voxels; maximal t-value 3.85). There were no significant voxels in the posterior STG/MTG region that includes Wernicke’s area (i.e., between the middle portion of the temporal lobe and the termination of the Sylvian fissure).
Figure 7.
VLPM analysis of s-weight (red-yellow) thresholded with a false discovery rate correction (q = .05), rendered on the MNI-space Colin27 template. The critical t-vlaue was t = 2.25. Panel A shows a sagittal slice at MNI coordinate x = −54 and panel B shows a coronal slice at MNI coordinate y = −16.
It is apparent in Figure 7 that the s-map also includes voxels in the anterior dorsal stream territory where we had earlier identified effects for p. We determined that 10,568 voxels were significant in both analyses; this is 34.7% of the p-weight significant voxels and 9.6% of the s-weight significant voxels. The overlapping voxels were in the supramarginal gyrus extending into the post-central gyrus. This result initially surprised us for two reasons: first, the lack of correlation between the actual s and p parameters; second, a recent finding from our group that the VLSM maps for semantic and phonological errors in naming did not overlap at all (Schwartz et al., 2012). To address this point, we first note that random sets of significant voxels will always have some overlap if there are many significant voxels for each variable, which is true here, particularly for s. Using the Phi coefficient as a measure of the strength of the overlap controls for these effects, and here, Phi between s and p is essentially zero (+.02). Nonetheless, the overlapping s-p voxels are in the central p region. Consequently, we explored the s map further. It turned out that s is unique among the parameters in that it was significantly correlated with lesion volume (r = −.35), thus raising the possibility that some s associated voxels are just those that tend to occur in large lesions. We regressed lesion volume out of the maps for all three parameters and examined them at an arbitrary threshold of t = 2.0. The p and nl maps were largely unchanged. The s map, though, no longer exhibited suprathreshold voxels in the region that had originally overlapped with p. It did, however, retain voxels in the anterior temporal lobe, frontal lobe, and parietal-temporal junction and angular gyrus.
4. General Discussion
We determined the p, nl and s parameters of the dual-route interactive two-step model for 103 aphasic individuals, and sought their neural correlates using voxel-based lesion parameter mapping. We reached several conclusions:
The model’s s and p parameters were uncorrelated and mapped for the most part to different brain areas. The s parameter, though, did not map to a single region. This was not entirely unexpected. Prior VLSM studies of semantic errors in naming had also identified voxel clusters in multiple regions, including portions of the anterior temporal lobe (ATL), prefrontal cortex, and angular gyrus (the latter effect was specific to thematically-related semantic errors; Schwartz et al., 2011; also Schwartz et al., 2012; Schwartz et al., 2009; Walker et al., 2011). The map for the s parameter identified all these regions, and another in the anterior parietal region that we argued was probably explained by the correlation of s with lesion size.
Overall, the VPLM of s-weight questions the model’s characterization of s as a single cognitive function. The estimated value of s likely picks up on error generation processes that are not strictly associated with the transmission of activation from semantic to lexical representations (see, e.g. Rapp & Goldrick, 2000; Schwartz et al., 2006). This could include semantic representations that serve both comprehension and production; the ATL and angular gyrus are considered major sites for these core semantic representations (e.g., Patterson et al., 2007; Binder & Desai, 2011; Binder et al., 2009). It could also include attentional or working memory processes that control such representations; this would help explain the large concentration of s-associated voxels in prefrontal cortex (Badre & Wagner, 2007; Hamilton, Martin, & Burton, 2009; Schnur, et al., 2009; Thompson-Schill, et al., 1997) and possibly in parietal-temporal cortex as well (Jefferies, Baker, Doran, & Lambon Ralph, 2007; Jefferies, Crisp, & Lambon Ralph, 2006; Noonan, et al., 2012).
In contrast to the stark differences between the s and p maps, nl and p were closely related, both behaviorally and in their associated brain regions. Although they represent separate sets of connections in the model, they did not so clearly dissociate, either behaviorally or in the brain. The behavioral regressions, however, confirmed the prediction from the dual-route aspect of model that word repetition is carried out through both nl and p. In the brain maps, there was a close correspondence between the voxels that are associated with word repetition and those associated with nl and p.
There were some differences between nl and p, though. Parameter p was correlated with speech apraxia, but not auditory discrimination, whereas nl’s correlation with these was just the opposite. Moreover, nl’s uniquely significant voxels were concentrated in the superior temporal gyrus and the temporo-parietal junction at the termination of the Sylvian fissure, involving area Spt. These areas are strongly associated with auditory discrimination and auditory-motor integration, capacities that are crucial in word repetition.
Although the findings support aspects of the model such as the independence of the s and p parameters and the contribution of both nl and p to word repetition, they also challenge the model in significant ways. The data suggest that nl and p are not as separate as the model originally claimed, and moreover, that parameter s may have functions in addition to that of connecting between semantics and lexical items. Given this, we offer in Figure 8 a tentative reinterpretation of the cognitive functions of the parameters. First, as the figure illustrates, s is not just the lexical-semantic connections, but includes semantic representations and processes that control them. This treatment of s retains the original model’s claim that s is a separate parameter from p, as supported by the finding of no correlation between the parameter values, and little similarity in their brain maps. Second, both p and nl’s functions are expanded so that they have a common function and distinct ones. We see the common function as including phonological representations, that is, ordered sets of phonological units that abstract over purely auditory or articulatory features. This claim is supported by the correlation between the two parameters and the similarity of their brain maps. In the implemented model, the units of phonological representations were phonemes, but as we discuss later, other conceptions of phonology should be considered. nl’s distinct function includes the auditory processing of speech and its translation into phonological units. This proposal is supported by nl’s correlation with auditory discrimination. p’s distinct functions include the original p-weights, as well as aspects of articulation, thus explaining the link between p and apraxia of speech. One should note in the figure that the lexical nodes themselves are not assigned to any particular parameter. By drawing the figure this way, we do not imply that direct lexical-node damage should necessarily be viewed as another parameter (but see below for a proposal along these lines). Rather, the current evidence, which does not concern lexical-grammatical features or whole-word omissions, does not provide sufficient constraint about what parameter(s) should be associated with this representation.
Figure 8.
Reinterpretation of cognitive functions of model parameters. Each parameter corresponds to a colored box, which identifies representations and connections that contribute to the value of that parameter. Production from meaning involves representations arranged vertically on the left. Repetition maps from auditory input to articulation with involvement of lexical and even semantic levels if the repeated stimulus is meaningful. There is considerable overlap between the boxes for the nl and p parameters, but the s parameter does not overlap with the others. The nl and p parameters differ in the relative involvement of audition and articulation, respectively. Their overlap corresponds to phonological representations. Note that in the original model (Figure 1), each parameter is associated with the strength of a distinct set of connections, labeled here as s weight, p weight, and nl weight.
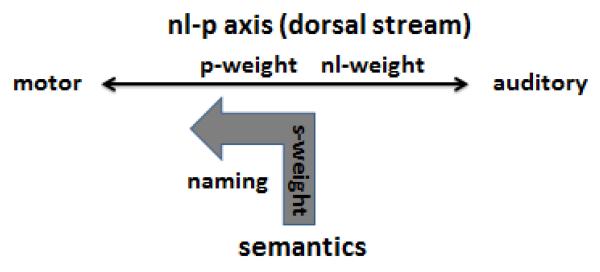
Our proposal (Figure 8) that nl and p act as a system linking auditory and articulatory aspects of speech, with their common component representing the brain’s extraction of phonology enables us to join our amended cognitive model with dual-stream neurocognitive models, as illustrated in Figure 9.
Figure 9.
Model parameters nl and p together represent the dorsal stream.
In essence, our proposal is that p and nl together represent the action of the dorsal stream and its role in the repetition of verbal stimuli (Baldo, Katseff, & Dronkers, 2012; Buchsbaum, et al., 2011; Fridriksson et al., 2010; Hickok & Poeppel, 2004). The dorsal stream is distinct from the processing associated with parameter s, which indexes semantic processes (in the ventral stream) and their use during production. Given that parameter p is derived solely from performance in the naming task, this means that the part of the dorsal stream associated with p plays an important role during language production from meaning.
To flesh out this proposal, we now turn to two recent computational models that address the contribution of the dorsal stream to language production, the Lichtheim2 model of Ueno et al. (2011) and Hickok’s (2012) hierarchical state feedback control (HSFC) model. These models have four useful features for our purposes: First, they are not just concerned with sensory-motor aspects of speech production, but rather have attempted to link up speech production and processing with the language system. Because our data and model parameters concern both semantic and phonological aspects of production, this is critical. Second, these models have computational implementations. Although the implementations have not been developed to the point that speech error patterns are simulated, they nonetheless make claims about processing levels and how processing flows among the levels. Third, the models have something to say about both word retrieval from meaning and the repetition of phonological forms. Finally and crucially, the proposed processing levels in the models are assigned to brain regions.
Before we present and discuss these two neurocognitive models and their relation to our model and data, it is important to note that all three models are quite different. They are inspired by different facts and they account for different data. For example, our model is the only one that simulates production error-type patterns and fits individual patients, as opposed to patient syndromes. Thus, the models should not be seen as strict competitors, but rather as attempts to characterize different aspects of the behavioral and neuroscientific data associated with lexical processing. As in the classic Indian parable, they are descriptions of an elephant by three different blind men. Given this, our discussion aims more for points of concurrence than to defend one model at the expense of the others. Models are difficult enough to understand and evaluate when considered singly. Yet, theoretical progress is only made when multiple models, each from a limited perspective, are considered in concert.
The Lichtheim2 model of Ueno et al. (2011) combines a multi-leveled parallel-distributed processing (PDP) approach to cognition (e.g. Rogers et al., 2004) with the notion that the neuroanatomical organization of the language system includes dorsal and ventral pathways. In Ueno et al.’s implementation, the dorsal path includes auditory cortex and surrounding areas, the inferior SMG, and motor cortex. The ventral path also links auditory and motor cortex, but through the temporal and frontal lobes. The model’s connections link the various layers of these pathways, and their strengths are learned through training. The model was trained to repeat words (map from the auditory input to motor output layers) and to produce words from meaning (map from the model’s “semantic” layer (associated with the ventral anterior temporal lobe), to motor output). Because of the model’s learning algorithm and its interactive architecture, which allows for activation to flow bidirectionally, both the dorsal and ventral paths contribute to both repetition and naming. There is, nonetheless, some specialization in the paths. The dorsal path is more important for the systematic mappings between sound and articulation, whereas the ventral path becomes specialized for the unsystematic mapping between word meaning and word form.
The fact that Lichtheim2’s dorsal path is the major factor in the repetition task makes it well suited to explaining our finding that nl and p were mapped to dorsal stream areas, and particularly the SMG, which the Lichtheim2 model associates with extracting and representing the statistical structure shared between speech sounds and phonotactics. Moreover, the role of p in naming is expected in that model from the fact that the dorsal path also makes a contribution to naming. The model also provides a good account of our finding that parameter s is strongly associated with temporal and frontal cortex. Finally, the fact that verbal semantic ability has a positive effect on word repetition is expected from the interactive property of the model.
Hickok’s (2012) HSFC model links psycholinguistic approaches to production to motor control theory. Word forms are retrieved and spoken through a control network involving phonological targets at both the syllable and phoneme level, corresponding motor programs for these units, and acoustic and somatosensory feedback to the target representations (see also Guenther et al., 2006). The crucial part of the model for our purposes is that it hypothesizes different brain circuits for programming syllable and phoneme-level units. The retrieval of whole syllable units involves a mapping between Wernicke’s area (posterior superior temporal gyrus), which contains auditory syllable targets, and BA44, which is part of Broca’s area containing syllable motor programs. With regard to phoneme units, the retrieval processes proceed through the anterior SMG, which contains somatosensory phoneme targets, to vBA6-M1 for phoneme motor programs.
Given that the p and nl parameters derive largely from phonemic errors, that is, responses such as “cap” or “cag” for CAT, it makes sense that these parameters would line up more with the phonemic rather than the syllabic control circuits in the HFSC model. We hypothesized that p and nl together represent the strength of the mapping from audition to motoric processes and that their common voxels may represent neural tissue that encodes regularities in this mapping. These regularities could involve phonemic or phonemic feature units. The p-nl overlap voxels clearly implicate the supramarginal gyrus, which the HFSC model associates with somatosensory representations of phoneme targets. Damage to such targets would thus be expected to generate phonological errors in production. In this way, the current study is consistent with the HFSC model.
When we say that damage to the p-nl overlap area leads to phonemic or phonological errors, it raises the question of how or even whether abstract linguistic representations can be distinguished from auditory and articulatory ones. Finding that the p-nl overlap localizes to sensory-motor areas of the brain, as opposed to traditional linguistic regions such as Wernicke’s area and other temporal regions associated with phonological retrieval, suggests either that the production errors that go into p and nl are not abstract phonological errors, or that phonological representations have a sensory-motoric character. The former possibility is likely true for some of the “phonological” errors that the patients make. Some may arise from faulty auditory perceptual processes (e.g. the correlation of nl with auditory discrimination abilities) or from faulty motoric planning (e.g. the correlation of p with apraxia). The latter possibility, however, also has merit because, within linguistic theory, the role of audition and articulation in shaping phonological generalizations is readily acknowledged (e.g. Cole & Hualde, 2003). Moreover, the theory of articulatory phonology (Browman & Goldstein, 1992) assumes that phonological forms consist of temporally coordinated gestures rather than abstract discrete segments. This approach to phonology is increasingly being used to interpret both linguistic and psycholinguistic data (e.g. Goldstein et al., 2007).
Gow (2012) makes a similar move towards an articulatory-based account of phonological processes in production in his arguments for a “dorsal route lexicon” that localizes to the left SMG and houses articulatorily organized word-form representations. This is in opposition to the popular view that lexical-phonological representations are auditory-based and centered on posterior temporal cortices in and around Wernicke’s area (e.g., Graves et al., 2007; Indefrey & Levelt, 2000, 2004; Wilson et al., 2009; de Zubicaray et al., 2002). Our evidence that parameter p associates with lesions to SMG and not STG favors Gow’s position.
However, let us accept the more traditional view that lexical-phonological forms inhabit the posterior temporal lobe and consider another possibility for why lesions here did not correlate with p. Our proposal is that deficits in the retrieval of lexical-phonological forms may manifest in errors of omission or, more generally, in errors in which little of the word form is present in the response. These errors can be contrasted with the target-related phonemic errors that comprise the bulk of errors that go into the determination of p. Thus, damage to the phonological lexicon in the posterior temporal lobe may lead to different kinds of errors than damage to the area linked to parameter p. This proposal is in line with Hickok (2012)’s HFSC, which assumes a role for Wernicke’s area in the retrieval of whole syllables and syllable sequences, as opposed to parietal areas that play a role at the phonemic level. Damage to the whole-syllable level may lead to omission of entire words and syllables, whereas damage to the finer phonemic level leads to perturbation, omission, or deletion of phonemes. Our proposal is also consistent with studies of aphasia that distinguish between lexical-phonological deficits and post-lexical phonological deficits (e.g. Goldrick & Rapp, 2007; Buchwald & Miozzo, 2011). For now, the claim that omission errors arising during retrieval of lexical-phonological forms will be predicted by voxels in posterior temporal regions must remain a question for future research. It is not easy to clearly identify the functional locus of aphasic omission errors, and even so, power issues confound attempts to identify the neural correlates of uncommon errors using VLSM, even in a sample of 103.
5. Conclusions
Mapping the parameter values of the dual-route interactive two-step model to the brain has supported the model in some ways and challenged it in other ways. The relation between the neural correlates of word repetition and those of the model’s nl and p parameters was consistent with the model. Also, as expected, the model’s s and p parameters were, to a large extent, neurally and behaviorally distinct. The nl and p parameters, however, were found to be correlated in the patient sample, and associated with many of the same voxels in the brain, notably in central and parietal areas, as opposed to temporal areas that have previously been associated with lexical phonological forms. This is unexpected from the model. Consequently, we reinterpreted the nl and p parameters as overlapping aspects of the dorsal stream proposed in current theorizing about language processing in the brain (e.g. Hickok & Poeppel, 2004; Hickok, 2012; Ueno et al., 2011).
As a final point, we note the value of mapping the parameters of cognitive models of pathological behavior to lesion locations. Not only can one do conventional cognitive neuroscience and locate cognitive functions in the brain, one can also use the lesion data to directly test the models and stimulate changes in them. Ultimately, such data can help bridge the gap between cognitive models, which offer sophisticated processing accounts of behavior, and neurocognitive models, which focus on brain anatomy and functional imaging data.
Highlights.
-
-
Parameters of a computational model of lexical access in production, the dual-route interactive two step model, were mapped to the brain in a voxel-based lesion parameter mapping (VLPM) study of 103 aphasic individuals. This is the first study mapping individual model-derived parameters to lesioned voxels.
-
-
The dual-route aspect of the model was supported by finding that voxels associated with patient word repetition ability overlapped to a large extent with voxels associated with the model’s nl (non-lexical) and p (phonological) parameters.
-
-
The model’s s (semantic) parameter was, to a large extent, behaviorally and neurally distinct from the p parameter.
-
-
The p and nl parameters were closely associated behaviorally and in the brain.
-
-
The p parameter, which is largely derived from phonological errors made in the picture naming task, was associated with sensory-motor areas rather than Wernicke’s area, challenging the standard assumption that such errors arise from poor access of lexical-phonological forms stored in the posterior temporal lobe.
-
-
The joint operation of the p and nl parameters was identified with the dorsal stream in dual-stream (dorsal-ventral) neurocognitive models of language processing.
Acknowledgements
This research was supported by a grant from the National Institutes of Health’s National Institute for Deafness and Other Communication Disorders: DC000191. The authors thank Daniel Y. Kimberg and Grant W. Walker for contributions to the image analysis and VLSM methods, and Adelyn Brecher and Gabriella Garcia for their role in patient recruitment and testing. We also thank the many research assistants who gathered, scored, and analyzed behavioral data and the research participants and caregivers who made this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous applications of the models had not constrained the fitting in this way and, as a result, a rare fit would identify a supranormal value for a patient, which was well outside of the distribution of parameters. In all such cases, it turns out that a fit of nearly equal quality can be obtained by constraining the parameters to be .06 or below.
References
- Abel S, Huber W, Dell GS. Connectionist diagnosis of lexical disorders in aphasia. Aphasiology. 2009;23:1–26. [Google Scholar]
- Antonucci SM, Beeson PM, Labiner DM, Rapcsak SZ. Lexical retrieval and semantic knowledge in patients with left inferior temporal lobe lesions. Aphasiology. 2007;22:281–304. doi: 10.1080/02687030701294491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20:529–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26:338–354. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Hanley JR, Dell GS, Kay J. Testing single- and dual-route computational models of auditory repetition with new data from six aphasic patients. Aphasiology. 2008;22:62–76. [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends In Cognitive Sciences. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119:1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJS. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges J. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Browman CP, Goldstein L. Articulatory phonology: an overview. Phonetica. 1992;49:155–80. doi: 10.1159/000261913. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Baldo JV, Okada K, Berman KF, Dronkers N, D’Esposito M, Hickok G. Conduction aphasia, sensory-motor integration, and phonological short-term memory - An aggregate analysis of lesion and fMRI data. Brain and Language. 2011;119:119–128. doi: 10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald A, Miozzo M. Finding levels of abstraction in speech production: Evidence from sound-production impairment. Psychological Science. 2011;22:1113–1119. doi: 10.1177/0956797611417723. [DOI] [PubMed] [Google Scholar]
- Budd M, Hanley JR, Nozari N. Evidence for a non-lexical influence on children’s auditory repetition of familiar words. Journal of Psycholinguistic Research. 2012;41(4):253–266. doi: 10.1007/s10936-011-9189-8. [DOI] [PubMed] [Google Scholar]
- Chang F, Dell GS, Bock K. Becoming syntactic. Psychological Review. 2006;113:234–272. doi: 10.1037/0033-295X.113.2.234. [DOI] [PubMed] [Google Scholar]
- Cloutman L, Gottesman R, Chaudhry P, Davis C, Kleinman JT, Pawlak M, et al. Where (in the brain) do semantic errors come from? Cortex. 2009;45:641–649. doi: 10.1016/j.cortex.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JS, Hualde JI. Papers in Laboratory Phonology. Mouton de Gruyter; New York: 2003. p. 9. [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: Dual-route and parallel distributed processing approaches. Psychological Review. 1993;100:589–608. [Google Scholar]
- Compston A. From the Archives. Brain. 2006;129(6):1347–1350. [Google Scholar]
- Costa A, Strijkers K, Martin C, Thierry G. The time course of word retrieval revealed by event-related brain potentials during overt speech. Proceedings of the National Academy of Sciences. 2009;106:21442–21446. doi: 10.1073/pnas.0908921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabul BL. Apraxia battery for adults. second edition Pro-Ed; Austin, TX: 2000. [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio A. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dell GS. A spreading activation theory of retrieval in sentence production. Psychological Review. 1986;93:283–321. [PubMed] [Google Scholar]
- Dell GS, Lawler EN, Harris HD, Gordon JK. Models of errors of omission in aphasic naming. Cognitive Neuropsychology. 2004;21:125–145. doi: 10.1080/02643290342000320. [DOI] [PubMed] [Google Scholar]
- Dell GS, Martin N, Schwartz MF. A case-series test of the interactive two-step model of lexical access: Predicting word repetition from picture naming. Journal of Memory and Language. 2007;56:490–520. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Oppenheim GM, Kittredge AK. Saying the right word at the right time: Syntagmatic and paradigmatic interference in sentence production. Language and Cognitive Processes. 2008;23:583–608. doi: 10.1080/01690960801920735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in aphasic and nonaphasic speakers. Psychological Review. 1997;104:801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery. 2008;109:461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test, Revised. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Foundas A, Daniels SK, Vasterling JJ. Anomia: Case studies with lesion localization. Neurocase. 1998;4:35–43. [Google Scholar]
- Foygel D, Dell GS. Models of impaired lexical access in speech production. Journal of Memory and Language. 2000;43:182–216. [Google Scholar]
- Fridriksson J, Kiartansson O, Morgan PS, Hialtason H, Magnusdottir S, Bonilha L, Rorden C. Imparied Speech Repetition and Left Parietal Lobe Damage. Journal of Neuroscience. 2010;30:11057–11061. doi: 10.1523/JNEUROSCI.1120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldrick M. Theory selection and evaluation in case series research. Cognitive Neuropsychology. 2011;28:451–465. doi: 10.1080/02643294.2012.675319. [DOI] [PubMed] [Google Scholar]
- Goldrick MA, Rapp B. Lexical and post-lexical phonological representations in spoken production. Cognition. 2007;102:219–260. doi: 10.1016/j.cognition.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Goldstein L, Pouplier M, Chen L, Saltzman E, Byrd D. Dynamic action units slip in speech production errors. Cognition. 2007;103:386–412. doi: 10.1016/j.cognition.2006.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow DW. The cortical organization of lexical knowledge: A dual lexicon model of spoken language processing. Brain and Language. 2012;121:273–288. doi: 10.1016/j.bandl.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: Distinguishing the effects of word frequency from familiarity and length in overt picture naming. Journal of Cognitive Neuroscience. 2007;19:617–631. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gupta P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience. 2008;20:1698–1710. doi: 10.1162/jocn.2008.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. Examining the relationship between word learning, nonword repetition, and immediate serial recall in adults. Quarterly Journal of Experimental Psychology. 2003;56:1213–1236. doi: 10.1080/02724980343000071. [DOI] [PubMed] [Google Scholar]
- Gupta P, Tisdale J. Does phonological short-term memory causally determine vocabulary learning? Toward a computational resolution of the debate. Journal of Memory and Language. 2009;61:481–502. [Google Scholar]
- Hamilton AC, Martin RC, Burton PC. Converging fMRI evidence for a role of the left inferior frontal lobe in semantic retention during language comprehension. Cognitive Neuropsychology. 2009;26:685–70. doi: 10.1080/02643291003665688. [DOI] [PubMed] [Google Scholar]
- Hanley JR, Dell GS, Kay J, Baron R. Evidence for the involvement of a nonlexical route in the repetition of familiar words: A comparison of single and dual route models of auditory repetition. Cognitive Neuropsychology. 2004;21:147–158. doi: 10.1080/02643290342000339. [DOI] [PubMed] [Google Scholar]
- Hanley JR, Kay J, Edwards M. Imageability effects, phonological errors, and the relationship between auditory repetition and picture naming: Implications for models of auditory repetition. Cognitive Neuropsychology. 2002;19:193–206. doi: 10.1080/02643290143000132. [DOI] [PubMed] [Google Scholar]
- Hanley JR, Nickels L. Are the same phoneme and lexical layers used in speech production and comprehension? A case-series test of Foygel and Dell’s (2000) model of aphasic speech production. Cortex. 2009;45(6):784–790. doi: 10.1016/j.cortex.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nature Reviews Neuroscience. 2012;13:135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Okada K, Serences JT. Area Spt in the human planum temporale supports sensory-motor integration for speech processing. Journal of Physiology. 2009:101. doi: 10.1152/jn.91099.2008. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis A, Caramazza A. Mechanisms for Accessing Lexical Representations for Output - Evidence from a Category-Specific Semantic Deficit. Brain and Language. 1991;40:106–144. doi: 10.1016/0093-934x(91)90119-l. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker, et al. Restoring cerebral blood flow reveals neural regions critical for naming. Journal of Neuroscience. 2006;26:8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test. Thames Valley Test Company; Bury St. Edmunds: 1992. [Google Scholar]
- Indefrey P. Brain-Imaging studies of langauge production. In: Gaskell G, editor. Oxford handbook of psycholinguistics. Oxford University Press; Oxford: 2007. pp. 547–564. [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Isenberg AL, Vaden KI, Jr., Saberi K, Muftuler LT, Hickok G. Functionally distinct regions for spatial processing and sensory motor integration in the planum temporale. Human Brain Mapping. 2012;33:2453–2463. doi: 10.1002/hbm.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: A consequence of poor semantic control. Neuropsychologia. 2007;45:1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Crisp J, Lambon Ralph MA. The impact of phonological or semantic impairment on delayed auditory repetition: Evidence from stroke aphasia and semantic dementia. Aphasiology. 2006;20:963–992. [Google Scholar]
- Jefferies E, Jones RW, Bateman D, Lambon Ralph MA. A semantic contribution to nonword recall? Evidence for intact phonological processes in semantic dementia. Cognitive Neuropsychology. 2005;22:183–212. doi: 10.1080/02643290442000068. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland J, Patterson K, Galton C, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22:1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Martin N, Dell GS, Saffran EM, Schwartz MF. Origins of paraphasias in deep dysphasia: Testing the consequences of a decay impairment to an interactive spreading activation model of lexical retrieval. Brain and Language. 1994;47:609–660. doi: 10.1006/brln.1994.1061. [DOI] [PubMed] [Google Scholar]
- Martin N, Saffran EM, Dell GS. Recovery in deep dysphasia: Evidence for a relation between auditory verbal STM capacity and lexical errors in repetition. Brain and Language. 1996;52:83–113. doi: 10.1006/brln.1996.0005. [DOI] [PubMed] [Google Scholar]
- Martin N, Saffran EM. Language and auditory-verbal short-term memory impairments: Evidence for common underlying processes. Cognitive Neuropsychology. 1997;14:641–682. [Google Scholar]
- Martin N, Schwartz MF, Kohen FP. Assessment of the ability to process semantic and phonological aspects of words in aphasia: A multi-measurement approach. Aphasiology. 2005;20:1–13. [Google Scholar]
- Martin RC, Freedman ML. The neuropsychology of verbal working memory: The ins and outs of phonological and lexical-semantic retention. In: Roediger HL, Nairne JS, Neath I, Surprenant AM, editors. The nature of remembering: Essays in honor of Robert G. Crowder. American Psychological Association Press; Washington, DC: 2001. [Google Scholar]
- Mirman D, Strauss T, Brecher A, Walker GM, Sobel P, Dell GS, Schwartz MF. A large, searchable, web-based database of aphasic performance on picture naming and other tests of cognitive function. Cognitive Neuropsychology. 2010;27:495–504. doi: 10.1080/02643294.2011.574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the nature of deregulated semantic cognition in semantic aphasia: Evidence for the roles of prefrontal and temporo-parietal cortices. Journal of Cognitive Neuroscience. 2012;22:1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Nozari N, Kittredge AK, Dell GS, Schwartz MF. Naming and repetition in aphasia: Steps, routes, and frequency effects. Journal of Memory and Language. 2010;63:541–559. doi: 10.1016/j.jml.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Graham N, Hodges JR. The impact of semantic memory loss on phonological representations. Journal of Cognitive Neuroscience. 1994;6:57–69. doi: 10.1162/jocn.1994.6.1.57. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of langauge: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F. Motor cortex maps articulatory features of speech sounds. Proceedings of the National Academy of Sciences. 2006;103:7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Goldrick M. Discreteness and interactivity in spoken word production. Psychological Review. 2000;107:460–499. doi: 10.1037/0033-295x.107.3.460. [DOI] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia naming test: Scoring and rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. The structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski TJ. Thresholding lesion overlap difference maps: Application to category-related naming and recognition deficits. Neuroimage. 2008;41:970–984. doi: 10.1016/j.neuroimage.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruml W, Caramazza A, Capasso R, Miceli G. Interactivity and continuity in normal and aphasic language production. Cognitive Neuropsychology. 2005;22:131–168. doi: 10.1080/02643290442000031. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreherb W, Schnellb S, Kümmerer D, Kellmeyer P, Vrya M-S, et al. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Dell GS. Case series investigations in cognitive neuropsychology. Cognitive Neuropsychology. 2010;27:477–494. doi: 10.1080/02643294.2011.574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Dell GS, Martin N, Gahl S, Sobel P. A case-series test of the interactive two-step model of lexical access: Evidence from picture naming. Journal of Memory and Language. 2006;54:228–264. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012 doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Brecher A, Faseyitan O, Dell GS, et al. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proceedings of the National Academy of Sciences 108. 2011;8520:8524. doi: 10.1073/pnas.1014935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. Anterior temporal involvement in semantic word retrieval: VLSM evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, Lambon Ralph MA. Lichtheim 2: Synthesising aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72:385–396. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. American Speech Language Hearing Association. 1983;25:37–42. [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, Coslett HB. Support for anterior temporal involvement in semantic error production in aphasia: New evidence from VLSM. Brain and Language. 2011;117:110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Wise RJS, Warren JD. Sounds do-able: Auditory-motor transformations and the posterior temporal plane. Trends in Neuroscience. 2005;28(12):636–643. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Wernicke C. The aphasic symptom complex: A psychological study on a neurological basis. Cohn and Weigert; Breslau: 1874/1969. Reprinted in R.S. Cohen & M.W. Wartofsky (Eds.), Boston studies in the philosophy of science, vol. 4. Boston: Reidel. [Google Scholar]
- Wilson SM, Isenberg AL, Hickok G. Neural correlates of word production stages delineated by parametric modulation of psycholinguistic variables. Human Brain Mapping. 2009;30:3596–3608. doi: 10.1002/hbm.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]