Abstract
Hepatocellular carcinoma (HCC) or liver cancer is one of the fastest growing cancers in the United States. Current liver ablation methods are thermal-based and share limitations due to the heat sink effect from the blood flow through the highly vascular liver. In this study, we demonstrate the feasibility of using histotripsy for non-invasive liver ablation in the treatment of liver cancer. Histotripsy is a non-thermal ablation method that fractionates soft tissue through the control of acoustic cavitation. Twelve histotripsy lesions ~1cm3 were created in the livers of six pigs through an intact abdomen and chest in vivo. Histotripsy pulses of 10 cycles, 500 Hz pulse repetition frequency (PRF), and 14-17 MPa estimated in situ peak negative pressure were applied to the liver using a 1 MHz therapy transducer. Treatments were performed through 4-6 cm of overlying tissue with 30-50% of the ultrasound pathway covered by the ribcage. Complete fractionation of liver parenchyma was observed with sharp boundaries after 16.7 minute treatments. In addition, two larger volumes of 18 cm3 and 60 cm3 were generated within 60 minutes in two additional pigs. As major vessels and gallbladder have higher mechanical strength and are more resistant to histotripsy, the major hepatic vessels and gallbladder remained intact while the liver surrounding these structures was completely fractionated. This work demonstrates that histotripsy is capable of non-invasively fractionating liver tissue while preserving critical anatomical structures within the liver. Results suggest histotripsy has potential for the non-invasive ablation of liver tumors.
Keywords: Liver cancer, therapeutic ultrasound, cavitation, non-invasive tissue ablation, histotripsy
Introduction
Hepatocellular carcinoma (HCC) was the fastest growing cancer in the United States over the last decade with this trend expected to continue (El-Serag and Mason 1999; Bosch et al. 2004). While liver transplantation may be curative, only a small patient population will receive this treatment as tumors must be < 5 cm in diameter or 3 tumor nodules < 3 cm in diameter and confined to regions without major hepatic vessels. The limited donor availability also greatly constrains the number of liver transplants (El-Serag and Mason 1999; Bosch et al. 2004; Pelletier et al. 2009). Surgical resection of liver tumors is a proven treatment option but is associated with high rates of morbidity and mortality (Livraghi et al. 2011). Further, surgical resection or transplantation is not possible in many cases such as patients with decompensated cirrhosis (Parikh and Hyman 2007; Livraghi et al. 2011). Radiofrequency ablation (RFA) is currently the standard local ablation therapy for liver cancer and has had success for selected populations in reducing mortality and morbidity when compared to surgical resection (Kudo 2010; Livraghi et al. 2011). Transarterial chemoembolization (TACE) and percutaneous ethanol injection (PEI) have also shown success in treating small liver tumors but are not as safe and effective as minimally invasive ablation methods (Cho et al. 2009; Davis 2010; Kudo 2010; Izumi 2011).
Currently available minimally invasive ablation methods are mostly thermal based, including RFA, microwave therapy, cryoablation, and laser ablation (Charnley et al. 1989; Erce and Parks 2003; Head and Dodd 2004; Liapi and Geschwind 2007). While these minimally invasive therapies have shown some success, these methods share inherent limitations due to the heat sink effect originating from blood flow (Patterson et al. 1998; Curley 2001; Lu et al. 2003; Marrero and Pelletier 2006). Thermal ablation is inconsistent in tissue with non-uniform heat dissipation patterns, which is common in hypervascular liver tumors (Livraghi et al. 2003). In particular, for tissue near major vessels, thermal ablation often results in incomplete tumor necrosis (Aschoff et al. 2001; Kudo 2010). In addition, these methods are unsuitable for treating tumors larger than 3 cm or more than 3 nodules due to the excessive treatment time (Patterson et al. 1998; Curley 2001; Lu et al. 2003; Marrero and Pelletier 2006). Another limitation facing these methods is the lack of imaging feedback during treatment. The treatment is evaluated before and after by computer tomography (CT) or magnetic resonance imaging (MRI) while no real-time imaging provides monitoring during treatment (Curley 2001; Erce and Parks 2003; Lu et al. 2003). There remains an unmet clinical need for a local ablation method capable of overcoming these limitations.
High intensity focused ultrasound (HIFU) is a non-invasive thermal ablation technique (ter Haar 2001; Leslie and Kennedy 2006). The current HIFU systems use MRI thermometry to provide real-time monitoring of the thermal dose during treatment (Okada et al. 2006; Cochard et al. 2009; Bobkova et al. 2010; Khokhlova et al. 2010). With its non-invasiveness, real-time MRI feedback, the ability to scan the focal zone over a large tumor volume, HIFU has the promise to improve upon the current liver ablation methods (Li et al. 2003; Zhang et al. 2009; McWilliams et al. 2010). However, as a thermal-based method, HIFU is still affected by the heat sink effect, resulting in the reduced effectiveness in ablating tissue near major blood vessels and extended treatment time for larger hypervascular liver volumes, (Okada et al. 2006; Leslie et al. 2008).
Histotripsy is a non-invasive non-thermal ultrasonic ablation method that fractionates tissue through the precise control of acoustic cavitation (Xu et al. 2004; Parsons et al. 2006a; Roberts et al. 2006). Using microsecond, high-pressure pulses applied by an ultrasound transducer outside the body and focused to the target tissue, a cluster of microbubbles are generated and the energetic expansion and collapse of the microbubbles fractionates cells at the target. With a sufficient number of pulses, the target tissue can be completely fractionated to a fluid homogenate with no recognizable cellular structures. Since histotripsy is non-thermal, it is not affected by the heat sink effect from blood vessels and does not have the limitations associated with thermal ablation methods. Histotripsy can produce consistent and fast fractionation of tissue with different heat dissipation patterns, even when the tissue is in proximity to major vessels (Hall et al. 2009; Styn et al. 2010; Kim et al. 2011a; Maxwell et al. 2011). The fractionation is often self-limited at the boundaries of major vessels with surrounding tissue completely homogenized. As a non-invasive ablation method, the therapy focus can be scanned to treat a large tumor volume (>3cm) and multiple nodules in a reasonably short period of time. Further, the histotripsy cavitation cloud can be visualized with ultrasound imaging, allowing precise targeting (Kim et al. 2011a; Maxwell et al. 2011; Owens et al. 2011). The change in tissue during treatment can be also be directly monitored using standard imaging modalities such as ultrasound and MRI, which allows histotripsy to be guided in real time (Hall et al. 2007; Wang et al. 2009).
A major challenge facing the non-invasive treatment of liver cancer using ultrasound is to overcome the rib obstruction. Skin burns and subcostal edema have been reported in clinical HIFU liver ablation cases (Wu et al. 2004; Illing et al. 2005; Jung et al. 2011). For transthoracic ablation of the liver using HIFU, ribs in the ultrasound pathway cause periodic blockage of ultrasound, resulting in a significantly decreased main lobe and increased grating lobes (Wang et al. 1994; Bobkova et al. 2010; Khokhlova et al. 2010). Moreover, due to the high ultrasound absorption coefficient of bone and reflection effects at the bone-tissue interface, overheating of ribs and surrounding tissue often results in unwanted tissue damage. Phased arrays and aberration correction algorithms have been developed to switch off the elements blocked by the ribs to reduce overheating to the ribs and associated tissue (Cochard et al. 2009; Bobkova et al. 2010). Even with these improvements, grating lobes may still remain producing undesired heating and collateral damage. Histotripsy is more resistant to the grating lobes caused by rib aberration as the cavitation cloud is only generated when the pressure exceeds a distinct threshold. By using an appropriate pressure where the main lobe is above the threshold while the grating lobes are not, a confined cloud within the main lobe and a precise lesion can be produced despite the intervening ribs (Kim et al. 2011b). Thermal damage to the overlying and surrounding tissue is also prevented by using prolonged cooling times between pulses.
In this study, we investigate the feasibility of developing histotripsy for non-invasive liver ablation. Previous in vivo animal studies have investigated histotripsy for many applications where non-invasive tissue removal is desired including treatment for benign prostatic hyperplasia (Lake et al. 2008; Hempel et al. 2011), congenital heart disease (Xu et al. 2010; Owens et al. 2011), thrombolysis (Maxwell et al. 2011), renal tumor ablation (Styn et al. 2010), and fetal interventions including the treatment of fetal liver (Kim et al. 2011a). However, liver fractionation using histotripsy involves challenges not present in these previous studies, such as considerable bone obstruction and the ability to treat large lesions (>3cm) adjacent to major blood vessels. In this work, an in vivo porcine liver model was used due to its size and anatomic similarities to its human counterpart. First, histotripsy was used to generate consistent and complete fractionation of hepatic parenchyma through ribs and overlying tissue in various locations spanning all major regions of the liver. Second, the capability of ablating large regions in the liver was investigated. Finally, the effectiveness of tissue-selective ablation using histotripsy to fractionate the liver surrounding major blood vessels and gallbladder while preserving these critical structures was tested.
Materials and Methods
Porcine Surgical Procedures
A total of 8 healthy 60-90 pound mixed breed pigs were used. Each pig was pre-medicated with Telazol (6 mg/kg, Fort Dodge) and Xylazine (2.2 mg/kg, small animal AnaSed from Lloyd Labs), and an intravenous catheter was placed in the auricular ear vein. Pigs underwent endotracheal intubation with a 6-8 mm diameter endotracheal tube and were maintained under full anesthesia on isoflurane gas (1.5-2.0%, Vet One) for the duration of the surgical procedure. Vital signs monitored throughout the experiments included SpO2, heart rate, respiration, and core body temperature with a Heska vet/ox plus 4800. Anesthetized pigs were placed in a dorsal recumbent position on the surgical table, and the skin over the targeted tissue was treated with a depilatory cream to improve ultrasound coupling. To ensure ultrasound propagation to targeted tissue, a degassed water bolus was coupled to the skin with a thin plastic membrane and ultrasound coupling gel (Fig.1A). An 8 MHz phased array ultrasonic imaging probe (Model S8, used with Sonos 7500 imaging system, Philips Electronics, Andover, MA) was fixed coaxially with the histotripsy therapy transducer. The focal position of the therapy transducer in the imaging field of view was found prior to therapy by generating a bubble cloud in degassed water and identifying the location of the hyperechoic region. The location of the bubble cloud was marked on the ultrasound imager screen as an “x” to indicate the transducer focal position in free field. The therapy transducer was subsequently submerged in the water bowl for treatment. Targeting was achieved by aligning the focus marker (“x”) to the selected treatment region in the liver. Histotripsy treatment was applied transcutaneously with a portion of the rib cage within the acoustic pathway. Within three hours after treatment, the pigs were euthanized without recovery with a sodium pentobarbital (140-160 mg/kg, Vet One) intravenous injection. The treated livers were harvested for examination and tissue layers overlying the treatment target were visually inspected for signs of injury. All procedures used in this work were reviewed and approved by the University Committee on Use and Care of Animals at the University of Michigan.
Figure 1. Histotripsy in vivo porcine liver ablation experimental setup.

(A) A 1 MHz histotripsy therapy transducer with coaxially aligned ultrasound imaging probe (insert) was attached to a motorized 3D positioning system controlled using a PC console and coupled to the pig with a degassed water bolus. (B) Histotripsy was applied transcutaneously through ribs and overlying tissue with treatments targeted to all positions within the liver. Specific treatment information is listed in Table 1.
Lesion Generation through Ribs and Overlying Tissue Guided by Ultrasound Imaging
To demonstrate the ability of histotripsy to achieve consistent and efficient liver fractionation through overlying ribs and other intervening tissues, 12 histotripsy lesions were created in 6 pigs with treatment locations spanning various regions of the liver (Fig.1B). Histotripsy pulses were generated by a 1 MHz focused ultrasonic transducer (Imasonic, Besançon, France) with an aperture of 100 mm, focal length of 90 mm, and focal volume of 2.2×2.2×15 mm (defined by - 6dB from the peak negative pressure). Histotripsy pulses of 10 cycles and 500 Hz pulse repetition frequency (PRF) were applied to the liver. The PRF used in this work has previously been used to successfully treat fetal liver in an in vivo sheep model through overlying tissue including fetal bones (Kim et al. 2011a).
Acoustic waveforms produced by the 1 MHz therapeutic transducer were measured in free field using a fiber optic hydrophone built in house (Parsons et al. 2006b). Pressure wave measurements were recorded in free-field in degassed 1,3-Butanediol (Sigma-Aldrich) at room temperature. Calibration was performed in 1,3-Butanediol to prevent cavitation at the fiber tip, which allowed the measurement of the acoustic waveforms at a high-pressure level. To avoid the attenuation by Butanediol, the fiber tip was placed such that the ultrasound only travelled through 5 mm Butanediol before reaching the tip. Free field peak negative pressure values used in this work were between 23 MPa and 27 MPa. The in situ focal pressures could not be directly measured non-invasively. With 30-50% of the aperture covered by rib cage, 4-6 cm overlying tissue, and using 0.5 dB/cm-MHz attenuation for the overlying tissue, the in situ peak negative pressure was estimated to 14-17 MPa. To apply the treatment, the acoustic pressure was incrementally increased until a confined bubble cloud was visualized on ultrasound imaging. As such, a peak pressure right above the threshold to generate a bubble cloud in that treatment tissue was used for each treatment, which also allowed us to limit the cavitation cloud within the main lobe and prevent forming cavitation in the grating lobes.
After cloud initiation, histotripsy lesions were generated by mechanically scanning the therapy focus to follow a 5×5×5 mm cubic grid. Each point in the grid was treated for 8 seconds (4,000 pulses) before the focus was moved to an adjacent spot 1 mm apart. The eight-second treatment time per location was used here instead of the two seconds used in the fetal sheep liver treatment in our previous study, because the mechanical strength of the adult porcine liver is significantly higher than that of the fetal sheep liver (Kim et al. 2011a). Due to the finite size of the bubble cloud, the effective treatment volume resulted from the scan was approximately 7.2×7.2×20 mm. The procedure was monitored in real-time with ultrasound imaging. Pre-treatment and post-treatment ultrasound images of the targeted region were collected for comparison purposes. After the pigs were euthanized, the ribs and overlying tissues were visually examined for signs of damage. The liver was harvested, imaged with MRI, and fixed in formalin for histological processing and evaluation.
Larger Lesion Ablation
To demonstrate the ability of histotripsy to ablate a larger region of liver tissue, two larger focal scans were performed in separate experiments covering scanned volumes of 12 cm3 (3×2×2 cm scan) and 18 cm3 (3×3×2 cm scan). Due to the size of the bubble cloud, the effectively treated volumes were estimated to be 3.2×2.2×3.5 cm and 3.2×3.2×3.5 cm. Histological analysis of the twelve lesions formed in the previous section was conducted before these experiments and suggested an overtreatment. Therefore, the treatment time per point was decreased to 4 seconds (2000 pulses). The spacing between points was also increased for the larger liver volume treatment, with 2 mm transverse spacing and 5 mm axial spacing. Prior to the in vivo experiments, these treatment parameters were validated in ex vivo porcine liver. The total treatment times for the two larger lesions formed here were 26.7 minutes and 60 minutes, respectively. The treatment region was examined with ultrasound imaging before, during, and after treatment including Doppler images of the blood vessels. After treatment, the livers were harvested for MRI and histological evaluation.
Tissue-selective Liver Ablation
To study the effectiveness of histotripsy to fractionate tissue near major vessels and the gallbladder while preserving these critical structures, treatments were selectively targeted to regions containing major vessels and gallbladder. We hypothesize that the relatively higher mechanical strength of these tissues compared to liver parenchyma will allow their preservation while the surrounding liver parenchyma with lower mechanical strength can be completely fractionated. The locations containing major hepatic blood vessels and gallbladder were identified using ultrasound imaging. The gallbladder cavity was hypoechoic compared to the surrounding liver. The major hepatic vessels were identified by detecting the blood flow using ultrasound Doppler imaging. After identifying these anatomical structures in the liver, histotripsy treatment was applied to the regions containing these structures using the acoustic parameters described above. The structures and surrounding liver tissue treated by histotripsy were evaluated by 2D ultrasound imaging during and after treatment to monitor any potential damage. Ultrasound Doppler images of blood vessels within the treated region were taken before and after treatment to determine if blood flow patterns changed after therapy. After treatment, the integrity of vital anatomical structures was assessed with MRI and histological analysis.
Liver MRI
MRI is the standard clinical imaging method to diagnose and evaluate tumor response to treatment. To evaluate the acute aspect of histotripsy-induced lesions in the liver by MRI, porcine liver samples were harvested and imaged ex vivo by a 7T small animal scanner (7.0 Tesla, 310 mm bore, Varian, Inc, Walnut Creek, CA) prior to fixing for histological evaluation. The reason why the pig liver was not imaged by MRI in vivo is because our small animal scanner was not large enough to accommodate the entire pig. The samples were embedded in a gelatin mold prior to imaging. Both T1 and T2 weighted multi-slice spin-echo images of the liver were acquired along coronal and axial planes. MRI parameters were optimized for each sample but stayed within the following ranges: repetition time of 900-3000 ms, echo delay time of 13-30 ms, field of view of (50-150)×(50-150)×(1-40) mm, and acquisition times between 2-45 minutes.
Histological Evaluation
Treated porcine liver samples were harvested after experiments and fixed in 10% buffered formalin (Fisher Scientific). Lesion gross morphology was examined prior to histological analysis. Samples were then stained with hematoxylin and eosin (H&E) and examined under a microscope (Nikon Eclipse 50i) using 4x, 10x, 20x, and 40x objective lenses. To quantitatively assess the distribution of intermediate sized blood vessels remaining after histotripsy, the number of vessels between 50-1000 μm in diameter was counted using four H&E slides of the largest lesion, as it was the only lesion containing sufficient number of vessels for quantitative assessment. For each H&E slide, one hundred 1 mm2 subareas (10x fields of view) within the treatment region and in the intact liver tissue outside the treatment region (control) were observed under microscope. Vessels between 50-1000 μm were counted, recorded, and organized into six groups: vessels <50 μm, 50-100 μm, 100-200 μm, 200-300 μm, 300-400 μm and 400-1000 μm. The total number of vessels over the one hundred subareas (total 100 mm2) for each group was calculated on each slide. The mean and standard deviation of the number of vessels for each group over four slides were calculated for both treatment and control samples. To compare the treatment and control samples, a Student’s t-test was conducted for each vessel group between treatment and control samples (n=4) and p <0.01 was considered statistically significant.
Results
Lesion Generation through Ribs and Other Overlying Tissue Guided by Ultrasound Imaging
To demonstrate the ability of histotripsy to non-invasively generate precise liver lesions through ribs and overlying tissue, twelve histotripsy treatments were performed on six pigs. Therapy was targeted to locations spanning the entire liver with lesions formed in the superior and inferior regions of the left, middle, and right lobes. Treatments were performed through 3-6.5 cm of overlying tissue with rib cage covering 30-50% (including intercostal space) of the transducer aperture (Table 1).
Table 1. Histotripsy lesion generation through ribs and overlying tissue parameters.
Table shows an overview of the ribcage coverage (including intercostal space), overlying tissue depth, and liver location for 12 histotripsy lesions. Treatments covered all regions of the liver and were further targeted to areas containing critical anatomical structures such as the gallbladder (GB) or major hepatic blood vessels (BV). Bubble cloud initiation was achieved for all treatments resulting in histotripsy lesions with a maximum diameter between 1 cm and 2.2 cm.
| Treatment | Pig Weight | Rib Coverage | Depth | Liver Location | Proximity to Vital Structures |
|---|---|---|---|---|---|
| 1 | 76# | 30% | 5 cm | L-1 | Near BV |
| 2 | 76# | 45% | 5 cm | M-2 | Near BV |
| 3 | 60# | 45% | 6 cm | M-4 | Near BV |
| 4 | 60# | 30% | 6.5 cm | M-3 | Near BV |
| 5 | 85# | 40% | 6 cm | R-1 | Near BV |
| 6 | 85# | 30% | 3 cm | L-2 | Near BV |
| 7 | 80# | 35% | 4.5 cm | R-2 | Containing BV |
| 8 | 80# | 30% | 4 cm | M-GB | Near BV and GB |
| 9 | 80# | 35% | 6 cm | R-1 | Near BV |
| 10 | 80# | 40% | 6.5 cm | L-1 | Containing BV |
| 11 | 88# | 50% | 6.5 cm | M-1 | Containing BV |
| 12 | 88# | 45% | 6 cm | M-GB | GB Surface |
A bubble cloud was successfully generated in the liver for all twelve treatments and visualized as a hyperechoic zone on ultrasound imaging allowing for real-time treatment monitoring (Fig. 2A-B). In all treatments, the bubble cloud locations in the liver tissue (i.e. the in situ focal position) were within 3 mm of the marker representing the free-field focal position as shown in Figure 2. The creation of liver lesions of ~1 cm3 from histotripsy therapy was confirmed for all twelve treatments. The treatment time for all lesions in this section was 16.7 minutes.
Figure 2. Liver ablation was guided by ultrasound imaging in real-time.
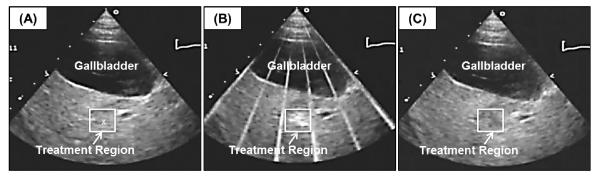
Images show the histotripsy liver treatment monitored by ultrasound imaging before (A), during (B), and after (C) treatment. Prior to each treatment, the bubble cloud was generated in free water with the free-field focal location marked as an “x” on the ultrasound image (A). The histotripsy bubble cloud appears as a dynamically changing hyperechoic region on ultrasound imaging (B) while the resulting lesion appears hypoechoic (C) resulting from fractionation of liver parenchyma.
Lesions were assessed using ultrasound imaging after histotripsy. Histotripsy-induced fractionation often appears as a hypoechoic zone on ultrasound imaging due to the reduced size and density of the sound scatterers present in the fractionated zone. After each treatment, the average speckle amplitude within the treatment region was calculated and compared to that of surrounding intact liver tissue (control). In 8 of 12 lesions, there was a statistically significant decrease in average speckle amplitude (>3 dB, p-value <0.001; Student’s t-test) of the treatment region compared to control. As such, the treated regions appeared as hypoechoic zones for these 8 lesions with an average decrease in backscatter intensity within the treated regions of 3.9±0.7 dB. In the remaining 4 lesions, the average speckle amplitude of the treatment region was not significantly different from control (<3 dB, p-value >0.001) with an average decrease in backscatter intensity within the treated regions of 1.2±1.6 dB. The detailed steps to calculate the average backscatter intensity in the treatment region and in the surrounding tissue have been described in a previous study (Wang et al. 2009).
After treatment, the excised liver was assessed with MRI. All twelve lesions were observed as bright, hyper-intense regions on MRI with increased T2 relaxation times for fractionated homogenate. There was a significant increase in average T2 relaxation times which was 24±1.3 ms for the histotripsy lesion compared to 17±1.8 ms for the surrounding intact liver (p=0.0015). The location and size of the lesions shown in MRI correlated well with those evaluated from gross morphology (Fig. 3A). Additionally, dark, hypo-intense regions within the lesion were observed on MRI for certain samples corresponding to blood accumulation.
Figure 3. Histotripsy treatment caused complete removal of liver parenchyma.
Histotripsy liver lesions appear hyper-intense on MRI imaging (A) corresponding to the fractionated liver tissue. Histological evaluation (B) demonstrates histotripsy completely fractionated liver parenchyma into acellular debris (F) without damaging surrounding liver tissue (E). Histotripsy lesions contained sharp boundaries of approximately a few hundred micrometers between homogenized and intact liver tissue (C, D).
Histological evaluation of histotripsy lesions showed complete fractionation of hepatic parenchyma inside the treated volume (Fig. 3B-F). The histotripsy lesions contained sharp boundaries with only a small area of partially ablated liver tissue <500 μm for all treatments likely caused by respiratory motion. Additionally, localized hemorrhage was often observed inside the histotripsy lesion or accumulated along the lesion boundary. No hemorrhage was observed in surrounding intact liver tissue. Overlying tissues in the acoustic path were examined after treatment for signs of potential damage. Gross morphological examination showed no signs of damage to overlying skin, muscle, or ribs. The vital signs of the pigs showed no negative responses to histotripsy. During and after treatment, the SpO2 remained above 95%. The fluctuation of the heart rate and respiration rate was maintained within ±10% of the baseline values observed before treatment. The core body temperature did not drop more than 1° throughout the duration of the treatment.
Larger Lesion Ablation
To demonstrate the ability of histotripsy to ablate larger sections of liver, two larger lesions were created. MRI multi-slice analysis showed lesion volumes of approximately 2.2×2.3×3.5 cm (18 cm3) and 5×4×3 cm (60 cm3) (Fig.4). Treatment times were 26.7 minutes and 60 minutes for the 18 cm3 and 60 cm3, respectively, with no negative responses in the vital signs of the pigs observed during treatment. While the 18 cm3 lesion was slightly smaller than the predicted scan volume plus the estimated bubble cloud size (3.2×2.2×3.5 cm), the 60 cm3 lesion was significantly larger than the scan volume with the bubble cloud size included (3.2×3.2×3.5 cm) as a result of substantial respiratory motion. Liver movement from breathing during the 60 cm3 lesion treatment showed significantly larger displacement (~1 cm) compared to the 18 cm3 lesion (~2-4 mm) and the twelve smaller lesions, which resulted in the significantly larger lesion volume than the scanned volume. Histological analysis demonstrated completely fractionated liver parenchyma throughout the treated volume with a transition zone between the fractionated tissue and intact region of <500 μm for both larger lesions.
Figure 4. Large lesion ablation.
Results show histotripsy-generated fractionation of large volumes in the liver. MRI multi-slice analysis demonstrated liver volume of approximately 5×4×3 cm (60 cm3) (A) was ablated. Histological evaluation (B) demonstrated histotripsy caused complete ablation throughout the large focal regions (D) without causing damage to surrounding tissue (C).
Tissue-selective Liver Ablation
To determine the ability of histotripsy to fractionate the liver tissue near major blood vessels and gallbladder while preserving these critical structures within the treatment region, multiple histotripsy treatments were targeted to regions near or containing major hepatic vessels and gallbladder. Results demonstrated that blood vessels and gallbladder remained structurally intact inside the completely fractionated region (Fig. 5). There were no vital sign alterations in any of the pigs undergoing treatments performed near critical vessels or the gallbladder.
Figure 5. Gallbladder and hepatic vessels remained intact and functional within histotripsy treated region.
MRI images demonstrate blood vessels (A) and gallbladder (D) remain structurally intact within the histotripsy focal volume while surrounding liver parenchyma was completely ablated. Doppler ultrasound images before (B) and after (C) histotripsy indicated that large vessels remained functional after treatment. Morphological observation of histotripsy lesions bordering the gallbladder (E, F) showed no signs of damage to the gallbladder itself.
Histotripsy completely fractionated hepatic parenchyma surrounding blood vessels without inducing perforation or visible damage to the vessel wall. MRI images showed that large intact blood vessels >1mm in diameter remained within the fractionated region (Fig.6A). Post-treatment Doppler images demonstrated that the large vessels had continued blood flow with no observed perforation (Fig.6B-C). Histological evaluation showed that liver parenchyma was completely fractionated into acellular debris surrounding these vessels while no observable damage was seen in any of the large vessels inside the treated region.
Figure 6. Histological evaluation of histotripsy lesions surrounding hepatic blood vessels.
Results show that the tissue surrounding blood vessels was completely fractionated while blood vessels remained intact (A-D). All large and medium sized arteries (A) and veins (B) were observed to remain intact with no observed perforations. Multiple smaller blood vessels (B, C, D) and bile ducts (D) were also observed inside the histotripsy lesion. The smallest vessels observed to remain intact were approximately 50-100 μm in diameter. Localized hemorrhage within lesion resulted from rupture of capillaries and small vessels <100 μm in diameter.
Histological evaluation further demonstrated an extensive network of undamaged intermediate vessels below 1 mm diameter throughout the completely fractionated liver lesion (Fig. 6A-B). Additionally, many smaller blood vessels and bile ducts were observed inside the lesion often accompanied by a surrounding connective tissue (Fig. 6C-D). Six groups of vessels (<50 μm, 50-100 μm, 100-200 μm, 200-300 μm, 300-400 μm and 400-1000 μm in diameter) were counted in four H&E slides of the 60 cm3 lesion. Figure 7 shows the number of vessels (mean ± standard deviation) for six vessel groups in treatment and control samples (n=4). Results demonstrated no vessels below 50 μm remained in the treatment region. For the 50-100 μm vessel group, there was a statistically significant decrease in the number of vessels (p<0.01). The mean number of vessels was 68.5 and 5 for the control and treatment samples, respectively, reduced by 92.7%. The mean number of vessels in 100-200 μm and 200-300 μm groups in the treatment region was decreased by 47.2% and 34.1% in comparison to the control, but these decreases were not statistically significant (p>0.1). The change in the mean number of vessels for 300-400 μm or 400-1000 μm vessel groups was both low, within 15% with no statistically significant difference (p>0.3). The small vessels (<100 um) that survived were mostly located surrounding larger hepatic structures that were protected by the extensive connective tissue. Localized hemorrhage was visible within the histotripsy lesion due to the rupture of capillaries and smaller vessels.
Figure 7. Large lesion vessel size comparison.
The number of vessels between 50-1000 μm were counted within a 100 mm2 region (n=4) within the histotripsy lesion and intact liver tissue. Results indicate a significant decrease in blood vessels between 50-100 μm (p<0.01). A decrease was observed for 100-200 μm and 200-300 μm vessels but was not significant (p>0.1) as a result of the high variability between samples. No significant differences in vessel count were observed between the histotripsy lesion and intact liver tissue for 300-400 μm and 400-1000 μm vessels. No blood vessels <50 μm were observed to remain within the histotripsy lesion after treatment.
Results for treatments applied near the gallbladder demonstrated that histotripsy completely fractionated the liver up to the gallbladder without causing perforation even when the bubble cloud was actively targeted to a region encompassing the gallbladder-liver interface (Fig. 5D-F). No visible damage or perforation to the gallbladder was observed under gross inspection of harvested samples. This finding was verified with MRI showing that the histotripsy lesion extended up to gallbladder wall without perforation.
Discussion
In this study, we investigated the feasibility of using histotripsy for non-invasive liver ablation in an in vivo porcine model. Results demonstrate that histotripsy created precise lesions at locations throughout the entire liver through ribs and overlying tissue without using aberration correction. Bubble clouds were successfully initiated and lesions were formed in all treatment attempts. The different heat dissipation patterns associated with regions containing different vasculatures did not affect the consistency of histotripsy liver ablation. Within the main treatment region, the liver tissue was completely fractionated to acellular debris with only small regions of partially damaged tissue present near the lesion boundary.
Overcoming rib obstruction is a major concern for the non-invasive treatment of liver cancer using ultrasound. Recent advancement in aberration correction algorithms has allowed liver ablation using extracorporeal HIFU (Marquet et al. 2011). As the cavitation cloud is only generated when pressure exceeds a distinct threshold, histotripsy is more resistant to the increased grating lobes caused by the aberration effect from ribs in comparison to thermal therapy (Kim et al. 2011b). Previous studies have shown histotripsy ablation through neonatal porcine (Owens et al. 2011) and fetal sheep (Kim et al. 2011a) ribs, but the neonatal and fetal ribs were not well calcified. This is the first time histotripsy lesions have been generated without using aberration correction through intact ribs in an adult large (porcine) animal model. In this work, the hyperechoic bubble cloud generated through the ribs and other overlying tissue was always within 3-mm of the transducer free-field focal position, as viewed on the ultrasound image. If needed, the focal position of the transducer can be adjusted to ensure the hyperechoic bubble cloud is right on the targeted tissue before treatment is applied. The ultrasound imaging pulses can be triggered by the therapy pulses, and, as such, only a few therapy pulses are sufficient to generate and view the bubble cloud for targeting before any significant damage is produced. No visual damage was observed to the ribs and overlying tissue, but a more in depth study using thermocouple measurements may be needed to investigate the potential temperature increases to the ribs and surrounding tissue. However, with the prolonged cooling time between histotripsy pulses, the temperature increase to the overlying tissue is expected to be low.
The histotripsy cavitation cloud in this work was monitored as a hyperechoic region using ultrasound imaging with treatment scans guided in real-time. Lesion assessment after treatment indicated that damage only occurred in regions exposed to the cavitating bubble cloud. After treatment, the fractionated zone generally appears hypoechoic on ultrasound imaging, although this was not the case for 4 of the 12 initial treatments as a result of the degraded ultrasound image quality through significant overlying tissue. Current work in our laboratory is investigating methods to improve the ultrasound imaging feedback for histotripsy lesions, including ultrasound elastography-based feedback (Wang et al. 2011). Further, results demonstrate that histotripsy lesions can be clearly visualized on spin-echo MRI for all lesions after treatment. While histotripsy lesions have been viewed extensively on ultrasound in the previous studies (Roberts et al. 2006; Lake et al. 2008; Wang et al. 2009; Kim et al. 2011a; Maxwell et al. 2011), only one study used MRI to image the histotripsy lesions generated in vitro (Hall et al. 2007). This study is the first time a histotripsy-induced in vivo liver lesion has been visualized using MRI.
This work also studied the effectiveness of histotripsy to fractionate liver tissue near major vessels and gallbladder while preserving these critical hepatic structures. Thermal ablation methods are often ineffective in ablating tissue near major vessels or produce incomplete ablation surrounding vessels (Chen et al. 1993). Our previous work has shown that tissues of increased mechanical strength are more resistant to histotripsy damage (Lake et al. 2008; Vlaisavljevich et al. 2011). In this work, the major hepatic vessels (>1 mm) and the gallbladder remained intact while the surrounding hepatic parenchyma was fractionated. This finding suggests that histotripsy can be effective and safe for treating tumors near major vessels and gallbladder. Leaky tumor vessels, which generally have a lower mechanical strength in comparison to normal vessels (Jain 1988), are expected to be ablated along with the tumor tissue while the normal large hepatic vessels will be preserved.
In addition to large blood vessels, an extensive network of smaller vessels remained inside the treatment region. While the results of this study demonstrated all vessels <50 μm and the majority of 50-100 μm vessels were removed, there was no significant decrease in vessels larger than 200 μm. In this study, we only performed measurement for one lesion that was large enough to contain sufficient vessel number for statistical analysis. The size distribution of remaining vessels inside a histotripsy lesion likely depends upon the acoustic parameters and treatment times. Future work will be needed to further study the effects of histotripsy on intermediate sized blood vessels. It is worth noting that, while preferable, the preservation of intermediate sized vessels (<1mm) is not a primary clinical concern for liver ablation as it is with the major vessels (>1mm).
One major concern for current liver ablation therapy, including HIFU, is the extended treatment time for large liver tumor volumes. The treatment time for recent clinical HIFU cases ranged from 2-4 hours for tumors >4cm in diameter (Wu et al. 2004) or 1-6 hours for tumors >1.7 cm in diameter (Park et al. 2009). When combined with TACE to inject chemotherapy agents in patients to decrease tumor blood flow, the HIFU treatment time is comparable to RFA, the current clinical standard (Zhang et al. 2009). Current research is underway to decrease HIFU treatment times by improving MRI imaging feedback during treatment or using temperature sensitive contrast agents to aid in the treatment effectiveness (Fischer et al. 2010). As histotripsy is unaffected by heat perfusion from blood flow, it has the potential to achieve fast and consistent ablation of large volumes and a greater number of tumor nodules. In this initial study, a larger porcine liver volume (60 cm3) close to a 5 cm diameter volume (65.4 cm3) was ablated. By optimizing the acoustic and scanning parameters, the treatment time is expected to decrease. As histotripsy pulses are only a few microseconds long separated by several milliseconds, a phased array could be utilized to electronically steer the focus to other locations in the treatment region during the time between pulses to further shorten the treatment time. Future work will aim to optimize acoustic parameters and investigate phased array transducers to minimize the treatment time for large liver volumes.
Another concern of liver ablation is the effect of respiratory motion. The 60 cm3 volume lesion created here was significantly larger than the treatment scan volume likely because of the large displacement (~1 cm) from respiratory motion observed during the treatment. There are multiple approaches that may be used to address this issue. To avoid the effect of motion on therapy, patients in HIFU liver tumor clinical trials were trained to hold their breath during sonication, with HIFU therapy halted during breaths. Sophisticated motion-tracking methods are being investigated for MRI guided HIFU (Pernot et al. 2004; Ries et al. 2010; Arnold et al. 2011; de Senneville et al. 2011). Ultrasound-based tracking has also been developed using pulse-echo sequences from a subset of the therapy phased array elements to track the liver speckle (Hein and O’Brien 1993; Marquet et al. 2011). Our lab is investigating a histotripsy system integrated with ultrasound image-based motion tracking algorithms that can dynamically adjust the focal zone of a 2D phased-array transducer to compensate for respiratory or other motion. Our tracking algorithm uses ultrasound images but significantly accelerates the computation by limiting the search iterations for the moving target (Miller et al. 2011). Although not used in this study, a motion tracking method will be utilized in the future to improve the precision of histotripsy for liver ablation.
Conclusions
This study demonstrates the feasibility of using histotripsy as a non-invasive ablation method for liver treatment. Histotripsy was capable of creating precise lesions within the liver through the intact chest without the need for aberration correction. Additionally, results show that histotripsy completely ablated a 60 cm3 liver volume within an hour. Treatments were shown to fractionate the liver tissue surrounding major vessel and the gallbladder while the fractionation was self-limiting at the boundaries of these critical structures. The results of this work suggest that histotripsy has potential as an innovative non-invasive liver ablation method with improvement over the current tissue ablation methods. Future work will aim to further optimize histotripsy for liver ablation and demonstrate the ability of histotripsy to treat tumors in a relevant liver cancer model.
Acknowledgements
This work was supported by NIH grant R01 CA134579, a National Science Foundation Graduate Research Fellowship, and Dr. Zhen Xu’s research fund from the Department of Biomedical Engineering at the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold P, Preiswerk F, Fasel B, Salomir R, Scheffler K, Cattin PC. 3D organ motion prediction for MR-guided high intensity focused ultrasound. Med Image Comput Comput Assist Interv. 2011;14:623–30. doi: 10.1007/978-3-642-23629-7_76. [DOI] [PubMed] [Google Scholar]
- Aschoff AJ, Merkle EM, Wong V, Zhang Q, Mendez MM, Duerk JL, Lewin JS. How does alteration of hepatic blood flow affect liver perfusion and radiofrequency-induced thermal lesion size in rabbit liver? J Magn Reson Imaging. 2001;13:57–63. doi: 10.1002/1522-2586(200101)13:1<57::aid-jmri1009>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bobkova S, Gavrilov L, Khokhlova V, Shaw A, Hand J. Focusing of high-intensity ultrasound through the rib cage using a therapeutic random phased array. Ultrasound Med Biol. 2010;36:888–906. doi: 10.1016/j.ultrasmedbio.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Charnley RM, Doran J, Morris DL. Cryotherapy for liver metastases: a new approach. Br J Surg. 1989;76:1040–1. doi: 10.1002/bjs.1800761018. [DOI] [PubMed] [Google Scholar]
- Chen L, ter Haar G, Hill CR, Dworkin M, Carnochan P, Young H, Bensted JP. Effect of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys Med Biol. 1993;38:1661–73. doi: 10.1088/0031-9155/38/11/011. [DOI] [PubMed] [Google Scholar]
- Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–9. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- Cochard E, Prada C, Aubry JF, Fink M. Ultrasonic focusing through the ribs using the DORT method. Med Phys. 2009;36:3495–503. doi: 10.1118/1.3159755. [DOI] [PubMed] [Google Scholar]
- Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6:14–23. doi: 10.1634/theoncologist.6-1-14. [DOI] [PubMed] [Google Scholar]
- Davis CR. Interventional radiological treatment of hepatocellular carcinoma. Cancer Control. 2010;17:87–99. doi: 10.1177/107327481001700204. [DOI] [PubMed] [Google Scholar]
- de Senneville BD, Ries M, Maclair G, Moonen C. MR-guided thermotherapy of abdominal organs using a robust PCA-based motion descriptor. IEEE Trans Med Imaging. 2011;30:1987–95. doi: 10.1109/TMI.2011.2161095. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- Erce C, Parks RW. Interstitial ablative techniques for hepatic tumours. Br J Surg. 2003;90:272–89. doi: 10.1002/bjs.4091. [DOI] [PubMed] [Google Scholar]
- Fischer K, Gedroyc W, Jolesz FA. Focused ultrasound as a local therapy for liver cancer. Cancer J. 2010;16:118–24. doi: 10.1097/PPO.0b013e3181db7c32. [DOI] [PubMed] [Google Scholar]
- Hall TL, Hempel CR, Wojno K, Xu Z, Cain CA, Roberts WW. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74:932–7. doi: 10.1016/j.urology.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head HW, Dodd GD., 3rd Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127:S167–78. doi: 10.1053/j.gastro.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Hein IA, O’Brien WR. Current time-domain methods for assessing tissue motion by analysis from reflected ultrasound echoes-a review. IEEE Trans Ultrason Ferroelectr Freq Control. 1993;40:84–102. doi: 10.1109/58.212556. [DOI] [PubMed] [Google Scholar]
- Hempel CR, Hall TL, Cain CA, Fowlkes JB, Xu Z, Roberts WW. Histotripsy fractionation of prostate tissue: local effects and systemic response in a canine model. J Urol. 2011;185:1484–9. doi: 10.1016/j.juro.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, Middleton MR. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–5. doi: 10.1038/sj.bjc.6602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi N. Recent advances of radiofrequency ablation for early hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(Suppl 1):115–22. doi: 10.1111/j.1440-1746.2010.06543.x. [DOI] [PubMed] [Google Scholar]
- Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–58. [PubMed] [Google Scholar]
- Jung SE, Cho SH, Jang JH, Han JY. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36:185–95. doi: 10.1007/s00261-010-9628-2. [DOI] [PubMed] [Google Scholar]
- Khokhlova VA, Bobkova SM, Gavrilov LR. Focus Splitting Associated with Propagation of Focused Ultrasound through the Rib Cage. Acoust Phys. 2010;56:665–74. doi: 10.1134/S106377101005012X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Gelehrter SK, Fifer CG, Lu JC, Owens GE, Berman DR, Williams J, Wilkinson JE, Ives KA, Xu Z. Non-invasive pulsed cavitational ultrasound for fetal tissue ablation: feasibility study in a fetal sheep model. Ultrasound Obstet Gynecol. 2011a;37:450–7. doi: 10.1002/uog.8880. [DOI] [PubMed] [Google Scholar]
- Kim Y, Wang TY, Xu Z, Cain CA. Lesion generation through ribs using histotripsy therapy without aberration correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2011b;58:2334–43. doi: 10.1109/TUFFC.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78(Suppl 1):113–24. doi: 10.1159/000315239. [DOI] [PubMed] [Google Scholar]
- Lake AM, Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72:682–6. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie TA, Kennedy JE. High-intensity focused ultrasound principles, current uses, and potential for the future. Ultrasound Q. 2006;22:263–72. doi: 10.1097/01.ruq.0000237259.25885.72. [DOI] [PubMed] [Google Scholar]
- Leslie TA, Kennedy JE, Illing RO, Ter Haar GR, Wu F, Phillips RR, Friend PJ, Roberts IS, Cranston DW, Middleton MR. High-intensity focused ultrasound ablation of liver tumours: can radiological assessment predict the histological response? Br J Radiol. 2008;81:564–71. doi: 10.1259/bjr/27118953. [DOI] [PubMed] [Google Scholar]
- Li CX, Xu GL, Li JJ, Luo GY. [High intensity focused ultrasound for liver cancer] Zhonghua Zhong Liu Za Zhi. 2003;25:94–6. [PubMed] [Google Scholar]
- Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol. 2007;25:978–86. doi: 10.1200/JCO.2006.09.8657. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Makisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22–9. doi: 10.1177/145749691110000105. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–74. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- Marquet F, Aubry JF, Pernot M, Fink M, Tanter M. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys Med Biol. 2011;56:7061–80. doi: 10.1088/0031-9155/56/22/005. [DOI] [PubMed] [Google Scholar]
- Marrero JA, Pelletier S. Hepatocellular carcinoma. Clin Liver Dis. 2006;10:339–51. doi: 10.1016/j.cld.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr., Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol. 2011;22:369–77. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, Kee ST. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol. 2010;21:S204–13. doi: 10.1016/j.jvir.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Okada A, Murakami T, Mikami K, Onishi H, Tanigawa N, Marukawa T, Nakamura H. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn Reson Med Sci. 2006;5:167–71. doi: 10.2463/mrms.5.167. [DOI] [PubMed] [Google Scholar]
- Owens GE, Miller RM, Ensing G, Ives K, Gordon D, Ludomirsky A, Xu Z. Therapeutic ultrasound to noninvasively create intracardiac communications in an intact animal model. Catheter Cardiovasc Interv. 2011;77:580–8. doi: 10.1002/ccd.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Hyman D. Hepatocellular cancer: a guide for the internist. Am J Med. 2007;120:194–202. doi: 10.1016/j.amjmed.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Park MY, Jung SE, Cho SH, Piao XH, Hahn ST, Han JY, Woo IS. Preliminary experience using high intensity focused ultrasound for treating liver metastasis from colon and stomach cancer. Int J Hyperthermia. 2009;25:180–8. doi: 10.1080/02656730802641949. [DOI] [PubMed] [Google Scholar]
- Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006a;32:115–29. doi: 10.1016/j.ultrasmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Parsons JE, Cain CA, Fowlkes JB. Cost-effective assembly of a basic fiber-optic hydrophone for measurement of high-amplitude therapeutic ultrasound fields. J Acoust Soc Am. 2006b;119:1432–40. doi: 10.1121/1.2166708. [DOI] [PubMed] [Google Scholar]
- Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–65. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, Magee JC, Lok AS, Fontana RJ, Marrero JA. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–68. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]
- Pernot M, Tanter M, Fink M. 3-D real-time motion correction in high-intensity focused ultrasound therapy. Ultrasound Med Biol. 2004;30:1239–49. doi: 10.1016/j.ultrasmedbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Ries M, de Senneville BD, Roujol S, Berber Y, Quesson B, Moonen C. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn Reson Med. 2010;64:1704–12. doi: 10.1002/mrm.22548. [DOI] [PubMed] [Google Scholar]
- Roberts WW, Hall TL, Ives K, Wolf JS, Jr., Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–8. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- Styn NR, Wheat JC, Hall TL, Roberts WW. Histotripsy of VX-2 tumor implanted in a renal rabbit model. J Endourol. 2010;24:1145–50. doi: 10.1089/end.2010.0123. [DOI] [PubMed] [Google Scholar]
- ter Haar G. High intensity ultrasound. Semin Laparosc Surg. 2001;8:77–89. [PubMed] [Google Scholar]
- Wang H, Ebbini ES, Odonnell M, Cain CA. Phase Aberration Correction and Motion Compensation for Ultrasonic Hyperthermia Phased-Arrays - Experimental Results. Ieee T Ultrason Ferr. 1994;41:34–43. [Google Scholar]
- Wang TY, Hall TL, Xu Z, Fowlkes JB, Cain CA. Imaging feedback of histotripsy treatments using ultrasound shear wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59:1167–81. doi: 10.1109/tuffc.2012.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Xu Z, Winterroth F, Hall TL, Fowlkes JB, Rothman ED, Roberts WW, Cain CA. Quantitative ultrasound backscatter for pulsed cavitational ultrasound therapy-histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:995–1005. doi: 10.1109/tuffc.2009.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, Zheng G, Zhou Y, Xu G, Li M, Zhang C, Ye H, Feng R. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–54. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, Cain CA. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–36. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121:742–9. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H, Chen W, Bai J, Wang Z. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437–45. doi: 10.1007/s00330-008-1137-0. [DOI] [PubMed] [Google Scholar]







