Abstract
Background
Poor sleep contributes to adult morbidity and mortality.
Purpose
The study examined the extent to which trait positive affect (PA) and PA reactivity, defined as the magnitude of change in daily PA in response to daily events, were linked to sleep outcomes.
Methods
Analyses are based on data from 100 respondents selected from the National Survey of Midlife in the United States (MIDUS).
Results
Multilevel analyses indicated that higher levels of trait PA were associated with greater morning rest and better overall sleep quality. In contrast, PA reactivity was associated with diminished sleep efficiency. Finally, interactions between PA reactivity and trait PA emerged on all three sleep measures, such that higher event-related change in daily positive affect was associated with impaired sleep, especially among individuals high in trait PA.
Conclusions
Results suggest that high trait PA, when coupled with high PA reactivity, may contribute to poor sleep.
Keywords: trait positive affect, positive affect reactivity, sleep
Introduction
Changes in fundamental aspects of sleep, including poorer sleep efficiency and greater sleep disturbances, can have profound health effects that contribute to increased risk for adult morbidity and all-cause mortality [1–3]. While progressive loss of sleep adversely affects health and well-being, recent empirical evidence demonstrates that positive affect (PA) may be conducive to adaptive sleep patterns. In an illustrative study, Steptoe, O’Donnell, Marmot, and Wardle [4] reported an inverse association between trait PA and sleep problems among a sample of healthy adults. Other studies conducted with clinical samples and healthy controls show similar associations between PA and sleep quality indicators, including increases in sleep duration and decreases in fragmented rapid eye-movement sleep [5, 6]. The available evidence, thus, suggests that the restorative benefits of sleep may be enhanced by high trait PA. Moreover, these associations appear to be independent of negative affect (NA), suggesting that high trait PA may have a salutary health effect that is distinct from that associated with low NA [7].
Although previous research suggests that high trait PA is associated with improved sleep [8, 9], little is known about how day-to-day changes in PA are connected to sleep. Whereas trait PA refers to people’s characteristic global levels of positive affect, PAreactivity can be conceptualized as the within-person or intraindividual covariation between daily events and daily PA [10, 11]. High reactivity theoretically reflects a diathesis that constitutes vulnerability [11]. Although much of the existing literature has focused on NA reactivity to daily stressors [12–16], recent research suggests PA reactivity to everyday situations that are positive as well as stressful may account for important variations in health and well-being [17]. For example, O’Neill, Cohen, Tolpin, and Gunthert [10] demonstrated that heightened PA reactivity to daily interpersonal stressors was a unique vulnerability factor in the development of later depressive symptoms. Along similar lines, Finan, Zautra, and Davis [18] observed that failure to maintain PA in the face of daily pain reflected a vulnerability for fibromyalgia patients. More recently, Mroczek et al. [19] showed that deficits in PA in response to daily stressors predicted mortality. Finally, and more specific to the issue of sleep, is a finding by Talbot, Hairston, Eidelman, Gruber, and Harvey [20] that among individuals with bipolar disorder, difficulties in regulating PA following a positive mood induction contributed to disturbances in sleep onset latency. These findings, in combination with previous research [11, 21], suggest that PA reactivity may function in a diathesis-stress-like manner in conferring differential vulnerability to poor sleep.
Beyond consideration of affective reactivity to putatively positive and negative experiences, studies examining affect variability also provide evidence relevant to individual differences in affective functioning. Affect variability refers to the amount of fluctuations in affective states and, in previous research, has been operationalized as the intraindividual standard deviation (iSD) of affect scores across time; the larger the standard deviation, the more variable an individual’s affect. A number of studies have shown that individual differences in affect variability are stable across time [22–24] and linked to personality traits such as neuroticism and extraversion [25, 26]. More recently, Gruber and colleagues [27] showed that independent of average levels of PA, variability in PA was associated with lower life satisfaction and higher depression and anxiety. It is important to point out, however, that as a potential vulnerability factor, affect reactivity should be differentiated from affect variability (e.g., iSD), because vulnerability in the latter sense does not directly account for the covariation between exogenous influences and daily affect that may be present in the former [11, 12, 28].
A better understanding of individual differences in PA reactivity has important theoretical and practical implications. First, it may clarify the extent to which affect reactivity is a distinct dynamic facet, separate from trait affect, along which individuals can be characterized [11]. Second, prior investigations have generally examined PA reactivity in response to negative events or stressors, thereby leaving open the question of whether as a potential vulnerability byproduct, PA reactivity is unique to negative events [10], positive events [29], or the overall ratio of positive to negative events [30]. Third, a focus on PA reactivity may help to provide a potential explanation for why, at very high levels, PA may sometimes confer detrimental outcomes [31, 32]. For instance, Diener, Colvin, Pavot and Allman [33] reported that people who experienced intense PA were more likely to experience intense NA as well. Likewise, Friedman and colleagues found that extremely cheerful people were more likely to engage in risky health behaviors [34] that increased their risk of early mortality [35]. Thus, examining changes in PA in response to daily positive and negative events may help to reveal trait vulnerabilities in PA and thereby point to the dynamics associated with fragile high PA. A similar emphasis on understanding the interactive roles of stable and dynamic processes has been offered by Kernis and Waschull [36] in their discussion of the distinction between secure and vulnerable self-esteem. For example, Kernis and colleagues demonstrated that individuals with characteristically high but unstable feelings of self-worth scored higher on measures of hostility [37] and defensiveness [38], and lower on measures of psychological well-being [39].
The Current Study
The current study aims to extend conceptual understanding of the relationship between PA and sleep. Extrapolating from previous research, we hypothesized that: (1) higher levels of trait PA would relate to more adaptive sleep outcomes (i.e., greater morning rest, better overall sleep quality, and increased sleep efficiency); (2) greater PA reactivity would be associated with poorer sleep outcomes; and (3) high PA reactivity would interact with high trait PA to exacerbate sleep problems.
Methods
Sample and Procedure
The data for the current study are from a subset of participants in the Midlife in the United States Survey (MIDUS II), a national probability survey of health and aging (n = 4,963, aged 35–85) conducted in the United States between January 2004 and August 2005. Starting in April 2004, a subsample of MIDUS II respondents (n = 2,022) were recruited for the second wave of the National Study of Daily Experiences (NSDE II), an 8-day protocol which consisted of a 10–15 minute telephone interview on eight consecutive evenings at approximately the same time each day [40]; the average time between the MIDUS II survey and NSDE II telephone interview was approximately 9 months (M = 9.1 months, SD = 7.6 months). Of these, 100 respondents (47 men and 53 women, aged 43–68) subsequently participated in the Biomarker Study (University of Wisconsin-Madison) that included a 7-day sleep study from which the current data were drawn; the average time between the NSDE II telephone interview and the Biomarker Study was approximately 23 months (M = 23.2 months, SD = 13.6 months). The current analysis, thus, used available data from all respondents who progressed through the MIDUS II, NSDE II, and Biomarker studies. Data collection for the national probability, telephone, and sleep studies were approved by the Institutional Review Boards at each participating site, and all respondents provided informed consent. More information on MIDUS II participants and subsamples are available elsewhere [41].
Measures
Trait positive affect
Data on trait PA was obtained in MIDUS II by self-administered questionnaire. Participants rated the amount of time they experienced various affective states over the past 30 days on a 5-point scale, ranging from 1 (none of the time) to 5 (all of the time). The 6-item trait PA scale (i.e., “cheerful,” “in good spirits,” “extremely happy,” “calm and peaceful,” “satisfied,” and “full of life”) was comprised of items from several well-validated measures of trait PA including the Affect Balance Scale [42] and General Well-Being Schedule [43]. In current sample, Cronbach’s alpha for the 6-item scale was .82.
Daily positive affect
Data on daily PA was obtained in NSDE II by telephone interview. Thirteen items were used to assess daily PA (i.e., “in good spirits,” “cheerful,” “extremely happy,” “calm and peaceful,” “satisfied,” “full of life,” “close to others,” “like you belong,” “enthusiastic,” “attentive,” “proud,” “active,” and “confident”). Each evening, participants indicated how frequently they felt each affective state during the past 24 hours using a 5-point scale (0 = none of the time, 4 = all of the time). Cronbach’s alpha for the 13-item scale was .92.
Daily events
During the NSDE II telephone interview, respondents completed a daily inventory of positive and negative events. Daily negative events were assessed through the Daily Inventory of Stressful Events [DISE; 44]. The inventory consists of seven stem questions used to obtain information about stressor occurrence in the past 24 hours: having arguments, avoiding arguments, work stressors, home stressors, and network stressors (i.e., stressors that occurred to friends and family). Participants also reported positive events that occurred in the previous 24 hours using five questions: a positive interaction with someone, a positive experience at work, a positive event at home, a positive event experienced by a close friend or relative, or anything else that was particularly positive.
Sleep quality
Data on sleep quality were obtained in the sleep protocol that was part of the larger Biomarker Study. For seven consecutive days, participants wore a Mini Mitter Actiwatch®-64 activity monitor and also completed a paper and pencil daily sleep diary over the same time period. Self-reported sleep measures included morning rest level and overall sleep quality. Upon awakening, respondents indicated how well-rested they felt using a 5-point scale (1 = well rested, 5 = poorly rested). Subjective ratings of overall sleep quality were similarly assessed using a 5-point scale (1 = very good, 5 = very poor). Items were reverse coded so that higher values represented more rest and better overall sleep quality. Sleep efficiency (the percent of time in bed spent asleep) was the primary objective measure of sleep and was measured using an Actiwatch® activity monitor, which calculates sleep efficiency by dividing the total sleep time by the total time between lights out and lights on.
Covariates
We examined the extent to which associations between sleep and components of PA (trait-level and daily reactivity) were independent of potential confounding demographic (e.g., age, gender, income), period (weekday vs. weekend), and psychological (e.g., self-rated health, trait NA) variables known to affect risk of sleep problems [4, 5]. Self-rated health was assessed by a single question: “In general would you say your physical health is excellent, very good, good, or fair.” Trait NA was rated over the past 30 days on a 5-point scale, ranging from 1 (none of the time) to 5 (all of the time), and measured with six items: “so sad nothing could cheer you up,” “nervous,” “restless or fidgety,” “hopeless,” “that everything was an effort,” and “worthless” (α = .65). In addition to the covariates above, analyses also controlled for the influence of daily exercise (in minutes), caffeine and alcohol consumption, total sleep time (in minutes), and use of sleep medication. Caffeine and alcohol use were determined by the number of drinks individuals reported consuming before bed. Use of sleep medication was determined by participants reporting whether they took any sleep medication (0 = did not use sleep medication, 1 = used sleep medication) before bed.
Overview of Analyses
Following Cohen et al. [11], we used a multilevel modeling (MLM) approach to compute estimates of daily PA reactivity by examining the unique relationships between the number of daily events and daily PA for each person. Three separate PA reactivity scores were estimated for each person, representing the amount of daily covariation between PA and positive events, negative events, and net events (positive-negative), respectively. The latter score provides an estimate of the daily net effect of positive and negative event occurrence on PA. All models were estimated by means of restricted maximum likelihood (REML). Under this estimation procedure, REML estimates for missing data at Level 1 are obtained via the expectation-maximization (EM) algorithm [45].
The MLM-derived slope estimates were then used as person-level independent variables in subsequent analyses of sleep. All person-level variables were standardized (i.e., mean centered and divided by their sample standard deviation) so that each coefficient reflects differences in the outcome per unit of change in the independent variable. In addition to the primary independent variables of trait PA and PA reactivity, analyses controlled for the effects of time-varying (e.g., weekday vs. weekend, exercise, total sleep time) and time-invariant (e.g., demographics, self-rated health, negative affect) covariates [4, 5]. Furthermore, because previous studies controlled for the intraindividual standard deviation and average levels of NA and PA [15, 46], we also included these variables in our models. Finally, interactions between trait PA and PA reactivity were included to examine trait-level differences in the association between PA reactivity and sleep. To reduce spurious moderator effects [47, 48], curvilinear trends of the two component PA variables (trait-level and daily reactivity) were also included. Significant interactions were probed using procedures described by Bauer and Curran [49] and Preacher, Bauer, and Curran [50]. The full Level-1 and Level-2 models, including all independent variables and covariates, are described in the Appendix.
Results
Descriptive Analyses
Of the 100 participants who completed the MIDUS II, NSDE II, and Biomarker studies, 3 had missing data on household income, trait PA, and trait NA. Comparisons between the 3 participants who had missing data on one or more of these covariates and the 97 participants who had complete data revealed no differences in baseline demographics of gender, χ2(1, N = 100) = 0.48, n.s., age, t(98) = −0.72, n.s., or level of educational attainment, χ2(1, N = 100) = 6.53, n.s. Descriptive statistics on the study participants are provided in Table 1. The average total number of positive events across the 8 days was 8.42 (SD = 5.54, range = 0 to 24). By comparison, the average total number of negative events was 3.34 (SD = 2.92, range = 0 to 15). The average total number of net events (derived by subtracting the total number of negative events from the total number of positive events) across the 8 days was 5.08 (SD = 5.36, range = −6 to 20). The mean PA reactivity coefficient (i.e., how much PA changed per unit increase in daily events) was −0.11 (SD = 0.05, range = −0.21 to 0.04) for negative events, 0.05 (SD = 0.04, range = −0.02 to 0.18) for positive events, and 0.07 (SD = 0.03, range = −0.06 to 0.11) for net events, respectively. Gender differences were also tested. On average, sleep efficiency was significantly higher in women (M = 84.8, SD = 6.91) than men (M = 79.5, SD = 9.70), t(98) = 3.19, p < .05. Women also reported higher rates of cigarette smoking (17.0%) than men (3.8%), χ2(1, N = 100) = 4.86, p < .05. Compared with men, women reported, on average, greater numbers of positive events (M = 1.31 vs. M = 0.88), t(98) = 3.33, p < .05, negative events (M = 0.51 vs. M = 0.36), t(98) = 2.01, p < .05, and net events (M = 0.80 vs. M = 0.52), t(98) = 2.15, p < .05, respectively. Table 2 shows the zero-order correlations among the major day- and person-level variables under investigation. In the current sample, trait PA and PA reactivity scores were moderately and inversely correlated with one another (rs ranged from −0.48 to −0.28).
Table 1.
Characteristics of Study Participants
| Men (n = 47) | Women (n = 53) | Overall (N = 100) | |
|---|---|---|---|
| Age (years) | 56.00 (12.62) | 55.45 (12.26) | 55.71 (12.37) |
| Currently employed | 59.57% | 56.60% | 58.00% |
| Currently married (n=97) | 82.69% | 73.33% | 78.35% |
| Household income (n=97) | |||
| < $45,000 | 35.56% | 30.76% | 32.99% |
| $45,000–80,000 | 33.33% | 46.15% | 40.21% |
| >$80,000 | 31.11% | 23.08% | 26.80% |
| Self-rated health | |||
| Excellent | 17.02% | 16.98% | 17.00% |
| Very good | 48.94% | 52.83% | 51.00% |
| Good | 21.27% | 28.30% | 25.00% |
| Fair | 12.77% | 1.89% | 7.00% |
| Current smoker | 3.77% | 17.02% | 10.00% |
| Body mass index (kg/m2) | 27.79 (4.64) | 27.15 (5.60) | 27.45 (5.15) |
| Trait PA (1–5) | 3.65 (0.67) | 3.77 (0.68) | 3.72 (0.67) |
| Trait NA (1–5) | 1.36 (0.36) | 1.37 (0.38) | 1.37 (0.33) |
| Daily PA (0–4) | 2.76 (0.61) | 2.96 (0.53) | 2.86 (0.58) |
| Daily NA (0–4) | 0.12 (0.14) | 0.13 (0.13) | 0.12 (0.14) |
| Positive events | 0.88 (0.53) | 1.31 (0.76) | 1.10 (0.71) |
| Negative events | 0.36 (0.33) | 0.51 (0.39) | 0.44 (0.38) |
| Net events | 0.50 (0.51) | 0.80 (0.78) | 0.66 (0.69) |
| Overall sleep quality (1–5) | 3.66 (0.75) | 3.79 (0.68) | 3.73 (0.72) |
| AM rested (1–5) | 3.70 (0.79) | 3.77 (0.65) | 3.73 (0.71) |
| Efficiency (%) | 79.5 (9.70) | 84.81 (6.91) | 82.31 (8.72) |
| Sleep time (in minutes) | 364.80 (51.80) | 403.10 (58.87) | 385.10 (58.58) |
| Reactivity to negative events | −0.10 (0.02) | −0.12 (0.04) | −0.11 (0.05) |
| Reactivity to positive events | 0.05 (0.04) | 0.05 (0.03) | 0.05 (0.04) |
| Net reactivity | 0.07 (0.02) | 0.07 (0.03) | 0.07 (0.03) |
Note. Standard deviations are shown in parentheses. NA = negative affect. PA = positive affect.
Table 2.
Summary Statistics of Person-level and Day-level Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Person-level Variables | |||||||
| 1. Trait Positive Affect | --- | −.32** | −.48** | −.28** | .21* | .41** | .34** |
| 2. Negative Reactivity | --- | .28** | .30** | −.02 | −.09 | −.04 | |
| 3. Positive Reactivity | --- | .79** | −.23* | −.33** | −.35** | ||
| 4. Net Reactivity | --- | −.08 | −.24* | −.31** | |||
| Day-level Variables | |||||||
| 5. Sleep Efficiency | --- | .03 | .06 | ||||
| 6. Morning Rest | --- | .51** | |||||
| 7. Overall Quality | --- | ||||||
Note.
p < .05,
p < .01, two-tailed.
N = 100 persons.
MLM Analyses of Sleep Data
Sleep efficiency
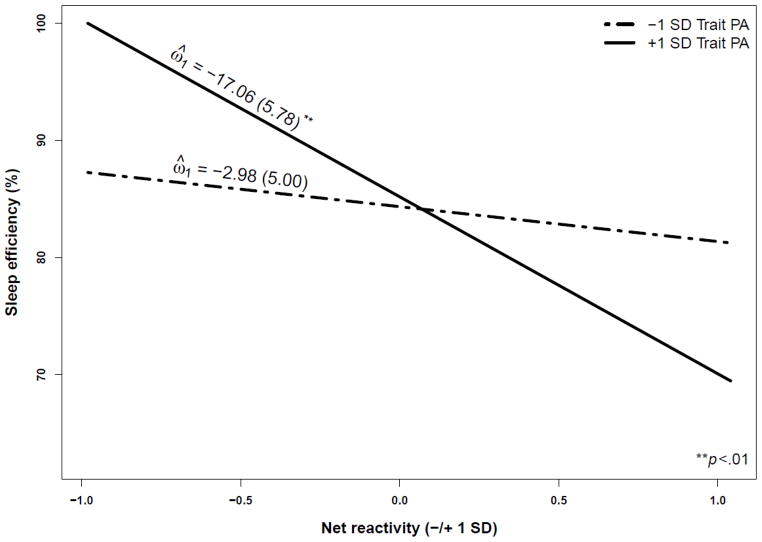
As seen in Table 3, net of other independent variables in the model, each PA reactivity coefficient had a significant effect on sleep efficiency. In each case, the greater the PA change in response to everyday events, the lower the sleep efficiency. The effect of net reactivity on sleep efficiency, however, was qualified by a significant interaction with trait PA (γ020 = −7.11, p < .05). To aid in the interpretation, parameter values were generated using values one standard deviation (SD) above and below the mean to represent high and low scores for net reactivity and trait PA (see Figure 1). Estimates of simple slopes from the two-way multilevel interaction [49, 50] confirmed that the increase in PA in response to net daily events was associated with lower sleep efficiency among persons high (ω̂ = −17.06, Z = −2.95, p < .01) but not low (ω̂= −2.98, Z = −0.59, n.s.) in trait PA.
Table 3.
Multilevel Model Estimates for Sleep Efficiency
| Fixed Effect | Coefficient | SE | t-value | p-value |
|---|---|---|---|---|
| Average level of sleep efficiency | ||||
| Intercept | 85.724 | 1.728 | 49.596 | <0.001 |
| Gender | −3.725 | 2.024 | −1.840 | 0.070 |
| Age | −1.683 | 1.075 | −1.566 | 0.122 |
| Income | 0.121 | 0.952 | 0.127 | 0.899 |
| Self-rated health | 0.085 | 1.108 | 0.077 | 0.939 |
| Mean PA | 2.231 | 2.225 | 1.003 | 0.319 |
| Mean NA | −1.784 | 1.589 | −1.122 | 0.265 |
| iSD PA | −1.727 | 1.496 | −1.154 | 0.252 |
| iSD NA | −2.039 | 1.736 | −1.174 | 0.244 |
| Trait NA | −1.106 | 1.196 | −0.924 | 0.358 |
| Trait PA | 1.443 | 1.255 | 1.150 | 0.254 |
| Negative reactivity | −6.030 | 2.481 | −2.430 | 0.018 |
| Positive reactivity | −11.373 | 4.612 | −2.466 | 0.016 |
| Net reactivity | −9.805 | 4.025 | −2.436 | 0.017 |
| Trait PA squared | −1.189 | 1.033 | −1.151 | 0.253 |
| Negative reactivity squared | −0.702 | 0.864 | −0.812 | 0.419 |
| Positive reactivity squared | −0.494 | 0.990 | −0.499 | 0.619 |
| Net reactivity squared | −0.409 | 1.188 | −0.345 | 0.731 |
| Trait PA × Negative reactivity | −0.567 | 2.328 | −0.244 | 0.808 |
| Trait PA × Positive reactivity | −5.965 | 3.718 | −1.604 | 0.113 |
| Trait PA × Net reactivity | −7.110 | 3.627 | −1.991 | 0.044 |
| Weekend slope | ||||
| Intercept | −1.336 | 0.460 | −2.904 | 0.004 |
| Exercise slope | ||||
| Intercept | −0.001 | 0.005 | −0.132 | 0.895 |
| Caffeine slope | ||||
| Intercept | −0.301 | 0.190 | −1.587 | 0.113 |
| Sleep medication slope | ||||
| Intercept | −1.494 | 2.106 | −0.709 | 0.478 |
| Sleep time slope | ||||
| Intercept | 0.058 | 0.004 | 15.675 | <0.001 |
Note. Gender is dichotomously coded (female=0, male=1). iSD = intraindividual standard deviation. NA = negative affect. PA = positive affect. All person-level variables were standardized (i.e., mean centered and divided by their sample standard deviation).
Figure 1.
Average sleep efficiency as a function of trait positive affect (PA) and net reactivity.
Morning rest
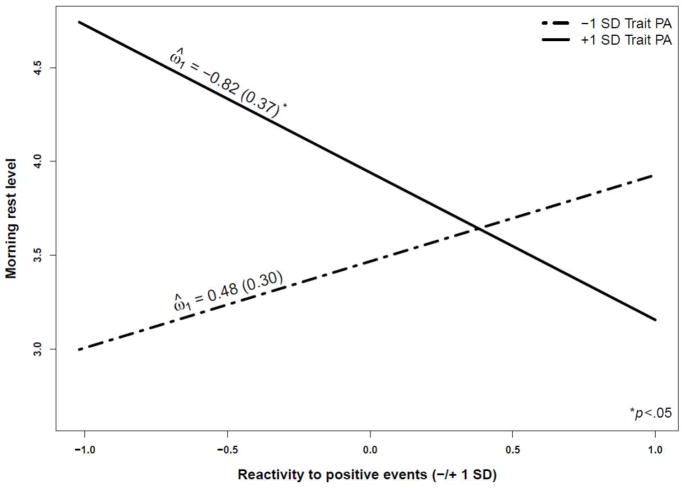
A significant effect emerged for trait PA (γ06 = 0.21, p < .01), indicating that those with higher trait PA reported greater rest the following morning. However, in each instance, this effect was qualified by a PA reactivity × trait PA interaction (see Table 4). More specifically, greater PA reactivity (dampened PA responses to negative events and heightened PA responses to positive or net events) was related to diminished morning rest among high trait PA individuals, but not among low trait PA individuals. For illustrative purposes, Figure 2 illustrates this relationship for reactivity to positive events. Post-hoc analyses of simple slopes confirmed significant differences among individuals high in trait PA (ω̂ = −0.82, Z = −2.23, p < .05) but not among those low in trait PA (ω̂ = 0.48, Z = 1.61, n.s.) as a function of increased PA reactivity to positive events.
Table 4.
Multilevel Model Estimates for Morning Rest
| Fixed Effect | Coefficient | SE | t-value | p-value |
|---|---|---|---|---|
| Average level of sleep efficiency | ||||
| Intercept | 3.691 | 0.121 | 30.575 | <0.001 |
| Gender | 0.240 | 0.138 | 1.743 | 0.085 |
| Age | 0.107 | 0.074 | 1.445 | 0.153 |
| Income | 0.164 | 0.056 | 2.908 | 0.005 |
| Self-rated health | −0.149 | 0.078 | −1.913 | 0.060 |
| Mean PA | 0.220 | 0.126 | 1.743 | 0.086 |
| Mean NA | −0.055 | 0.104 | −0.530 | 0.598 |
| iSD PA | −0.100 | 0.082 | −1.219 | 0.227 |
| iSD NA | −0.042 | 0.106 | −0.396 | 0.693 |
| Trait NA | −0.094 | 0.078 | −1.199 | 0.234 |
| Trait PA | 0.213 | 0.064 | 3.313 | 0.001 |
| Negative reactivity | −0.206 | 0.157 | −1.311 | 0.194 |
| Positive reactivity | −0.150 | 0.262 | −0.570 | 0.570 |
| Net reactivity | −0.227 | 0.230 | −0.985 | 0.328 |
| Trait PA squared | −0.002 | 0.053 | −0.046 | 0.963 |
| Negative reactivity squared | 0.001 | 0.052 | −0.004 | 0.997 |
| Positive reactivity squared | −0.105 | 0.045 | −2.319 | 0.023 |
| Net reactivity squared | 0.025 | 0.061 | 0.411 | 0.682 |
| Trait PA × Negative reactivity | −0.312 | 0.137 | −2.271 | 0.026 |
| Trait PA × Positive reactivity | −0.655 | 0.207 | −3.167 | 0.002 |
| Trait PA × Net reactivity | −0.599 | 0.206 | −2.901 | 0.005 |
| Weekend slope | ||||
| Intercept | 0.042 | 0.075 | 0.557 | 0.578 |
| Exercise slope | ||||
| Intercept | −0.001 | 0.001 | −0.607 | 0.544 |
| Caffeine slope | ||||
| Intercept | 0.020 | 0.017 | 1.131 | 0.259 |
| Sleep medication slope | ||||
| Intercept | −0.541 | 0.370 | −1.463 | 0.144 |
| Sleep time slope | ||||
| Intercept | 0.002 | 0.001 | 3.853 | <0.001 |
Note. NA = negative affect. PA = positive affect.
Figure 2.
Average morning rest level as a function of trait positive affect (PA) and reactivity to positive events.
Overall sleep quality
As predicted, trait PA was positively associated with overall sleep quality (γ10= 0.20, p < .01), even after controlling for the effects of trait NA (γ09= −0.15, p < .05). More importantly, PA reactivity moderated this relation, as revealed by multiple PA reactivity × trait PA interactions (see Table 5). As an illustration, predicted values for reactivity to negative events are plotted in Figure 3. Specifically, attenuated PA responses to negative events was related to reduced sleep quality among individuals high in trait PA, but not among those low in trait PA. Post-hoc analyses of simple slopes confirmed significant differences among high (ω̂ = −0.51, Z = −1.98, p < .05) but not low (ω̂ = 0.34, Z = 1.71, n.s.) trait PA individuals as a function of increased PA reactivity to negative events.
Table 5.
Multilevel Model Estimates for Overall Sleep
| Fixed Effect | Coefficient | SE | t-value | p-value |
|---|---|---|---|---|
| Average level of sleep efficiency | ||||
| Intercept | 3.753 | 0.122 | 30.887 | <0.001 |
| Gender | 0.080 | 0.132 | 0.610 | 0.544 |
| Age | 0.007 | 0.071 | 0.102 | 0.919 |
| Income | 0.165 | 0.055 | 2.990 | 0.004 |
| Self-rated health | −0.180 | 0.067 | −2.699 | 0.009 |
| Mean PA | 0.038 | 0.153 | 0.246 | 0.806 |
| Mean NA | −0.156 | 0.084 | −1.852 | 0.068 |
| iSD PA | −0.152 | 0.083 | −1.819 | 0.073 |
| iSD NA | −0.033 | 0.091 | −0.367 | 0.715 |
| Trait NA | −0.154 | 0.076 | −2.021 | 0.047 |
| Trait PA | 0.205 | 0.072 | 2.854 | 0.006 |
| Negative reactivity | −0.073 | 0.171 | −0.428 | 0.670 |
| Positive reactivity | −0.083 | 0.274 | −0.304 | 0.762 |
| Net reactivity | 0.077 | 0.233 | 0.331 | 0.742 |
| Trait PA squared | −0.048 | 0.053 | −0.906 | 0.368 |
| Negative reactivity squared | 0.067 | 0.061 | 1.095 | 0.277 |
| Positive reactivity squared | −0.085 | 0.044 | −1.913 | 0.060 |
| Net reactivity squared | −0.015 | 0.063 | −0.237 | 0.813 |
| Trait PA × Negative reactivity | −0.430 | 0.154 | −2.794 | 0.007 |
| Trait PA × Positive reactivity | −0.764 | 0.231 | −3.309 | 0.001 |
| Trait PA × Net reactivity | −0.645 | 0.231 | −2.789 | 0.007 |
| Weekend slope | ||||
| Intercept | 0.002 | 0.077 | 0.027 | 0.978 |
| Exercise slope | ||||
| Intercept | −0.001 | 0.001 | −0.547 | 0.585 |
| Caffeine slope | ||||
| Intercept | −0.018 | 0.025 | −0.701 | 0.484 |
| Sleep medication slope | ||||
| Intercept | −0.280 | 0.453 | −0.619 | 0.536 |
| Sleep time slope | ||||
| Intercept | 0.002 | 0.001 | 2.604 | 0.009 |
Note. NA = negative affect. PA = positive affect.
Figure 3.
Average overall sleep quality as a function of trait positive affect (PA) and reactivity to negative events.
In sum, the separate and interactive effects of PA reactivity and trait PA were robust, remaining significantly associated with multiple sleep outcomes even when adjustments for the person-level means and standard deviations in NA and PA were taken into account.1, 2
Discussion
Findings from the current research indicated that both trait PA and PA reactivity were meaningfully associated with sleep. In line with previous work, we found an overall positive relation between trait PA and sleep [7, 51]. Second, we found that the magnitude of event-related change in PA was inversely related to sleep: the more reactive participants’ contextually based PA, the less efficient their sleep quality. Furthermore, our results suggested that these relationships were best captured by considering the joint effects of PA reactivity and trait PA, with greater PA reactivity being associated with substantially poorer sleep quality, especially among individuals high in trait PA. Importantly, these associations occurred over a relatively long timescale and could be dissociated from the effects of curvilinearity, average levels, and person-level variability in NA and PA, respectively [27, 32].
Overall, results from the present research point to several conclusions. First, the findings join with previous work in underscoring the importance of attending to both stable and dynamic features of psychological functioning [48, 52]. In focusing on the degree to which high self-esteem is secure or vulnerable, for example, Kernis and Waschull [36] suggested that unstable high self-esteem reflects inflated feelings of self-worth that are associated with heightened sensitivity to external positive and negative experiences. Consistent with this view, our findings suggested that the decrease in sleep quality observed among high trait PA individuals may reflect a tendency on the part of some who are high in trait PA to view everyday events as having relevance to their daily PA. This finding may help to explain why, despite its documented benefits, there is also a “dark side” to high PA [31] that has been linked to intense psychological distress [33], risky health behaviors [34], and early mortality [35]. In this way, our findings suggest that high trait PA, when coupled with high PA reactivity, may set the stage for poor sleep outcomes.
Second, to the extent that PA reactivity reflects the tendency to place importance in everyday events as determinants of overall well-being, such ego-involvement may also represent a form of contingent self-worth [53]. This approach suggests that the significance of PA lies not in whether it is high or low, but rather in what it is contingent upon. As such, this reasoning suggests an empirically testable hypothesis. Inasmuch as reactivity may be a source of preexisting vulnerability that contributes to poor affect regulation, reactive individuals should be especially derailed by failure (and buoyed by success) in domains in which their PA is staked.
Third, while existing theoretical models of PA focus on stable individual differences [54, 55], our findings suggest that models that incorporate both trait and state (stable and dynamic) components may offer a more complete understanding of PA and its relationship to sleep. Recent research suggests that stable trait-like feelings of PA may serve to slow down the effects of aging by fortifying restorative sleep [7, 51]. Although our findings are complementary to this work, we extend this research by showing that depending on the magnitude of PA reactivity, high trait PA may be associated with either enhanced or impaired sleep.
Our conclusions are limited by some features of our methods and analyses. First, our sample consisted of a cross-section of relatively healthy adults. Both the restricted age range (aged 43–68) and sample size further limit the generalizability of results. Although we attempted to examine the extent to which associations between sleep and components of PA (trait-level and daily reactivity) were independent of potential confounding variables (e.g., self-rated health, education, employment status, BMI, trait NA), future research should replicate these results with larger samples of both younger and older adults and explore whether these effects are robust beyond the contribution of variables (e.g., cognitive and social control) that likely covary with age. Second, our analyses of PA reactivity relied heavily on self-report measures that were completed at the end of each day. It is well established the PA varies within day and across days [56, 57]. Thus, future research should include ecological momentary assessment approaches [58] that allow for modeling of diurnal and circadian effects of PA. Third, as with any cross-sectional study design, the directionality of the observed associations cannot be determined. It is possible, for example, that greater PA reactivity may result from disturbances in sleep [6, 59]. Similarly, our conceptualization of PA reactivity assumed unidirectional effects (i.e., daily events influencing daily PA). However, it is possible that relationships between daily events and daily PA reflect bidirectional or third-variable effects. Thus, longitudinal data are required to disentangle the complex associations between PA reactivity, trait PA, and sleep.
Another limitation includes the possibility that affective responses are more closely linked to how daily events are appraised than the absolute number of events on a given day. For example, Peeters, Nicolson, Berkhof, Delespaul, and deVries [60] reported a curious effect whereby depressed individuals, relative to controls, showed a marked increase in NA in response to positive events, particularly those that were appraised as stressful. Thus, future research examining event appraisals as a moderator might help to account for individual differences in PA responses to daily events. Furthermore, although PA reactivity was assumed to have trait-like characteristics, evidence of test-retest stability would provide stronger evidence for the validity of PA reactivity as having a basis in stable individual differences. A more fundamental methodological issue relates to the assessment of PA reactivity itself. The current study used a multilevel modeling approach to estimate the degree of PA reactivity over the course of a week. Although the results provided converging evidence that greater PA reactivity was associated with poorer sleep, other research using day reconstruction methods has found that PA reactivity is present among individuals scoring high on indicators of optimal mental health [61]. Given the different approaches to assessing PA reactivity between studies, it would be difficult to make strong statements as to how much PA reactivity is considered detrimental to health [31]. Thus, identifying the operative mechanisms that link PA reactivity to maladaptive health outcomes remains an important task for future work. Additionally, the role of reactivity among low trait PA individuals was less definitive in the current data. These findings should, therefore, be replicated with additional measures of low trait PA (e.g., anhedonia) before firm conclusions are drawn. Finally, it remains to be seen whether the effects of PA reactivity prove more consistent with a model of differential vulnerability (i.e., diathesis stress), differential susceptibility (i.e., biological sensitivity), or vantage sensitivity (i.e., individual differences in response to positive or enriching experiences) [62–64]. Future studies building on these findings should, therefore, examine the effects of PA reactivity across a range of positive and negative environmental influences. These limitations notwithstanding, these results extend the study of PA and sleep and suggest that the costs sometimes associated with the pursuit of happiness may, in part, be attributed to the possession of high but fragile positive affect.
Acknowledgments
We extend thanks to Gary Evans, Anthony Burrow, and Thomas Fuller-Rowell for their helpful comments on previous versions of this article.
This research was supported by a grant from the National Institute on Aging (P01 AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation and a National Research Service Award from the National Institute of Mental Health (T32 MH18931). Support was also given to the second author through a Doctoral Foreign Study Award from the Canadian Institutes of Health Research. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
Appendix
Full Level-1 and Level-2 Models
| Level 1: | Sleepij = β0j + β1j*(Weekend)ij + β2j*(Exercise)ij + β3j*(Caffeine)ij + β4j*(Sleep Medication)ij + β5j*(Sleep Time)ij + rij |
| Level 2: | β0j = γ00 + γ01*(Gender) j + γ02*(Age) j + γ03*(Income) j + γ04*(Self-rated Health) j + γ05*(Mean Daily PA) j + γ06*(Mean Daily NA) j + γ07*(Standard Deviation in Daily PA) j + γ08*(Standard Deviation in Daily NA) j + γ09*(Trait NA) j + γ010*(Trait PA) j + γ011*(Reactivity to Negative Events) j + γ012*(Reactivity to Positive Events) j + γ013*(Net Reactivity) j + γ014*(Trait PA2) j + γ015*(Reactivity to Negative Events2) j + γ016*(Reactivity to Positive Events2) j + γ017*(Net Reactivity2) j + γ018*(Trait PA * Reactivity to Negative Events) j + γ019*(Trait PA * Reactivity to Positive Events) j + γ020*(Trait PA * Net Reactivity) j + u0j |
| β 1j = γ10 | |
| β 2j = γ20 | |
| β 3j = γ30 | |
| β 4j = γ40 | |
| β 5j = γ50 |
Footnotes
Random effects for the Level 2 intercept were significant in the full model for sleep efficiency [u0 = 65.39, χ2(74) = 1263.19, p < .001], morning rest [u0 = 0.26, χ2(74) = 260.97, p < .001], and overall quality [u0 = 0.24, χ2(74) = 231.18, p < .001], respectively.
To probe the effect of nonevents, we set the slopes of participants who reported no negative events or positive events over the 8 day study period to zero, and re-ran all analyses. The pattern of results remained the same. It is also possible that negative affect (NA) reactivity might be driving our results [13]. To explore this possibility, we reran all analyses, controlling for NA reactivity effects. NA reactivity was not predictive of sleep efficiency (γ021 = 0.48, p >.10), morning rest (γ021 = 0.31, p = .06), or overall sleep (γ021 = 0.14, p >.10), and including NA reactivity did not alter the pattern of trait PA and PA reactivity results. From this, we conclude that the main and interactive effects of trait PA and PA reactivity on sleep are not attributable to NA reactivity.
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
References
- 1.Reid KJ, Marinovick Z, Finkel S, et al. Sleep: A marker of physical and mental health in the elderly. American Journal of Geriatric Psychiatry. 2006;14:860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 2.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives of General Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 4.Steptoe A, O’Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and good sleep. Journal of Psychosomatic Research. 2008;64:409–415. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Bardwell WA, Berry CC, Ancoli-Israel S, Dimsdale JE. Psychological correlates of sleep apnea. Journal of Psychosomatic Research. 1999;47:583–596. doi: 10.1016/s0022-3999(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 6.Fosse R, Stickgold R, Hobson A. Emotional experience during rapid-eye-movement sleep in narcolepsy. Sleep. 2002;25:724–732. doi: 10.1093/sleep/25.7.724. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. Journal of Personality. 2009;77:1747–1775. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong A. Pathways linking positive emotion and health in later life. Current Directions in Psychological Science. 2010;19:358–362. [Google Scholar]
- 9.Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill S, Cohen L, Tolpin L, Gunthert K. Affective reactivity to daily interpersonal stressors as a prospective predictor of depressive symptoms. Journal of Social and Clinical Psychology. 2004;23:172–194. [Google Scholar]
- 11.Cohen JH, Gunthert KC, Butler AC, O’Neill SC, Tolpin LH. Daily reactivity as a prospective predictor of depressive symptoms. Journal of Personality. 2005;73:1687–1713. doi: 10.1111/j.0022-3506.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolger N, Zuckerman A. A framework for studying personality in the stress process. Journal of Personality and Social Psychology. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- 13.Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Annals of Behavioral Medicine. doi: 10.1007/s12160-012-9423-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles ST, Piazza JR, Mogle J, Sliwinski MJ, Almeida DM. The wear-and-tear of daily stressors on mental health. Psychological Science. doi: 10.1177/0956797612462222. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrish B, Cohen L, Laurenceau J-P. Prospective relationship between negative affective reactivity to daily stress and depressive symptoms. Journal of Social and Clinical Psychology. 2011;30:270–296. [Google Scholar]
- 16.Wichers M, Geschwind N, Jacobs N, et al. Transition from stress sensitivity to a depressive state: Longitudinal twin study. British Journal of Psychiatry. 2009;195:498–503. doi: 10.1192/bjp.bp.108.056853. [DOI] [PubMed] [Google Scholar]
- 17.Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. Journal of Personality. 2005;76:1511–1538. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finan PA, Zautra AJ, Davis MC. Daily affect relations in Fibromyalgia patients reveal positive affective disturbance. Psychosomatic Medicine. 2009;71:474–482. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 19.Mroczek DM, Stawski RS, Turiano NA, et al. Emotional reactivity predicts mortality: Longitudinal findings from the VA Normative Aging Study. Unpublished manuscript. 2012 doi: 10.1093/geronb/gbt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talbot L, Hairston I, Eidelman P, Gruber J, Harvey A. The effect of mood on sleep onset latency and REM sleep in interepisode bipolar disorder. Journal of Abnormal Psychology. 2009;118:448–458. doi: 10.1037/a0016605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews. 2010;14:227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Eid M, Diener E. Intraindividual variability in affect: Reliability, validity, and personality correlates. Journal of Personality and Social Psychology. 1999;76:662–676. [Google Scholar]
- 23.Eaton LG, Funder DC. Emotional experience in daily life: Valence, variability, and rate of change. Emotion. 2001;1:413–421. doi: 10.1037/1528-3542.1.4.413. [DOI] [PubMed] [Google Scholar]
- 24.Penner LA, Shiffman S, Paty JA, Fritzsche BA. Individual differences in intraperson variability in mood. Journal of Personality and Social Psychology. 1994;66:712–721. doi: 10.1037//0022-3514.66.4.712. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz DS, Zuroff DC. Flux, pulse, and spin: Dynamic additions to the personality lexicon. Journal of Personality and Social Psychology. 2004;86:880–893. doi: 10.1037/0022-3514.86.6.880. [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz DS, Zuroff DC. Robust predictors of flux, pulse and spin. Journal of Research in Personality. 2005;39:130–147. [Google Scholar]
- 27.Gruber J, Kogan A, Quoidbach J, Mauss IB. Happiness is best kept stable: Positive emotion variability is associated with poorer psychological health. Emotion. doi: 10.1037/a0030262. in press. [DOI] [PubMed] [Google Scholar]
- 28.Ram N, Gerstorf D. Time-structured and net intraindividual variability: Tools for examining the development of dynamic characteristics and processes. Psychology and Aging. 2009;24:778–791. doi: 10.1037/a0017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalino LI, Fredrickson BL. Tuesdays in the lives of flourishers: The role of positive emotional reactivity in optimal mental health. Emotion. 2011;11:938–950. doi: 10.1037/a0024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oishi S, Diener E, Choi DW, Kim-Prieto C, Choi I. The dynamics of daily events and well-being across cultures: When less is more. Journal of Personality and Social Psychology. 2007;93:685–698. doi: 10.1037/0022-3514.93.4.685. [DOI] [PubMed] [Google Scholar]
- 31.Gruber J, Mauss I, Tamir M. A dark side of happiness? How, when and why happiness is not always good. Perspectives on Psychological Science. 2011;6:222–233. doi: 10.1177/1745691611406927. [DOI] [PubMed] [Google Scholar]
- 32.Grant AM, Schwartz B. Too much of a good thing: The challenge and opportunity of the inverted U. Perspectives on Psychological Science. 2011;6:61–76. doi: 10.1177/1745691610393523. [DOI] [PubMed] [Google Scholar]
- 33.Diener E, Colvin C, Pavot WG, Allman A. The psychic costs of intense positive affect. Journal of Personality and Social Psychology. 1991;61:492–503. [PubMed] [Google Scholar]
- 34.Martin LR, Friedman HS, Tucker JS, et al. A life course perspective on childhood cheerfulness and its relation to mortality risk. Personality and Social Psychology Bulletin. 2002;28:1155–1165. [Google Scholar]
- 35.Friedman HS, Tucker JS, Tomlinson-Keasey C, et al. Does childhood personality predict longevity? Journal of Personality and Social Psychology. 1993;65:176–185. doi: 10.1037//0022-3514.65.1.176. [DOI] [PubMed] [Google Scholar]
- 36.Kernis MH, Waschull SB. The interactive roles of stability and level of self-esteem: Research and theory. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 27. San Diego: Academic Press; 1995. pp. 93–141. [Google Scholar]
- 37.Kernis MH, Grannemann BD, Barclay LC. Stability and level of self-esteem as predictors of anger arousal and hostility. Journal of Personality and Social Psychology. 1989;56:1013–1023. doi: 10.1037//0022-3514.56.6.1013. [DOI] [PubMed] [Google Scholar]
- 38.Kernis MH, Greenier KD, Herlocker CE, Whisenhunt CR, Abend TA. Self-perceptions of reactions to doing well or poorly: The roles of stability and level of self-esteem. Personality and Individual Differences. 1997;22:845–854. [Google Scholar]
- 39.Paradise AW, Kernis MH. Self-esteem and psychological well-being: Implications of fragile self-esteem. Journal of Social and Clinical Psychology. 2002;21:345–361. [Google Scholar]
- 40.Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradburn NM. The structure of psychological well-being. Chicago: Aldine; 1969. [Google Scholar]
- 43.Fazio A. A concurrent validational study of the NCHS General Well-Being Schedule. 73. Washington, DC: Government Printing Office; 1977. [PubMed] [Google Scholar]
- 44.Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- 45.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks: Sage; 2002. [Google Scholar]
- 46.Wenze SJ, Gunthert KC, Forand NR. Cognitive reactivity in everday life as a prospective predictor of depressive symptoms. Cogntive Thereapy Research. 2010;34:554–562. [Google Scholar]
- 47.Lubinski D, Humphreys LG. Assessing spurious “moderator effects”: Illustrated substantively with the hypothesized (“synergistic”) relation between spatial and mathematical ability. Psychological Bulletin. 1990;107:385–393. doi: 10.1037/0033-2909.107.3.385. [DOI] [PubMed] [Google Scholar]
- 48.Kernis MH, Cornell DP, Sun CR, Berry AJ, Harlow T. There’s more to self-esteem than whether it is high or low: The importance of stability of self-esteem. Journal of Personality and Social Psychology. 1993;65:1190–1204. doi: 10.1037//0022-3514.65.6.1190. [DOI] [PubMed] [Google Scholar]
- 49.Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- 50.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- 51.Steptoe A, O’Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and good sleep. Journal of Psychosomatic Research. 2008;64:409–415. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Butler AC, Hokanson JE, Flynn HA. A comparison of self-esteem lability and low trait self-esteem as vulnerability factors for depression. Journal of Personality and Social Psychology. 1994;66:166–177. doi: 10.1037//0022-3514.66.1.166. [DOI] [PubMed] [Google Scholar]
- 53.Crocker J, Wolfe CT. Contingencies of self-worth. Psychological Review. 2001;108:593–623. doi: 10.1037/0033-295x.108.3.593. [DOI] [PubMed] [Google Scholar]
- 54.Fredrickson BL, Joiner T. Positive emotions trigger upward spirals toward emotional well-being. Psychological Science. 2002;13:172–175. doi: 10.1111/1467-9280.00431. [DOI] [PubMed] [Google Scholar]
- 55.Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. American Psychologist. 1989;44:513–524. doi: 10.1037//0003-066x.44.3.513. [DOI] [PubMed] [Google Scholar]
- 56.Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- 57.Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- 58.Steptoe A, Wardle J. Positive affect measured using ecological momentary assessment and survival in older men and women. Proceedings of the National Academy of Sciences. 2011;108:18244–18248. doi: 10.1073/pnas.1110892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton NA, Affeck G, Tennen H, et al. Fibromyalgia: The role of sleep in affect and in negative event reactivity and recovery. Health Psychology. 2008;27:490–494. doi: 10.1037/0278-6133.27.4.490. [DOI] [PubMed] [Google Scholar]
- 60.Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology. 2003;112:203–211. doi: 10.1037/0021-843x.112.2.203. [DOI] [PubMed] [Google Scholar]
- 61.Catalino LI, Fredrickson BL. A Tuesday in the life of a flourisher: The role of positive emotional reactivity in optimal mental health. Emotion. 2011;11:938–950. doi: 10.1037/a0024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellis BJ, Boyce WT. Differential susceptibility to the environment: Toward an understanding of sensitivity to developmental experiences and context. Development and Psychopathology. 2011;23:1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- 63.Belsky J, Pluess M. Beyond diathesis-stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 64.Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin. doi: 10.1037/a0030196. in press. [DOI] [PubMed] [Google Scholar]