Abstract
Background and Objective
Gingival keratinocytes are used in model systems to investigate the interaction between periodontal bacteria and the epithelium in the initial stages of the periodontal disease process. Primary gingival epithelial cells (GECs) have a finite lifespan in culture before they enter senescence and cease to replicate, while epithelial cells immortalized with viral proteins can exhibit chromosomal rearrangements. The aim of this study was to generate a telomerase-immortalized human gingival epithelial cell line and compare its in vitro behavior to that of human GECs.
Material and Methods
Human primary gingival epithelial cells were immortalized with a bmi1/hTERT combination to prevent cell cycle triggers of senescence and telomere shortening. The resultant cell-line, Telomerase Immortalized Gingival Keratinocytes (TIGKs), were compared to GECs for cell morphology, karyotype, growth and cytokeratin expression, and further characterized for replicative lifespan, expression of toll-like receptors (TLRs) and invasion by P. gingivalis.
Results
TIGKs showed morphologies, karyotype, proliferation rates and expression of characteristic cytokeratin proteins comparable to GECs. TIGKs underwent 36 passages without signs of senescence and expressed transcripts for TLRs 1-6, 8 and 9. A subpopulation of cells underwent stratification after extended time in culture. The cytokeratin profiles of TIGK monolayers were consistent with basal cells. When allowed to stratify, cytokeratin profiles of TIGKs were consistent with suprabasal cells of the junctional epithelium. Further, TIGKs were comparable to GECs in previously reported levels and kinetics of invasion by wild type Porphyromonas gingivalis and an invasion defective ΔserB mutant.
Conclusion
Results confirm bmi1/hTERT-immortalization of primary gingival epithelial cells generated a robust cell-line with similar characteristics to the parental cell type. TIGKs represent a valuable model system for the study of oral bacteria interactions with host gingival cells.
Keywords: Telomerase, Immortalization, hTERT, bmi1, Gingival Epithelial Cells, Porphyromonas gingivalis
Introduction
Gingival epithelial cells are an important tool to study interactions between periodontal pathogens such as Porphyromonas gingivalis and the host, and the consequences of this interaction for the progression of periodontal disease (1,2,3). Primary epithelial cells have a finite lifespan in culture of around 9 passages, after which they cease to replicate and enter a state of senescence (4,5). Furthermore, although cells from different donors exhibit consistent phenotypic properties (6,7,8), there can be donor-to-donor variability in transcriptomic or proteomic studies (9,10). Oral epithelial cell lines have previously been immortalized using viral oncoproteins, commonly through expression of human papilloma virus (HPV) E6 and E7 oncoproteins (5,11) or SV-40 large T antigen (12). However, such cells can display chromosomal abnormalities (13), in addition to altered expression of cell-cycle regulatory proteins (14).
The limited lifespan of somatic cells in culture can be triggered by progressive telomere shortening through a p53-/p21cip1-dependent senescence mechanism (15,16,17). The enzyme, telomerase, contains a catalytic subunit, hTERT, expressed solely in germ cells as well as immortal cancer cells (18,19), and introduction of hTERT into fibroblasts and some epithelial cells confers telomere maintenance and unlimited replicative potential (20). However, the expression of hTERT alone does not convey immortality in all cell types, including oral keratinocytes and mammary epithelial cells (21,22). The replication potential of these cell types during serial passaging is generally limited by a telomere independent mechanism mediated by the attenuated repression of CDKN2A (cyclin-dependent kinase inhibitor 2A), which encodes p16INK4A and p14ARF, (23). The function of p16INK4 is to attenuate Cdk4/6 phosphorylation of retinoblastoma protein and thus promote cell cycle arrest, whereas p14ARF functions in the p53 pathway to inhibit MDM2-mediated degradation of p53 and promote p53-mediated transcriptional activation (e.g., p53-/p21cip1-dependent senescence) (15). Transduction of cells with bmi1 (BMI1 polycomb ring finger oncogene), a natural repressor of CDKN2A, facilitates immortalization upon subsequent expression of hTERT (23,24). Keratinocytes immortalized by bmi1 + hTERT display unaffected cell morphology, growth control and differentiation potential (21), consistent with p16INK4a having no apparent role in epithelial homeostasis or development and with its up-regulation in rodent and human tissues with age and with cell senescence (15, 21).
We have generated an oral epithelial cell line immortalized upon introduction of hTERT into bmi1-transduced primary gingival epithelial cells by sequential transfection. The resultant cells were compared to GECs for cell morphology, growth and cytokeratin expression, and further characterized for replicative lifespan and the expression of toll-like receptors (TLRs). In addition, confirmation that bmi1/hTERT-immortalized cells could be used to model P. gingivalis interactions with gingival epithelial cells was achieved by assessing the internalization levels and kinetics of P. gingivalis strains with established invasive properties.
Material and methods
Isolation and routine culture of primary gingival epithelial cells
Gingival epithelial cells (GECs) were isolated and grown as previously described (4). Briefly, gingival tissue was obtained from a healthy human adult undergoing third molar extraction. Following washing in calcium- and magnesium-free phosphate-buffered saline (PBS) containing streptomycin-penicillin and Fungizone, 0.3 cm2 pieces of tissue were incubated overnight in 0.4% dispase at 4°C. Epithelial strips were then separated by mechanical separation and trypsinized for 10-15 min at 37°C in 0.05% trypsin/0.53 mM EDTA. After vigorous pipetting, the cell suspension was centrifuged for 5 min at 700 rpm and the cell pellet subsequently collected and resuspended in keratinocyte growth medium (Dermalife Basal Medium, Lifeline, Walkersville, MD). KGM is a serum-free medium supplemented with 10 ng/ml epidermal growth factor (EGF), 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 0.15 mM calcium and gentamicin/amphotericin B. Cells were cultured at 37°C, 5% CO2, with humidity, and utilized for assays between passages 4-6.
Immortalization of GECs with hTERT and culture of TIGK cells
GECs were immortalized with bmi1-transduction followed by human telomerase reverse transcriptase (hTERT) (21,23,24), by the Rheinwald laboratory at the Harvard Skin Disease Research Center. Briefly, GECs were cultured in keratinocyte serum-free medium (K-SFM, Invitrogen, Carlsbad, CA) supplemented with 0.4 mM calcium chloride, 25 μg/ml bovine pituitary extract (BPE) and 0.2 ng/ml epidermal growth factor (EGF). Cells were transduced with amphotropic BABE-bmi1.puro retroviral supernatants with 2 μg/ml polybrene (Sigma-Aldrich) for 6 hours. The following day, cells were subcultured in K-SFM and subjected to drug selection (0.5 μg/ml puromycin) for 7 days. GECs previously bmi1-transduced were then transduced in a similar manner with amphotropic Wzl-TERT.Bsd retroviral supernatants with polybrene followed by subculture and drug-selection (5 μg/ml blasticidin) in K-SFM. The bmi1/hTERT-immortalized gingival keratinocytes (TIGKs) were routinely cultured at 37°C, 5% CO2 in K-SFM. Cells were subsequently used for assays from passage 9-36. Karyotyping was performed by Applied Genetics Laboratories, Melbourne, FL.
P. gingivalis culture
Wild type P. gingivalis ATCC 33277 and isogenic ΔserB (2) were used in this study. Bacteria were cultured in trypticase soy broth supplemented with yeast extract (1 mg ml−1), hemin (5 μg ml−1), and menadione (1 μg ml−1), anaerobically, at 37°C. Overnight cultures were diluted 1:100 and grown to exponential phase (OD600 nm 0.7).
TIGK seeding/proliferation morphology
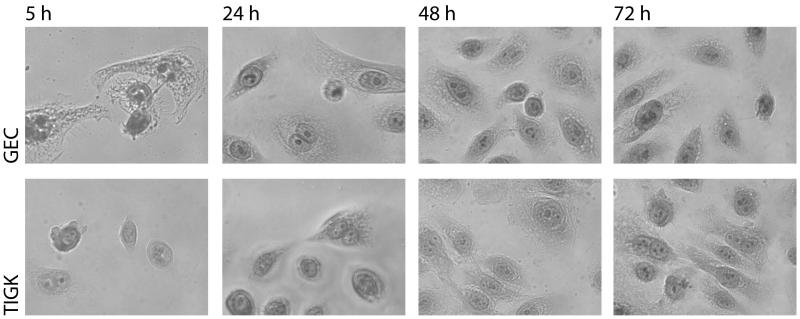
To compare the morphology of GECs with TIGKs from seeding through subsequent growth, glass coverslips (18 mm diameter in 12-well plates) were seeded with 2.0 × 104 cells and incubated under standard culture conditions. After 5 h, 24 h, 48 h and 72 h incubation, cells were fixed in 95 % methanol and stained with haematoxylin and eosin (H&E). Following a wash in dH2O, coverslips were mounted using Faramount aqueous mountant. Slides were left to dry for a minimum of 24 h prior to being photographed using a Nikon T120 inverted microscope.
Growth kinetics and replicative lifespan determination
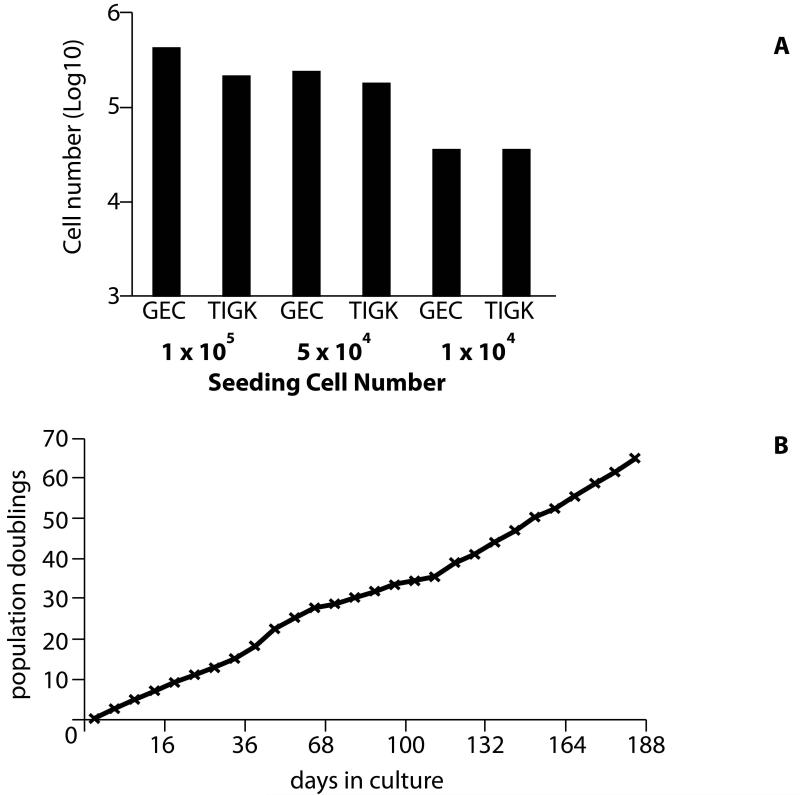
T75 culture flasks were seeded with TIGKs at varying densities: high density = 1.0 × 105 cells, medium density = 5.0 × 104 cells and low density = 1.0 × 104 cells; and incubated for 72 h. Cells were harvested by trypsinization and counted using a haemocytometer. For replicative lifespan determination, cells were seeded at 1 × 103 cells/cm2 in K-SFM and passaged every 4 days (passages 9-15) or 8 days (passages 16-36). The medium was changed every two days. Population doubling was calculated as log2 (cell number at subculture/cell number plated). Under these conditions, cells obtained less than 50% confluency.
Cytokeratin expression
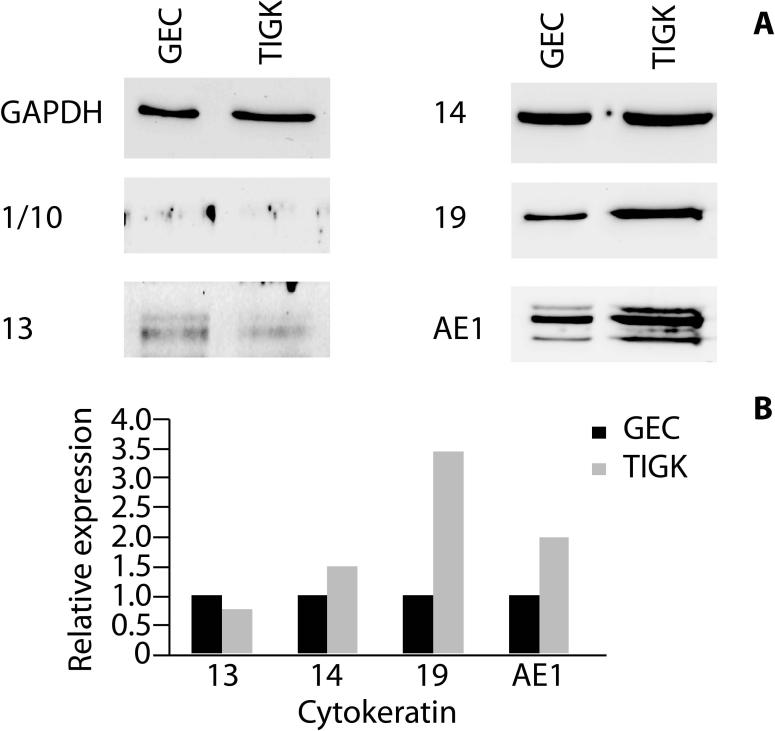
GECs and TIGKs were seeded at 5.0 × 104 cells per T75 culture flask and cultured to 60-70% confluence. The monolayer cultures were lysed in SDS-PAGE sample buffer, separated by SDS-PAGE, and transferred onto nitrocellulose membranes by electroblotting. Blots were blocked with 10% skimmed dry milk in Tris-buffered saline (TBS) overnight at 4°C. Primary antibodies were mouse monoclonal anti-cytokeratins 1/10 (clone LH1), 13 (clone Ks 13.1), 14 (clone LL002), 19 (clone BA 17) and AE1 (all from EMD Millipore, Billerica, MA), diluted 1:1000 and incubated for 2 h at room temperature. Antigen-antibody binding was detected using horseradish peroxidase-conjugated species-specific secondary antibodies followed by ECL Western Blotting detection reagents (Thermo Scientific, Waltham, MA). Blots were stripped and probed with GAPDH antibodies as a loading control.
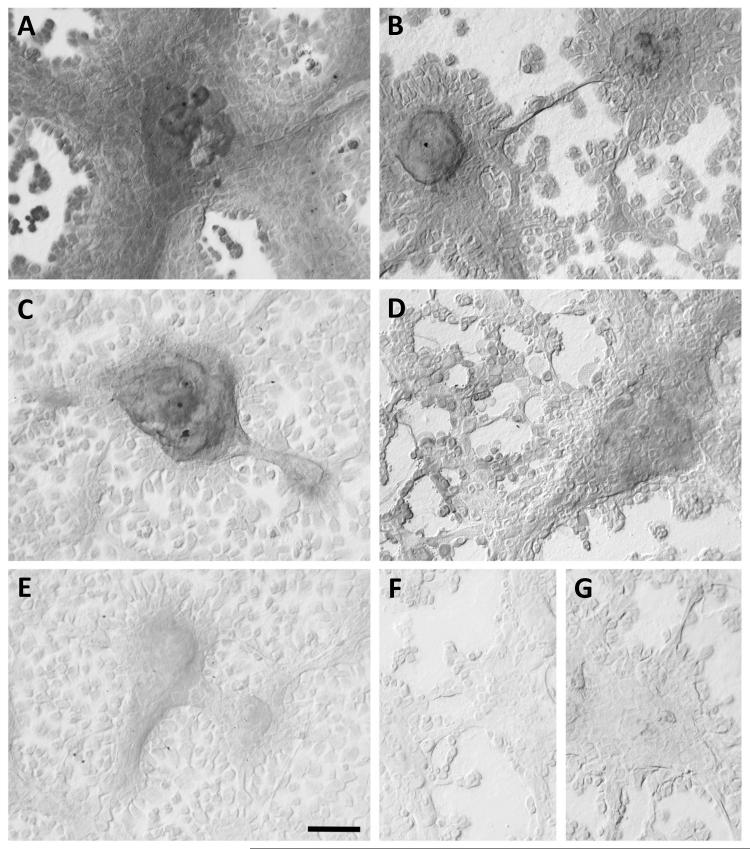
Immunohistochemical detection of cytokeratins was performed with TIGKs cultured under conditions to promote stratification of cell colonies. Cells were seeded into two-well Lab-Tek Permanox plastic chamber slides (Thermo Scientific) at 104 cells/cm2 in K-SFM medium and cultured for 19 days, then fixed with acetone:methanol (3:2). Immunohistochemistry was performed as described previously, with minor modifications (25). Cells were rehydrated in graded ethanol solutions and permeabilized in 0.4% Tween 20 in PBS. After inhibition of endogenous peroxidase activity (100% methanol containing 0.3% H2O2) and incubation with 10% normal goat serum (NGS, Vector Laboratories, Burlingame, CA) in PBS, cells were incubated overnight (4° C) with primary antibody or negative control IgG at the same concentration (diluted in 10% NGS in PBS). Primary antibodies were mouse monoclonal anti-cytokeratin 10 (clone DE-K10), 13 (clone 2D7), 19 (clone Ks19.1), 16 (clone LL025) and rabbit polyclonal IgG anti-cytokeratin 14 (all from Thermo Scientific). Non-immune negative controls (mouse IgG and rabbit IgG) were from (Santa Cruz Biotechnology, Santa Cruz, CA). Mouse and rabbit IgGs were used at 2 μg/ml and 1 μg/ml, respectively. Cells were washed (PBS with 0.05% Tween 20) and incubated 2 h with either biotinylated goat anti-mouse or biotinylated goat anti-rabbit (both from Vector Laboratories) at 2 μg/ml in PBS. Immunodetection was performed using the avidin-biotin-peroxidase complex method with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as peroxidase substrate and Ni enhancement according to manufacturer’s instructions (Vectastain Elite kit, Vector Laboratories). Following antibody detection, cells were washed, dehydrated and mounted in VECTASHIELD® HardSet™ Mounting Media (Vector Laboratories). Images were captured with a Micropublisher 5.0 RTV digital camera (Q Imaging, Pleasanton, CA) using QCapture Pro software (v5.01, Q Imaging) on a Leica DM LB2 microscope (Leica Microsystems, Bannockburn, IL) under DIC (differential interference contrast) optics.
Expression of toll-like receptor transcripts
RT-PCR was as described previously with slight modifications (26). Briefly, cell cultures were homogenized directly in TRIzol® Reagent (Invitrogen), total RNA isolated using standard protocols and then treated with DNase I using Ambion’s DNA-free™ Reagent Kit (Applied Biosystems, Foster City, CA). DNase I treated RNA was reverse transcribed with random primers (High Capacity cDNA Archive kit, Applied Biosystems), resultant cDNA purified (QIAquick® PCR purification system, Qiagen, Valencia, CA) and quantified (Quant-iT™ dsDNA HS assay kit, Invitrogen). PCR reactions (25 μl) contained as template 10 ng cDNA, equivalent DNase I-treated RNA (negative controls), or 200 ng human genomic DNA (Promega, Madison, WI) as positive controls. We used Accuprime™ High fidelity Taq DNA polymerase (Life Technologies, Carlsbad, CA) with cycling conditions of: 3 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 52 °C and 30 s at 68 °C followed by 5 min at 68 °C. Products were resolved in 3.0 % agarose gels made in TAE buffer. Digital images of gels were obtained with a Scion Grayscale 1394 Digital Camera (Fotodyne, Hartland, WI). PCR products were confirmed by direct sequencing of gel-purified bands (QIAquick Gel Extraction Kit; Qiagen). Sequencing was performed at the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR) using Applied Biosystems 3100 Genetic Analyzer ABI and Big Dye version 3.1 chemistry. Primer pairs are shown in Table 1 and are within the terminal exon of each TLR gene. The terminal exons encode a majority of each potential mRNA sequence and are included in all currently known splice variants. Each template cDNA was run in duplicate reactions.
Table 1.
Primers for human toll-like receptors 1 to 10.
| TLR | Forward Primer Reverse Primer |
Product (bp) |
Exon |
|---|---|---|---|
| 1 | 5′-GTCTTGCTGGTCTTAGGAGAGACTTATG-3′ 5′-GGGGAACACAATGTGCAGACTC-3′ |
93 | 3 |
| 2 | 5′-CCAAAAG GAAG C C CAG GAAAG-3′ 5′-GACCATAAGGTTCTCCACCCAGTAG-3′ |
101 | 4 |
| 3 | 5′-GGTACATCATGCAGTTCAACAAGC-3′ 5′-CATTCCTCIIICGCAAACAGAGTG-3′ |
118 | 5 |
| 4 | 5′-GGATGAGGACTGGGTAAGGAATG-3′ 5′-ATGGATGATGTTGGCAGCAATG-3′ |
124 | 4 |
| 5 | 5′-GGTGCTGGAGATACTAGAATGTTTCTG-3′ 5′-GGTGGGCAAGAATCAAAGAGAAG-3′ |
110 | 6 |
| 6 | 5′-GGGAGGAGATCCAACAGAACTTTG-3′ 5′-GGGATTATAGATGTGAGCCAACGTG-3′ |
127 | 2 |
| 7 | 5′-CCATACGGAGGTGACTATTCCTTACC-3′ 5′-CAGATCCAGGGAGATCACACIIIG-3′ |
94 | 3 |
| 8 | 5′-CAAAGGTCTCAAGAATCTGACACG-3′ 5′-CGAGGAAACTGCTGGAGTAATG-3′ |
162 | 2 |
| 9 | 5′-CTGGAAGAGCTAAACCTGAGCTAC-3′ 5′-AACAGTTGCCGTCCATGAATAG-3′ |
163 | 2 |
| 10 | 5′-CTTTGCCCACCACAATCTCTTC-3′ 5′-CCCACAIIIACGCCTATCCTTG-3′ |
163 | 4 |
Bacterial invasion
TIGKs were seeded at 1.0 × 105 cells on 18 mm diameter glass coverslips in 12-well plates and grown until ≈40% confluent. Cells were infected with P. gingivalis 33277 or ΔserB at MOI 100 for 5 or 60 min. Coverslips were washed 4 times in phosphate-buffered saline (PBS) and fixed for 10 min in 4% paraformaldehyde. Permeabilization was with 0.2% Triton X-100 for 10 min at room temperature, prior to blocking in 10% goat serum for 20 min. Bacteria were detected by reacting with primary rabbit anti-P. gingivalis antibodies at 1:1000 dilution for 1 h, followed by Alexa-568-conjugated anti-rabbit secondary antibody (1:200) for 1 h in the dark. Following a 20 min block in 0.1% goat serum, actin was labelled using 1:100 FITC-phalloidin (Sigma) for 40 min at room temperature. After 4 washes in PBS, coverslips were mounted using ProLong Gold with DAPI mounting medium (Invitrogen).
Images were acquired on an Olympus FV1000 inverted fluorescent microscope, with a spectral confocal scanner and Fluoview software, using a 60x oil immersion objective. Briefly, z-stacks were obtained (10 layers/stack, 2 μm between layers) through the z-axis of cells (3 z-stacks/coverslip). Internal P. gingivalis were enumerated by counting intracellular bacteria for each z-stack, thus allowing bacteria to be visualized throughout cytoplasmic layers and ensuring that the bacteria counted were within the host cell cytoplasm.
Results
Cell morphology and karyotype
The post-seeding morphology of GECs was compared with TIGKs. Following seeding at 2.0 × 104 cells/coverslip, the cells underwent similar patterns of growth and morphological changes (Fig. 1). The GECs were slightly faster to attach and begin cytoplasmic spreading, apparent at 5 h post-seeding. At this time-point, TIGKs were attached but still had a rounded morphology, with little evidence of cytoplasmic spreading. At all other time points the GECs and TIGKs showed very similar morphology. Cell clusters were visible 24 h post-seeding with active cell division evident. Cells then continued to divide and spread until an ≈80% confluent monolayer was achieved after 72 h incubation with both cell types. The normal karyotype of the TIGKs is shown in Fig. S1, no chromosomal aberrations were found in 100 cells analyzed at passage 30.
Figure 1. Morphology of TIGKs.
Glass coverslips were seeded with 2.0 × 104 TIGKs and cultured for the indicated times prior to fixation and subsequent staining with haematoxylin and eosin (H & E). Magnification ×100. Data are representative of three independent experiments.
Growth kinetics replicative lifespan
Cells were seeded at varying densities and harvested after 72 h to ascertain the affect of cell seeding density on subsequent proliferation (Fig. 2A). GECs and TIGKs showed similar rates of growth, but there were small differences in the sensitivity of each cell type to initial seeding density. At the highest seeding density, GECs proliferated slightly more efficiently compared with the TIGKs, producing around 3.5 × 105 cells in a 72 h period, compared to ≈1.25 × 105 cells produced by TIGKs. At medium seeding density the difference between the two cell types was less, with TIGKs producing only 5.0 × 104 cells fewer than the primary GECs. At the low seeding density, proliferation in both cell types was reduced, though the final number of cells produced in 72 h was virtually identical between the two cell types (2.75 × 104 cells). As shown in Fig. 2B, TIGKs failed to demonstrate senescence over a 188 day period, from passage 9 thru passage 36. The doubling rate was mostly consistent although there was an apparent slight increase after passage 25.
Figure 2. Growth kinetics and replicative lifespan.
A) Primary GECs and TIGKs were seeded at the indicated cell numbers in T75 flasks and recovered cell numbers assessed after 72 h by hematocytometer counts. Data are representative of three independent experiments. B) Cumulative population doublings of TIGKs plotted against total time during continuous culture and passage (passage 9 through 36) in K-SFM medium.
Cytokeratin expression
Western blot analyses were undertaken to compare the expression of cytokeratins between primary GECs and TIGKs in monolayer cultures (Fig. 3). Cytokeratins 13, 14 and 19 were expressed in both cell types. Cytokeratin 13 was expressed at slightly higher levels in the primary GECs, compared with TIGKs. Similar expression levels of cytokeratin 14 were apparent in both cell types. TIGKs expressed higher levels of cytokeratin 19 compared with GECs. Neither of the two cell types expressed cytokeratin 1/10. GECs and TIGKs expressed similar AE1-reactive cytokeratin proteins, although higher expression levels of these proteins were apparent in TIGKs.
Figure 3. Immunoblots of cytokeratin expression by primary GECs and TIGKs.
A) Whole cell lysates of GECs and TIGKs were examined by Western blotting with antibodies to cytokeratins as indicated. GAPDH was used as a loading control. Data are representative of three independent experiments. B) Scanning densitometry of Western blots showing relative expression levels of cytokeratins in TIGK cells compared to GECs, normalized to GAPDH.
With extended culture of TIGKs in K-SFM medium the cells seldom reach 100% confluence, due likely to the higher level of calcium (0.4 mM) in K-FSM compared to less than 0.1 mM in culture medium designed for clonal cell growth. Instead, cells within the central region of colonies start to stratify with cell layers 2-3 cells thick, suggesting they may be transitioning to a program of gene expression characteristic of suprabasal cells. Under these conditions, both the stratified cells and surrounding non-stratified cells expressed both cytokeratin 14 and 19 (Fig. 4A and 4B). Stratified cells expressed cytokeratin 13 (Fig. 4C), however cytokeratin 10 was barely detected (Fig. 4E). Cytokeratin 16 was also expressed by stratified cells as well as by some adjacent non-stratified cells (Fig. 4D).
Figure 4. Immunohistochemical detection of cytokeratins in TIGKs.
TIGKs were cultured in K-SFM medium for 19 days to allow cells within the central regions of colonies to form stratified layers 2-3 cells thick. Cells were probed with rabbit polyclonal IgG to cytokeratin 14 (A) and with mouse monoclonal antibodies to cytokeratin 19 (B), cytokeratin 13 (C), cytokeratin 16 (D) and cytokeratin 10 (E). Non-immune rabbit (F) and mouse (G) IgGs served as negative controls. Immunodetection was performed using the avidin-biotin-peroxidase complex. Cells were imaged under differential interference contrast optics without counterstaining. Bar in lower right of panel E = 100 μm for all panels.
Expression of transcripts for toll-like receptors
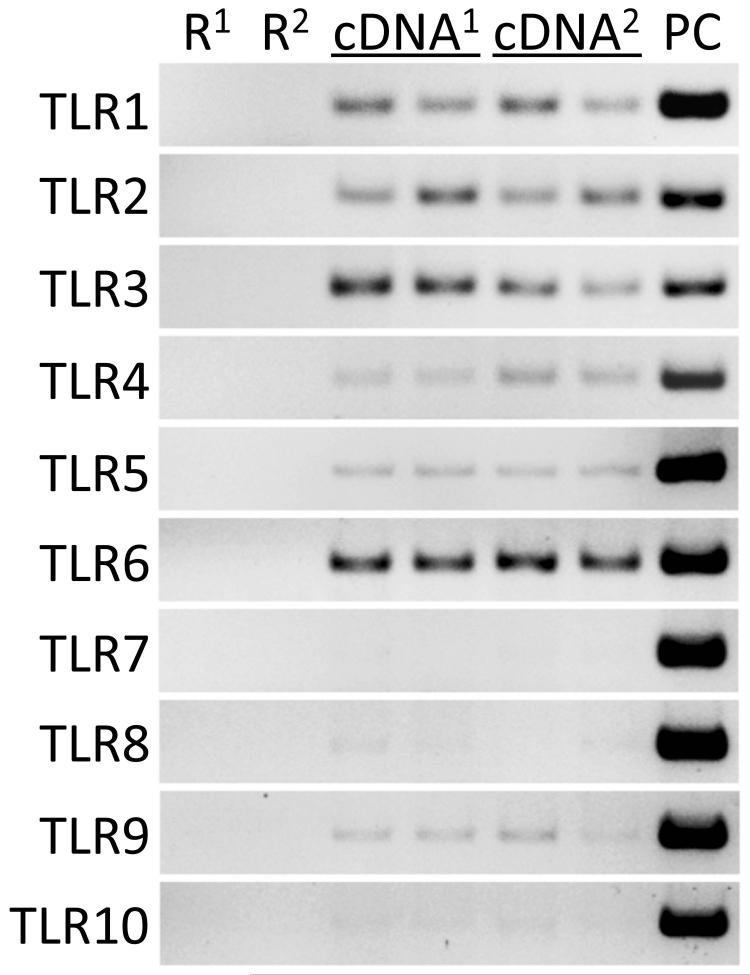
The gingival epithelium undergoes continuous exposure to microorganisms associated with the plaque biofilm. Surveillance of the plaque microbiota by toll-like receptors functions in epithelial innate immune responses to help maintain periodontal health. Epithelial cells in healthy gingival tissues were shown by immunohistochemistry to express TLRs 1-9, with expression observed consistently in the basal and lower spinous cell layers (27). As shown in Fig. 5, transcripts for TLRs 1 through 6 were readily detected in TIGKs by RT-PCR. TLR8 and TLR9 mRNA was barely detectable, whereas expression of TLR7 was absent.
Figure 5. Expression of transcripts for toll-like receptors (TLR) in TIGKs.
RT-PCR of TIGK mRNA from two separate experiments, superscript 1 and 2, using primers for the TLRs indicated. Genomic DNA templates serve as positive controls (PC), and negative controls are DNase I-treated total RNA (R). Each template cDNA was run in duplicate reactions for each experiment.
Internalization of P. gingivalis within TIGKs
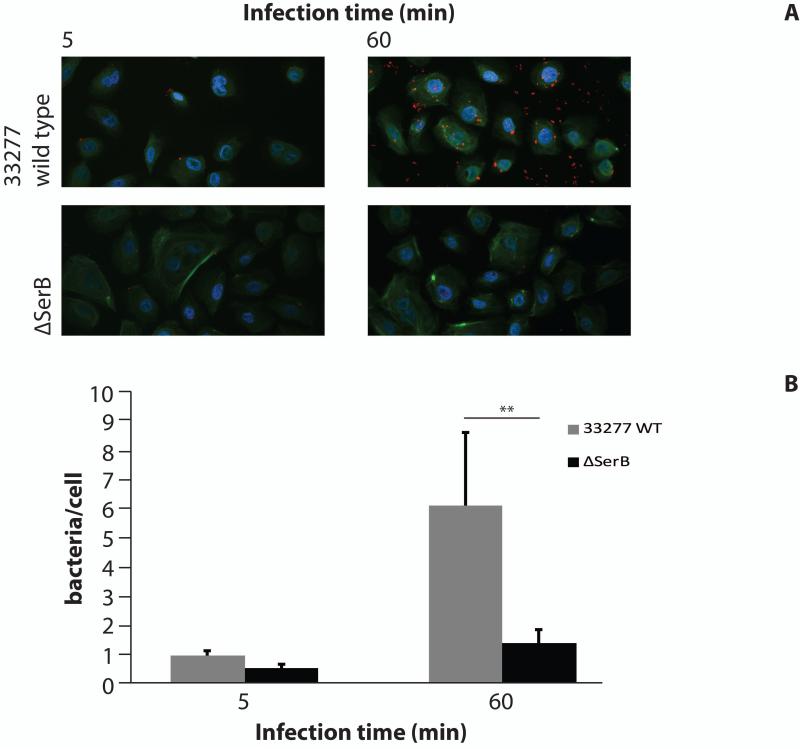
Internalization of P. gingivalis 33277 and ΔserB was compared using CSLM (Fig. 6). Following 5 min infection both P. gingivalis wild type and ΔserB showed only low levels of internalization. By 60 min infection, however, there was a clear difference in numbers of intracellular P. gingivalis. TIGKs supported invasion of the parental strain to the level of >6 bacteria/cell. Wild-type P. gingivalis invasion of TIGKs thus showed a similar rate and level of invasion as has been previously demonstrated in primary GECs (1), with low invasion levels at 5 min and increasing by 60 min. The ΔserB mutant was severely abrogated in invasion of TIGKs, with levels of intracellular bacteria not significantly increasing from 5 min to 60 min of infection, similar to the characteristics of invasion into GECs (2).
Figure 6. Internalization of P. gingivalis in TIGKs.
TIGKs were infected with P. gingivalis 33277 or ΔSerB at MOI 100 for 5 or 60 min. A) CSLM images. Bacteria are shown as red, actin green and DAPI is blue. Magnification ×63. Results are representative of three independent assays. Data shown are maximum projections of z-stacks (10 slices/z stack, 3 coverslips/group). B) Invasion levels of P. gingivalis. Numbers of intracellular bacteria were counted throughout z-stacks (10 slices/stack; 3 coverslips/group). Results are representative of three independent assays. Data are mean, with error bars indicating standard deviation. ** indicates p < 0.01.
Discussion
Primary cells have long been utilized in the elucidation of the mechanisms involved in P. gingivalis interactions with host epithelial cells (1,28,29,30). However, these cells have a finite life-span of only 8-9 passages before the cell population begins to senesce. Hence immortalized cell lines, utilizing viral oncoproteins to affect host cell-cycle regulation, are also a valuable tool, and HPV E6/E7-immortalized oral keratinocytes have been utilized in the study of P. gingivalis interactions with the gingival epithelia (2,31,32,33). The disadvantage with HPV-immortalized cells is there are chromosomal abnormalities and alterations in cell-cycle control compared with primary cells (13,14). Based upon previous work with epithelial cells from other organ systems (23), we hypothesized bmi1/hTERT-immortalization of gingival epithelial cells would provide a robust, proliferative cell line allowing reproducibility of experiments in non-senescent cells, and enabling study of the interactions between periodontal pathogens and cells of the gingiva in vitro. The post-seeding morphology of TIGKs was similar to that of GECs. At 72 h the cells had a characteristic ‘cobblestone’ appearance previously described for oral epithelial cells (4). TIGKs were slightly slower in their proliferative rate than GECs, but the doubling rate increased slightly after 36 population doublings. This phenomenon is also seen with HPV-immortalized GECs (5), and stem-cell derived epithelial cells (23).
The gingival as well as palatal tissues of the oral cavity are stratified keratinized (cornified) epithelia. As daughter cells of the dividing basal cell layer transition through the different suprabasal cell strata they undergo different patterns of cell architecture and cytokeratin expression (34). There are more than 20 distinct cytokeratins, representing the predominant cytoskeletal proteins in all epithelia and which are expressed in pairs consisting of a type I and type II family proteins (34,35). Within gingiva, the pattern of cytokeratin expression differs between the keratinized region, the non-keratinized junctional epithelium and the intervening non-keratinized gingival margin and sulcular epithelium. Cytokeratin 14 is localized to basal cells in all regions of gingiva (36), but has also been reported in suprabasal cells in all regions, with strong immunohistochemical staining of all cells of the junctional epithelium (37). Although not expressed in keratinized gingiva, cytokeratin 19 is localized to basal cells of the non-keratinized regions and to many suprabasal cells of the junctional epithelium, especially those nearer the tooth surface (36,37). Suprabasal cells throughout the gingiva express cytokeratin 16, whereas the cytokeratin pairs 1/10 and 4/13 are each mainly restricted to suprabasal cells of the keratinized and non-keratinized regions, respectively (36,37,38).
Monolayers of GECs and TIGKs grown under the same culture conditions displayed similar patterns of cytokeratin expression. Both cell types expressed cytokeratins 13, 14 and 19, whereas neither of the cell types expressed cytokeratin 1/10. The monoclonal antibody AE1 recognizes most acidic type I keratins, including cytokeratins 10, 11, 14, 15, and 19 (38). AE1 displayed the same banding pattern in TIGKs as in GECs, although expression of each protein band was higher in the former cell type. The pattern of cytokeratin expression by both cell types in monolayer cultures is consistent with that of the non-keratinized regions of the gingiva, especially those cells of the basal cell layer. The apparent low level of detection of cytokeratin 13 suggests some cells have transitioned to a suprabasal pattern of gene expression. Cytokeratin expression and the potential for differentiation of TIGKs were further explored by immunohistochemistry of cells cultured for an extended period during which individual colonies developed stratified cell layers. Expression of cytokeratins 14 and 19 in non-stratified cells is consistent with their expression in cell monolayers in Western blots, and with the cytokeratin profile of basal cells of the non-keratinized regions of the gingiva, although basal cells of other non-keratinized oral epithelia (e.g., buccal mucosa) also display the same pattern of expression (36). However, the expression pattern of stratified cells (i,e., cytokeratins 14, 19, 13 and 16, with little detectable cytokeratin 10) is further consistent with the unique pattern of the suprabasal cell layers of non-keratinized gingival regions. In particular, the presence of cytokeratins 16 and 19 in stratified cells is analogous to suprabasal cells of the junctional epithelium, and is consistent with the expression of junctional epithelium cytokeratin markers by primary human gingival cells when cultured under conditions to promote stratification (8). Given that TIGKs retain the karyotype, morphology, growth and marker protein characteristics of primary GECs, as well as exhibiting stratification during prolonged culture suggests that, under more selective organotypic culture conditions, these cells may replicate the parent epithelium, as demonstrated for bmi1/hTERT-immortalized cells from other epithelia (23).
The etiologies of periodontal diseases are multifactorial and involve interactions between the subgingival microbiota and epithelial cells of the gingival crevice or periodontal pocket. Even in the absence of clinical disease, both the sulcular epithelium and cells at the coronal border of the junctional epithelium are continually exposed to the subgingival biofilm. Disruption of the balance between host and the subgingival microbiota during health leads to an ecological imbalance, dysregulation of immunity and disease. Gingival epithelial cells are used extensively to examine the interactions with periodontal pathogens such as P. gingivalis (2,32,33). Internalization of P. gingivalis into primary GECs has been studied in detail (1,28,29,30) and invasion effectors have been identified including the serine phosphatase SerB (2,32). To evaluate the suitability of TIGKs for P. gingivalis invasion studies, the internalization of a P. gingivalis SerB deficient mutant was compared with the parental strain. Similar to the results in GECs and HPV E6/E7 immortalized gingival keratinocytes, the ΔserB strain showed diminished invasion into TIGKs.
Because TIGKs retain normal growth and differentiation control systems, this new cell line will provide a valuable tool for studying the interactions of P. gingivalis and other species of the subgingival microbiota with epithelial cells resembling those encountered in vivo. Although these initial studies suggest TIGKs display the junctional epithelial phenotype, it will be of interest to determine appropriate culture conditions to recapitulate the sulcular epithelial phenotype to compare host-pathogen interactions between the two epithelial phenotypes of the gingival crevice or periodontal pocket. Moreover, expression of mRNA for multiple TLRs by TIGKs suggests these cells may function as a model to delineate mechanisms of immunity by the gingival epithelium. TIGKs further allow reproducibility of assays, with a proliferative cell-line that enables standardization between experiments over extended periods of time.
Supplementary Material
Acknowledgements
We thank the NIDCR for support through DE11111 (RJL) and DE20147 (DJC).
References
- 1.Lamont RJ, Chen A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tribble GD, Mao S, James CE, Lamont RJ. A Porphyromonas gingivalis haloacid dehalogenase family phosphatase interacts with human phosphoproteins and is important for invasion. Proc Natl Acad Sci USA. 2006;103:11027–11032. doi: 10.1073/pnas.0509813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz Ö , Yao L, Maeda K, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oda D, Watson E. Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell Dev Biol. 1990;26:589–595. doi: 10.1007/BF02624208. [DOI] [PubMed] [Google Scholar]
- 5.Oda D, Bigler L, Lee P, Blanton R. HPV immortalization of human oral epithelial cells: A model for carcinogenesis. Exp Cell Res. 1996;226:164–169. doi: 10.1006/excr.1996.0215. [DOI] [PubMed] [Google Scholar]
- 6.Schwab TS, Stewart T, Lehr J, Pienta KJ, Rhim JS, Macoska JA. Phenotypic characterization of immortalized normal and primary tumor-derived human prostate epithelial cell cultures. The Prostate. 2000;44:164–171. doi: 10.1002/1097-0045(20000701)44:2<164::aid-pros9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson BC, Moffatt CE, Hagerty D, et al. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol Oral Microbiol. 2011;26:210–220. doi: 10.1111/j.2041-1014.2011.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. doi 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart CE, Torr EE, Mohd Jamili NH, Bosquillon C, Sayers I. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy. 2012 doi: 10.1155/2012/943982. doi: 10.1155/2012/943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gröger S, Michel J, Meyle J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J Periodont Res. 2008;43:604–614. doi: 10.1111/j.1600-0765.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 12.Kusumoto Y, Hirano H, Saitoh K, et al. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2. J Periodontol. 2004;75:370–379. doi: 10.1902/jop.2004.75.3.370. [DOI] [PubMed] [Google Scholar]
- 13.Oda D, Bigler L, Mao C, Disteche CM. Chromosomal abnormalities in HPV-16-immortalized oral epithelial cells. Carcinogenesis. 1996;17:2003–2008. doi: 10.1093/carcin/17.9.2003. [DOI] [PubMed] [Google Scholar]
- 14.Sdek P, Zhang ZY, Cao J, Pan HY, Chen WT, Zheng JW. Alteration of cell-cycle regulatory proteins in human oral epithelial cells immortalized by HPV16 E6 and E7. Int J Oral Maxillofac Surg. 2006;35:653–657. doi: 10.1016/j.ijom.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39:1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Allsopp RC, Vaziri H, Patterson C, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura TM, Morin GB, Chapman KB, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 19.Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is upregulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 20.Bodnar AG, Ouelette M, Frolkis M, et al. Extension of lifespan by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 21.Dickson MA, Hahn WC, Ino Y, et al. Human keratinocytes that express hTERT and also bypass a p16INK4A-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4A inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 23.Dabelsteen S, Hercule P, Barron P, Rice M, Dorsainville G, Rheinwald JG. Epithelial cells derived from human embryonic stem cells display p16INK4A senescence, hypermotility, and differentiation properties shared by many P63+ somatic cell types. Stem Cells. 2009;27:1388–1399. doi: 10.1002/stem.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rheinwald JG, Hahn WC, Ramsey MR, et al. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latchney LR, Fallon MA, Culp DJ, Gelbard HA, Dewhurst S. Immunohistochemical assessment of fractalkine, inflammatory cells and human herpes virus 7 in human salivary glands. J Histochem Cytochem. 2004;52:671–681. doi: 10.1177/002215540405200511. [DOI] [PubMed] [Google Scholar]
- 26.Das B, Cash MN, Hand AR, Shivazad A, Culp DJ. Expression of Muc19/Smgc gene products during murine sublingual gland development: cytodifferentiation and maturation of salivary mucous cells. J Histochem Cytochem. 2009;57:383–396. doi: 10.1369/jhc.2008.952853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beklen A, Hukkanen M, Richardson R, Konttinen YT. Immunohistochemical localization of Toll-like receptors 1-10 in periodontitis. Oral Micro Immunol. 2008;23:425–431. doi: 10.1111/j.1399-302X.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuboniwa M, Hasegawa Y, Mao S, et al. P. gingivalis accelerates gingival epithelial cell progression throughout the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz Ö , Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handfield M, Mans JJ, Zheng G, et al. Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell Microbiol. 2005;7:811–823. doi: 10.1111/j.1462-5822.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa Y, Tribble GD, Baker HV, Mans JJ, Handfield M, Lamont RJ. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect Immun. 2008;76:2420–2427. doi: 10.1128/IAI.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffatt CE, Inaba H, Hirano T, Lamont RJ. Porphyromonas gingivalis SerB-mediated dephoshorylation of host cell cofilin modulates invasion efficiency. Cell Micro. 2012;14:577–588. doi: 10.1111/j.1462-5822.2011.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Presland RB, Jurevic RJ. Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of the oral mucosal and epidermal tissues. J Dent Educ. 2002;66:564–574. [PubMed] [Google Scholar]
- 35.Michel M, Török N, Godbout M, et al. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 36.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie IC, Rittman G, Gao Z, Leigh I, Lane EB. Patterns of cytokeratin expression in human gingival epithelia. J Periodont Res. 1991;26:468–478. doi: 10.1111/j.1600-0765.1991.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 38.Dale BA, Salonen J, Jones AH. New approaches and concepts in the study of differentiation of oral epithelia. Crit Rev Oral Biol Med. 1990;1:167–190. doi: 10.1177/10454411900010030201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.