Abstract
Because both androgens and estrogens have been implicated in penile morphogenesis, we evaluated penile morphology in transgenic mice with known imbalance of androgen and estrogen signaling using scanning electron microscopy (SEM), histology, and immunohistochemistry of androgen and estrogen receptors α/β. Penises of adult wild-type, estrogen receptor-α knockout (αERKO), estrogen receptor-β knockout (βERKO), aromatase knockout (Arom-KO), and aromatase overexpression (Arom+) mice were evaluated, as well as adult mice treated with diethylstilbestrol (DES) from birth to day 10. Adult penises were examined because the adult pattern is the endpoint of development. The urethral orifice is formed by fusion of the MUMP (male urogenital mating protuberance) with the MUMP ridge, which consists of several processes fused to each other and to the MUMP. Similarly, the internal prepuce is completed ventrally by fusion of a ventral cleft. In adult murine penises the stromal processes that form the MUMP ridge are separated from their neighbors by clefts. αERKO, βERKO, and Arom-KO mice have penises with a MUMP ridge clefting pattern similar to that of wild-type mice. In contrast, Arom+ mice and neonatally DES-treated mice exhibit profound malformations of the MUMP, MUMP ridge clefting pattern, and internal prepuce. Abnormalities observed in Arom+ and neonatally DES-treated mice correlate with the expression of estrogen receptor-beta (ERβ) in the affected structures. This study demonstrates that formation of the urethal orifice and internal prepuce is due to fusion of separate epithelial-surfaced mesenchymal elements, a process dependent upon both androgen and estrogen signaling, in which ERβ signaling is strongly implicated.
Keywords: mouse penis, prepuce, androgen receptor, estrogen receptor
The mouse penis develops from the embryonic genital tubercle through hormone-independent and hormone-dependent processes. In mice the embryonic ambisexual genital tubercle develops identically in males and females via a hormone-independent mechanism between 11 and 16 days of gestation (Yamada et al., 2003). Subsequent masculinization of the genital tubercle to form a penis requires signaling through the androgen receptor (AR). Impairment of androgen action via hormonal “androgen blockade” or mutation or absence of the AR can lead to varying degrees of feminization of the external genitalia (ExG) (Wilson et al., 1983; Wang et al., 2004; Galani et al., 2008). A potential role for estrogens in normal development of male ExG has been suggested by the findings that the developing penis expresses estrogen receptor-α (ERα), estrogen receptor-β (ERβ) [see Table 2 (Rodriguez et al., 2012)] and aromatase (Jesmin et al., 2004; Yonezawa et al., 2011), an enzyme that converts testosterone to estradiol. Thus, pharmacologic or physiologic perturbation of androgen and/or estrogen action may affect penile development.
TABLE 2.
Summary of the expression of AR, ERα, and ERβ in the developing penis of 10-day-old mice
| Structure | Subdivision | AR | ERα | ERβ |
|---|---|---|---|---|
| MUMP cartilage | N/A | + | + | +/− |
| Preputial lamina | Epithelium | + | − | + |
| Stroma | + | − | + | |
| MUMP ridge groove Lamina epithelium | N/A | + | − | + |
| Associated stroma | + | − | + | |
| Erectile bodies | CCG | + | − | + |
| CCUr | + | + | − | |
| MUMPCC | + | + | +/− | |
| Urethral epithelium | N/A | + | + | + |
Abbreviations: CCG = corpus cavernosum glandis; CCUr = corpus cavernosum urethrae; MUMPCC = MUMP corpus cavernosa.
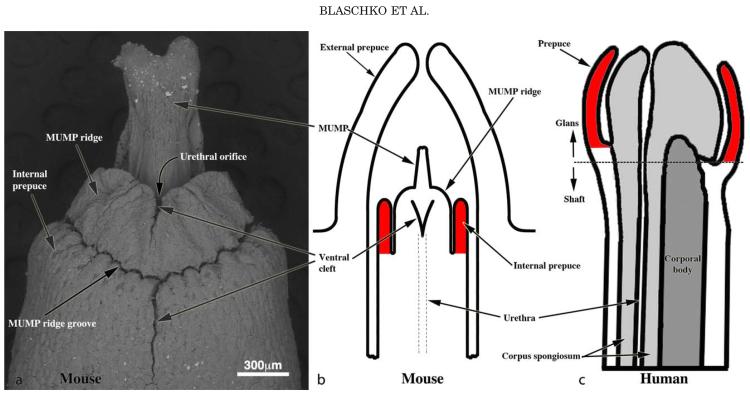
The elevation in the perineum of adult male mice is the prepuce, which is hair-bearing and covers the glans penis. While this historic description is essentially correct, we now are revising this terminology in recognition of homology with the human penis and accordingly will designate the hair-bearing mouse prepuce as external prepuce (see Results). In adulthood the glans extends beyond the external prepuce only during mating and urination. Extrusion of the penis from the external prepuce requires prior delamination of the preputial epithelial lamina, a process that occurs by 25 days postpartum, when the prepuce is completely freed and may be retracted from the glans penis. Upon retraction of the prepuce, the bifid distal tip of the mouse glans penis can be seen, which, is termed the male urogenital mating protuberance (MUMP) (Rodriguez et al., 2011). The MUMP is absent in female mice and in AR mutant mice (XTfm/Y and X/Y AR-null mice) (Yang et al., 2010; Rodriguez et al., 2012). The MUMP is reduced in length as a result of castration at birth or neonatal administration of diethylstilbestrol (DES) (Rodriguez et al., 2012), and thus development of the MUMP is responsive to the presence or absence of androgens and pharmacologic levels of estrogens. The MUMP is fused proximally to the MUMP ridge, which encircles and thus defines the urethral orifice (Rodriguez et al., 2011). The MUMP ridge is almost completely bisected by a ventral cleft (Yang et al., 2010; Rodriguez et al., 2011), but also displays several minor clefts on its surface. These minor clefts likely indicate earlier fusion events between several mesenchymal processes that form the MUMP ridge during development of the mouse penis. Perturbation in the location and formation of these clefts may be affected by androgen/estrogen signaling. Thus, the MUMP and the MUMP ridge clefts may serve as a measure of the androgen/estrogen action in development of the mouse penis.
Transgenic mouse models have been used to study perturbation of androgen and estrogen action. The importance of the androgen-signaling pathway is demonstrated by complete feminization of ExG in XTfm/Y and X/Y AR-null mice lacking functional androgen receptors (Murakami, 1987; Yang et al., 2010; Rodriguez et al., 2012). Estrogen receptor-α knockout (αERKO), estrogen receptor-β knockout (βERKO), aromatase knockout (Arom-KO), and aromatase over-expression (Arom+) mice may also clarify the roles of androgen and estrogen signaling in penile development since this group of mutant mice enhances and/or reduces androgen/estrogen signaling. While the basic phenotype of each of these mutant male mice has been described previously, detailed analysis of penile anatomy is lacking for all of these mutants.
Sex differentiation of the embryonic genital tubercle is initiated prenatally, but mostly occurs postnatally in mice (Rodriguez et al., 2012; Schlomer et al., 2013) as a result of exposure to endogenous androgens and estrogens (Pang et al., 1979; Pang and Tang, 1984). The balance of androgen versus estrogen signaling during this period is dependent upon serum androgen and estrogen levels, local androgen and estrogen levels, aromatase (the enzyme which converts testosterone to estradiol), and the tissue/cellular expression of AR, ERα, and ERβ. Accordingly, ExG development may be affected in transgenic mice having mutations affecting sex steroid production or sex steroid receptors. αERKO male mice are insensitive to estrogen, except via signaling through ERβ, are sensitive to androgens, express aromatase, and exhibit elevated serum testosterone levels in adulthood (Eddy et al., 1996; McDevitt et al., 2007; McDevitt et al., 2008). Adult βERKO male mice are deficient in ERβ signaling but retain ERα, AR, and aromatase. Adult βERKO male mice also have elevated to normal serum testosterone levels and have normal internal genitalia (Krege et al., 1998). Adult Arom-KO mice have elevated serum testosterone and reduced estradiol levels since conversion of testosterone to estradiol is absent. Arom-KO mice have normal internal genitalia, and express AR, ERα, and ERβ (Fisher et al., 1998; Honda et al., 1998; Simpson, 2004). Arom+ mice have two copies of the aromatase gene (mouse and human). Accordingly, overexpression of aromatase leads to elevated serum estradiol and reduced testosterone levels in the context of normal AR, ERα, and ERβ signaling pathways (Li et al., 2001). The consequence of these mutations on morphology of the penis is unknown and is the subject of this article. Because adult penile anatomy is the end point of development, we evaluated the adult penile anatomy of αERKO, βERKO, Arom-KO, and Arom+ mice by scanning electron microscopy (SEM), histology and immunohistochemistry.
MATERIALS AND METHODS
Animal care protocols were approved by the University of California San Francisco Institutional Animal Care and Use Committee (AN083370). Penises of adult wild-type and transgenic mice at 60 days of age (±7 days) were collected and processed for analysis. Strains of mice examined were CD1 (Charles River Laboratories, Wilmington, MA) and C57/6 (Jackson Labs, Bar Harbor, ME) wild-type, αERKONorth Carolina, and βERKO (provided by Dr. Dennis Lubahn, University of Missouri), αERKOStrasbourg (provided by Dr. Paul Cooke, University of Florida), Arom-KO and Arom+ (provided by Dr. Gail Risbridger, Monash University, Melbourne, Australia). Penises were fixed in formalin and were subsequently processed for scanning electron microscopic (SEM) and histologic analysis. Additionally, groups of 6 CD-1 wild-type mice were injected IP with sesame oil or sesame oil (20 μL) containing DES (200 ng g−1 body weight) every other day from birth to day 10. At 60 days postnatal these oil- and DES-treated mice were euthanized, and penises dissected and processed to generate serial 7-μm section sets. This study is based upon analysis of 20 wild-type, 8 αERKO, 7 βERKO, 6 Arom-KO, 10 Arom+, and 8 prenatally DES-treated and 6 prenatally oil-treated mice.
Scanning Electron Microscopy (SEM)
Adult mouse penises were placed in EM fixative (2.5% glutaraldehyde) and prepared for SEM evaluation by standard procedures (Yang et al., 2010). The TM-1000 electron microscope (Hitachi) was used for SEM analysis.
Three-Dimensional Reconstruction
Three-dimensional computer reconstructions were created from serial transverse sections utilizing SURF driver 3.5 software (SURF driver, University of Hawaii and University of Alberta). Sections were digitized to achieve linear tracings of relevant structures. Digital linear tracings were oriented using Photoshop software (Adobe, San Rafael, CA) and then were exported into SURF driver software for three-dimensional reconstruction.
Histologic Analysis
Mouse penises were fixed in formalin and embedded in paraffin. Seven micron sections were stained with hematoxylin and eosin. Serial tissue sections were matched to the corresponding location of SEM photographs for anatomic clarity.
Immunohistochemical Analysis
All immunohistochemistry was carried out on penises of 10-day mice when urethral development is occurring. Penile specimens were formalin fixed, paraffin embedded and serially sectioned at 7 mm. Immunohistochemistry was carried out as previously described (Rodriguez et al., 2012) utilizing the following antibodies: AR (rabbit monoclonal, diluted 1:200, GTX 62599, Genetex, Irving, CA), ERα (mouse monoclonal—clone 1D5, diluted 1:30, Dako, Carpinteria, CA) and ERβ (mouse monoclonal-clone EMR02, diluted 1:200, Leica Microsystems, Newcastle Upon Tyne, UK). Signal detection was achieved using the Vector ABC System (Vector Laboratories, Foster City, CA) followed by exposure to diaminobenzidine (Sigma®). Sections exposed to all steps except the application of the primary antibodies were used as negative controls.
RESULTS
Re-evaluation of Preputial Terminology
While past terminology regarding the mouse penis was devised in a logical fashion, new observations dictate a need for revision. Past terminology of the mouse penis described two elements: (a) a body deeply situated and attached to the pubic bones; and (b) a long glans abutting the penile body at a right angle bend (Rodriguez et al., 2011). The glans resides within an extensive preputial space defined by the attachment of the prepuce to the glans, which occurs proximally near the glans-body junction. Distally the prepuce is represented as the prominent hair-bearing elevation in the perineum (Rodriguez et al., 2011). This terminology is correct as the internal space created by this “external prepuce” houses the penis. In contrast, the human prepuce is attached to the distal aspect of the penis (Fig. 1). Thus, the hair-bearing “traditional mouse prepuce” is clearly not homologous to the human prepuce. Recent examination of the adult mouse penis revealed a series of ridges encircling the distal aspect of the glans penis. The distal projection of the mouse glans, the male urogenital mating protuberance (MUMP), is fused to the circumferential MUMP ridge to define the urethral orifice [Figs. 1–3 (Rodriguez et al., 2011)]. Proximal to the MUMP and MUMP ridge is the internal prepuce (originally called the glanular ridge [Rodriguez, 2011]), which is integral to and encircles the glans penis (Fig. 2). The internal prepuce, thus, has remarkable morphological homology with the human prepuce in so far as it is integral to the distal aspect of the glans and encircles and covers the glans penis (Fig. 1). Accordingly, the mouse actually has 2 prepuces: (a) the “traditional mouse prepuce” now called external prepuce and the internal prepuce (originally called the glanular ridge) (Figs. 1). The internal prepuce of the mouse contains the corpus cavernosum glandis, an erectile body (Rodriguez, 2011). The function of the internal mouse prepuce (like that of the human prepuce) is presumed to be male and/or female sexual stimulation.
Fig. 1.
Re-evaluation of penile terminology to establish mouse human homology. (a) SEM of the adult mouse penis. The penile glans lies within an extensive preputial space beginning at the opening of the preputial space distally in the hair-bearing prepuce [a prominent elevation in the perineum labeled External prepuce in (b)] and ending proximally near the glans-body junction. Drawings of mouse (b) and human (c) morphology demonstrating the homology of the human prepuce and the mouse “internal prepuce” (both red) in so far as both are integral to the distal penis and encircle the glans.
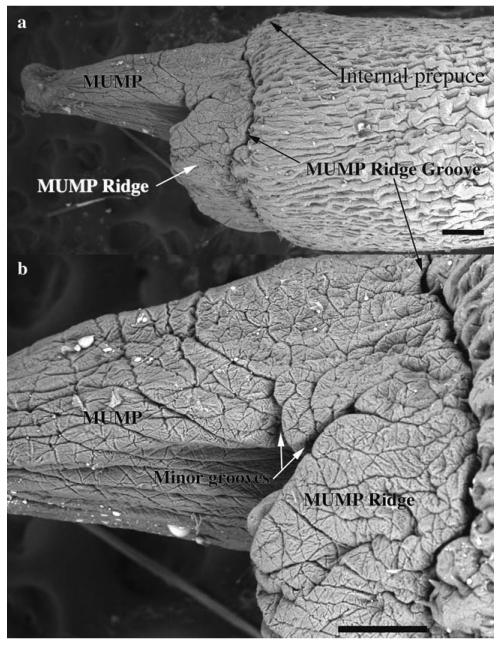
Fig. 3.
Scanning electron micrographs of an adult mouse penis, lateral view. Note that the MUMP fuses proximally with the MUMP Ridge, which is demarcated from the internal prepuce by the MUMP Ridge Groove. Scale bar = 200 μm for (a) and 150 μm for (b).
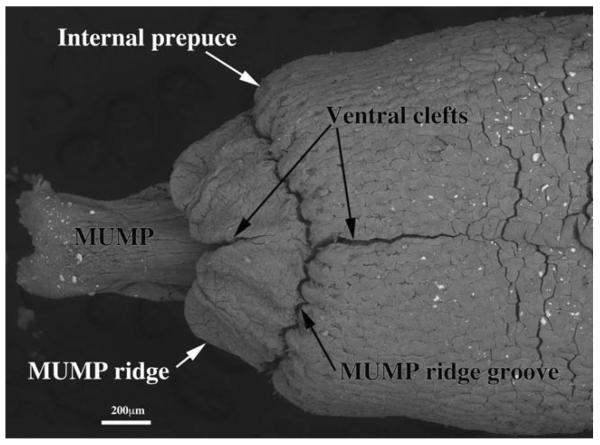
Fig. 2.
Scanning electron micrograph of an adult mouse penis, ventral view. The MUMP (male urogenital mating protuberance) is bifid distally and terminates proximally by joining the MUMP ridge, which is almost completely bisected by a ventral cleft as is the internal prepuce. The MUMP ridge is separated from the internal prepuce (also having a ventral cleft) by the MUMP ridge groove. Minor clefts are not seen in this view.
CD-1 Wild-type Mice
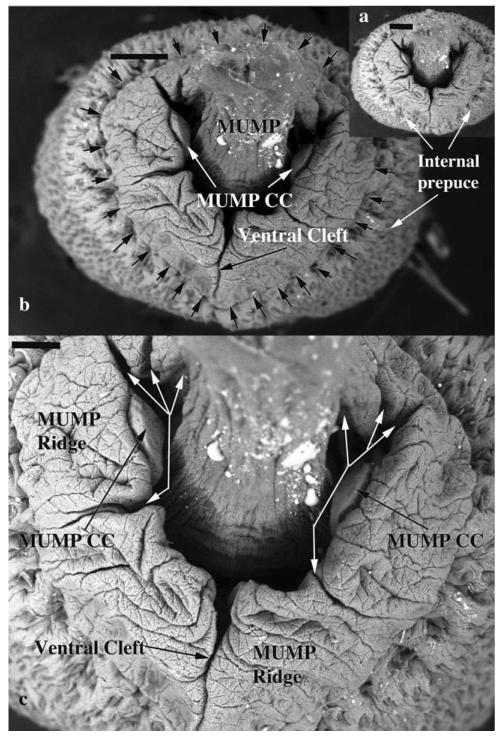
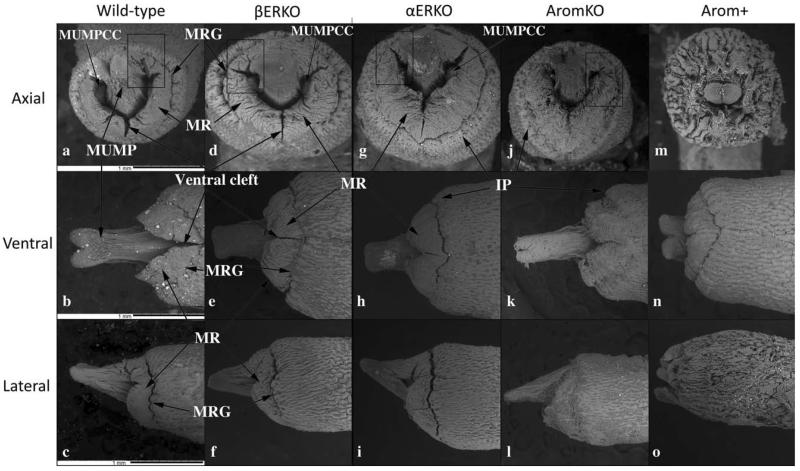
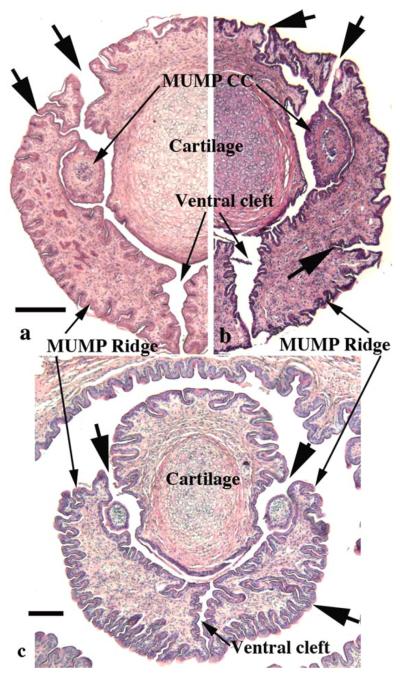
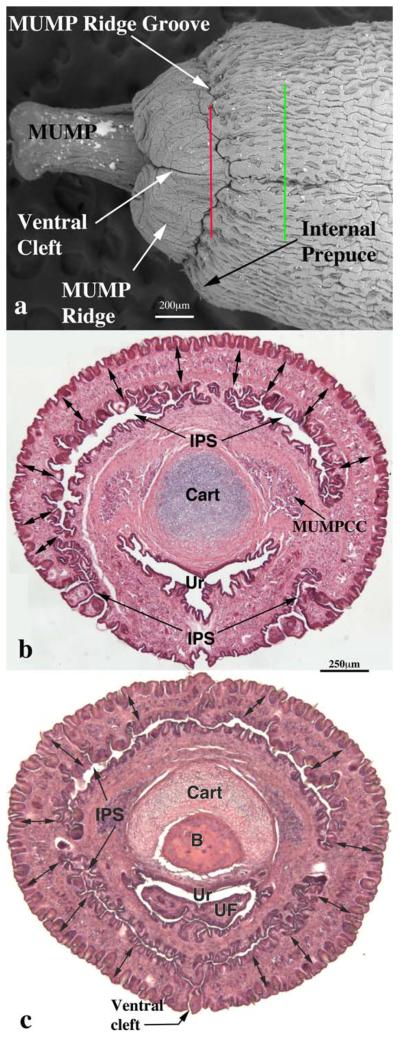
The SEM evaluation of the wild-type adult mouse penis with the external prepuce removed demonstrates the glans with the MUMP extending distally (Figs. 1–4 and 8b–c). The MUMP is a semirigid distal projection of the glans, bifid at the tip (Figs. 1–4 and 8b), containing a fibrocartilagenous core (Fig. 7b–d) (Rodriguez et al., 2011). The MUMP terminates proximally by joining a circumferential ridge called the MUMP ridge (Figs. 4) (Yang et al., 2010; Rodriguez et al., 2011). The MUMP ridge is demarcated proximally by the MUMP ridge groove, which separates the MUMP ridge distally from the internal prepuce proximally (Figs. 1–4 and 8a–c). The internal prepuce is integral to and completely circumscribes the glans penis (Fig. 4) and thus is homologous to the human prepuce (Fig. 1). The MUMP ridge and internal prepuce are deficient ventrally due to ventral clefts, which partially bisect the MUMP ridge as well as the internal prepuce (Figs. 2–8). Seen end-on (Figs. 4 and 8a), the Y-shaped urethral meatus is bounded dorsally by the MUMP and ventral-laterally by the MUMP ridge to which the MUMP is fused. Other minor clefts indent the distal edge of the MUMP ridge (Figs. 4 and 8a). The minor clefts in the MUMP ridge are roughly bilaterally symmetrical, although artificial shrinkage of specimens during SEM processing can affect the prominence of individual minor clefts. There are typically 3 bilateral minor clefts per side in the MUMP ridge in the 1/11 o'clock positions adjacent to the MUMP as well as bilateral minor clefts in approximately the 3/9 o'clock positions (Fig. 4). While the ventral cleft is always discernable in histologic sections, visualization of the minor clefts is subject to the level and the plane of histologic section through the MUMP ridge. Given this caveat, however, the histologic sections through the MUMP ridge (Figs. 5 and 7) are in close agreement with the SEM images (Fig. 4). In some sections, minor clefts correspond precisely with the SEM images and penetrate from the penile surface to the urethral lumen (Figs. 5a and 7c) and in so doing define individual MUMP ridge processes that fuse with the MUMP or other MUMP ridge elements to complete the contour of the urethral orifice (Fig. 4). In other cases, the clefts in histological sections extend only partially into the interior of the MUMP ridge (Figs. 5b–c and 7c). Thus, the urethral orifice (which is Y-shaped at its distal terminus [Figs. 4 and 7c]) is bounded by the MUMP dorsally and the clefted MUMP ridge ventral-laterally. Likewise, the internal prepuce is open to the preputial space via a ventral cleft distally that closes in more proximal regions (Figs. 2 and 6a,c), implying a fusion event in the ventral midline to form a completely circumferential internal prepuce.
Fig. 4.
Scanning electron micrographs of an adult mouse penis, distal end-on view. Seen end-on the urethral orifice is Y-shaped with the ventral cleft being the ventral stem of the “Y.” The MUMP, which is projecting toward the viewer, is dorsally situated and is continuous laterally with the MUMP ridge. Thus, the MUMP plus the MUMP ridge demarcate the urethral orifice. The MUMP ridge is demarcated peripherally by the circumferential MUMP ridge groove (small black arrows in b). The MUMP Ridge is almost completely bisected by a ventral cleft. In addition, the MUMP Ridge has several minor clefts (White arrows in c). Attached to the lateral wall of the Y-shaped urethra are the distal termini of bilateral erectile bodies called the MUMP corpora cavernosa (MUMP CC). Scale bars = 200 μm for a and b, 100 μm for c.
Fig. 8.
Scanning electron micrographs of penises of adult wild-type, βERKO, αERKO, aromatase knockout (AromKO), and aromatase overexpression (Arom+) mice in end-on (Axial), ventral and lateral views. Surface architecture is remarkably similar in wild-type, βERKO, αERKO, and AromKO mice in end-on views (a, d, g, j). In contrast, penises of Arom+ mice (m) exhibit truncation of the MUMP and a highly abnormal pattern of clefts and grooves. All photographs are at the same magnification (scale bars in [a–c] are 100 mm). Abbreviations: MUMPCC=MUMP corpus cavernosa, MUMP=male urogenital mating protuberance, MRG=MUMP ridge groove, MR=MUMP ridge.
Fig. 7.
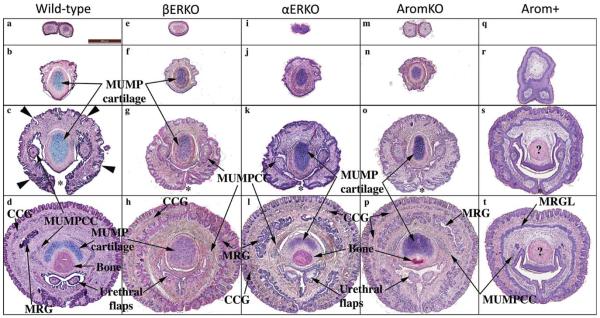
Hematoxylin-eosin-stained sections of penises from adult wild-type, βERKO, αERKO, aromatase knockout (AromKO), and aromatase over-expression (Arom+) mice selected from distal to proximal regions (top to bottom). Wild-type, βERKO, αERKO, and AromKO mice exhibit a similar normal pattern of structures and cell differentiation. In contrast, penises of Arom+ mice exhibit a variety of abnormalities of both pattern and cell differentiation. Note the absence of MUMP cartilage (r, s). The abnormal MUMP ridge groove (containing a slit-like lumen in all other groups) is represented as an undifferentiated solid MUMP ridge groove lamina (MRGL) in AROM+ mice (t). Also the pink body (?) in (t) is in the correct position to be cartilage, but is eosinophil instead of basophilic. All photographs are at the same magnification (scale bar in [a] is 100 μm). Abbreviations: CCG=corpus cavernosum glandis, MUMPCC=MUMP corpus cavernosa, MUMP=male urogenital mating protuberance, MRG=MUMP ridge groove, MRGL=MUMP ridge groove lamina.
Fig. 5.
Sections through the glans penis of three individual specimens illustrating the clefts (large arrows) in the MUMP ridge. In all cases the prominent ventral cleft is open into the preputial space. Minor clefts penetrate from the penile surface into the incipient urethral lumen in dorsal-lateral positions adjacent to the MUMP whose core contains cartilage. In all specimens, minor clefts demarcate separate processes, which in more proximal sections fuse with adjacent structures (MUMP or other processes constituting the MUMP ridge) to complete the MUMP ridge. Note in (b and c) minor clefts, which penetrate from the exterior and end blindly within the substance of the MUMP ridge in the 3 to 4 o'clock positions. Scale bar = 250 μm for a and b and 100 μm for c.
Fig. 6.
Scanning electron micrograph (a) of a penis of adult wild-type CD-1 mouse and two histological sections (b and c) taken at the levels specified by the red and green lines in (a). In (a) note the MUMP, the ventral cleft in the MUMP ridge and the internal prepuce. Section (b) corresponds to the red line in SEM (a). Double-headed arrows denote the MUMP ridge in (b and c). The internal preputial space (IPS) is seen and is confluent to the exterior ventrally. Note the MUMP corpus cavernosa (MUMPCC) flanking the MUMP cartilage (Cart) in (b). Section (c) corresponds to the green line in SEM (a). The ventral cleft in the MUMP ridge has close, but the ventral cleft in the internal prepuce is still open to the exterior. In (c) urethral flaps (UF) can be seen in the urethral (Ur) lumen. Scale bar in (b) also applies to (c).
Recently described bilateral erectile bodies called the MUMP corpora cavernosa (Rodriguez et al., 2011) (Figs. 4b,c, 5, and 7c,d) were also observed by SEM and histology. Proximally these bilateral erectile bodies flank the MUMP cartilage (Figs. 6b and 7d), but distally they project into the Y-shaped urethral lumen terminating proximally as tethered processes, which can be seen in end-on SEM images (Fig. 4b,c) and in transverse histologic sections (Figs. 5a–c and 7c).
A series of transverse sections through the distal aspect of penis of the adult wild-type mouse illustrates the overall organization of the glans (Fig. 7a–d). The most distal section demonstrates the bifid nature of the tip of the MUMP (Fig. 7a). More proximal sections (Fig. 7b–d) demonstrate the cartilaginous core of the MUMP. Figure 7c is a section through the MUMP ridge distal to closure of the ventral cleft in the MUMP ridge, and thus the Y-shaped urethral lumen is open ventrally (* in Fig. 7c,g,k,o,s). Figure 7d is a section proximal to closure of the ventral groove. In this position the urethral lumen is oval in contour and contains bilateral urethral flaps, which are distal extensions of the corpora cavernosa urethrae (Rodriguez et al., 2011). Note that the distal ends of the urethral flaps that project into the urethral lumen cannot be seen end-on in SEMs (Fig. 4). The proximal aspect of basophilic MUMP cartilage is dorsal to the distal end of the os penis and is flanked by the bilateral MUMP corpora cavernosa (Fig. 7d).
Estrogen Receptor-beta Knockout (βERKO) Mice
The glans penis of the adult βERKO mouse is remarkably similar to that of the wild-type mouse as judged by SEM and histological analyses (Figs. 7e–h and 8d–f). The length and appearance of the MUMP were comparable in length to the wild-type MUMP as judged by SEM and histologic analysis. The urethral meatus was Y-shaped and the MUMP ridge had a remarkably similar pattern of clefts (ventral cleft as well as minor clefts) in both βERKO and wild-type mice (Figs. 7e–h and 8d,e). The similarity of wild-type and βERKO penile morphology as seen by SEM was confirmed by histologic analysis in which the MUMP corpora cavernosa flanked the MUMP cartilage, and urethral flaps were present within the urethral lumen (Fig. 7e–h).
Estrogen Receptor-alpha Knockout (αERKO) Mice
Histological and SEM analysis of the adult αERKO mouse penis also demonstrated considerable similarity to that of the wild-type mice (cf., Figs. 7a–d vs. 7i–l and 8a–c vs. 8g–i). The MUMP, the Y-shaped urethral meatus, MUMP ridge, MUMP ridge groove, and ventral cleft of αERKO mice were all grossly similar to the wild-type glans penis (Fig. 8g). Prominent bilateral clefts were present in the 1/11 o'clock positions adjacent to the adult αERKO MUMP (Fig. 8g), even though some of the minor clefts typically in this position were poorly defined in SEM preparations (Fig. 8g). This is probably an artifact, since several minor clefts in the 1/11 o'clock positions were seen in histologic sections (Fig. 7k). The MUMP of αERKO mice (Fig. 8h,i) was similar in length and size to that of wild-type and βERKO mice. The basophilic MUMP cartilage was well developed as was the os penis of αERKO mice (Fig. 7j–l). Distally, the MUMP corpora cavernosa extended into the urethral lumen (Fig. 8g) and more proximally were in their normal positions flanking the MUMP cartilage (Fig. 7l). In proximal sections, urethral flaps were present in the urethral lumen (Fig. 7l). Thus, altered hormonal parameters in αERKO mice had minimal effects on development and differentiation of the glans penis, despite the absence of ERα signaling.
Aromatase Knockout (Arom-KO) Mice
The penis of adult Arom-KO male mice is also similar to that of adult wild-type mice by histologic and SEM analysis (Figs. 7m–p and 8j–l). The MUMP, urethral meatus, MUMP ridge groove, and MUMP ridge are grossly similar to those of wild-type mice in appearance, size, and pattern. The MUMP ridge has a pattern of clefts (ventral cleft and minor clefts) comparable to the wild-type counterparts. Minor clefts in the MUMP ridge near the junction of the MUMP with the MUMP ridge were comparable to that of the wild-type penis (cf., Figs. 8a,j). The basophilic MUMP cartilage was well developed/differentiated (Fig. 7n–p), with the MUMP corpora cavernosa and urethral flaps in their characteristic positions (Fig. 7o,p). As in wild-type mice, the os penis of Arom-KO mice is dorsally overlapped by the MUMP cartilage (Fig. 7p). Thus, the increased serum testosterone and decreased serum estradiol in Arom-KO male mice had little effect on the morphogenesis and differentiation of the glans penis.
Aromatase Overexpression (Arom+) Mice
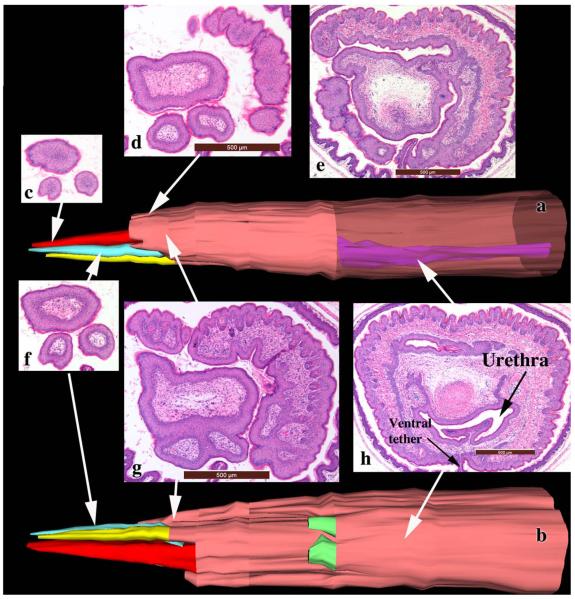
Arom+ mouse penises are profoundly different from wild-type penises as judged by histologic (Fig. 7q–t) and SEM (Fig. 8m–o) analyses and has a range of phenotypes. Arom+ mice typically have a substantially truncated MUMP (10/12) (Fig. 8n,o), which in some cases barely extended distal to the MUMP ridge (Fig. 8o) or had multiple distal processes (3/12) (Fig. 9). Figure 9 is a three-dimensional reconstruction of an Arom+ adult penis with associated histologic sections illustrating three distal processes and abnormality of MUMP cartilage differentiation. The MUMP ridge may be poorly defined due to an indistinct MUMP ridge groove that typically defines its proximal border with the internal prepuce (cf., Fig. 8a–c vs. 8m,o) or may be relatively normal but with a reduced or indistinct ventral cleft (Fig. 8n), even though the ventral cleft can be seen in histologic sections (Fig. 7s). Viewed end on, the typical Y-shaped urethral orifice may be profoundly distorted in Arom+ mouse penises (Fig. 8m). The typical pattern of minor clefts in the MUMP ridge can be absent or indistinct by SEM (Fig. 8m), an observation confirmed by histology (not illustrated). Instead, the distal aspect of the Arom+ MUMP ridge may display an extensive network of irregular deep furrows (Fig. 8m) whose pattern is completely different from that of wild-type mice (Fig. 4) or may be relatively normal (not illustrated). Whereas the initial distal sections of the wild-type mouse penis display the MUMP (Fig. 7a,b), the distal aspect of the Arom+ MUMP is typically absent or abnormal (12/12) (Figs. 7q and 9). Sections through “mid-MUMP' of wild-type mice reveal well differentiated basophilic MUMP cartilage (Fig. 7b), which is completely absent in Arom+ mice (Fig. 7r). MUMP cartilage differentiation is profoundly disturbed in most Arom+ male mice (8/9) in which the “MUMP cartilage” is represented as an eosinophilic fibrous connective tissue mass instead of the typically basophilic cartilaginous matrix (cf., Figs. 7c,g,k,o, vs. Figs. 7s,t and 9h). Morphogenesis/differentiation of the epithelium within the depth of the MUMP ridge groove (the internal preputial lamina or MUMP ridge groove lamina) is also impaired in most specimens (8/9). Note that in adult wild-type, αERKO, βERKO, and Arom-KO mice that the solid epithelial lamina of the neonatal MUMP ridge groove has canalized during development to create a complicated slit-like space within the depth of the MUMP ridge groove (Fig. 7d,h,l,p). Conversely, in Arom+ penises (Fig. 7t) the MUMP ridge groove epithelial lamina is undifferentiated, uncanalized and is devoid of a lumen. Likewise, creation of the external preputial space is impaired as a result of the presence of a ventral tether or frenulum linking the penile surface to the inner aspect of the external prepuce in many Arom+ penises (Fig. 9h). Thus, to reveal the penile surface, the adherent external prepuce must be forcibly removed with forceps. Finally, morphogenesis/differentiation of erectile bodies is impaired in the Arom+ penis. The corpus cavernosum glandis, MUMP corpora cavernosa, and the corpora cavernosa urethrae prominently displayed in wild-type, αERKO, βERKO, and Arom-KO mice (Fig. 7d,h,l,p), are either absent altogether or underdeveloped in Arom+ male mice (Fig. 7t). The urethral flaps, which are the distal extensions of the corpora cavernosa urethrae (Fig. 7t), are more proximally situated and shorter in proximal-distal length, and thus are present but seen in fewer transverse sections in the Arom+ penis (Fig. 7t). Finally, 7/9 Arom+ penises were tethered to the inner aspect of the prepuce by a frenulum-like structure (Fig. 9h). Table 1 is a summary of the histologic findings seen in wild-type and Arom+ mice.
Fig. 9.
Three-dimensional reconstructions (a and b) of an adult Arom+ mouse penis with accompaning tissue sections whose position is indicated on the 3DR by the tip of the white arrows. Similar to Fig. 8m–o, this specimen also has an abnormal MUMP, but in this case the MUMP is represented as three distal processes devoid of cartilaginous cores. The 3DRs have been artificially enlarged so as to stretch their dimensions to facilitate viewing. Surface skin is denoted by the tan color. The three distal MUMP-like projections are denoted in red, yellow and turquoise. A shorter epithelial-surfaced process is denoted in green. In (a) part of the skin has been rendered semitransparent to visualize the urethra in purple. Magnifications of the sections are variable and indicated by scale bars.
TABLE 1.
Comparison of features in wild-type versus Arom+ penises
| Wild-type | Arom+ | |
|---|---|---|
| MUMP shape | Normal (8/8) | Abnormal (12/12) |
| MUMP cartilage | Normal (8/8) | Abnormal (8/9) |
| MUMP length | 610μm | 221 μma |
| Urethral orifice | Normal (8/8) | Abnormal (12/12) |
| Erectile bodies | Normal (8/8) | Hypoplastic (9/9) |
| MUMP ridge groove | Lumen present (8/8) | Lumen absent (8/9) |
| Ventral tether | Absent (8/8) | Present (7/9) |
| Urethral flap position | Normal distal location (8/8) | Abnormal proximal location (9/9) |
| Bone position | Normal distal location (8/8) | Abnormal proximal location (9/9) |
Data is based upon both histologic and SEM specimens. Different denominators are a function of specimen orientation and ability to score.
Statistically significant P < 0.0001.
Neonatally Diethylstilbestrol-Treated Mice
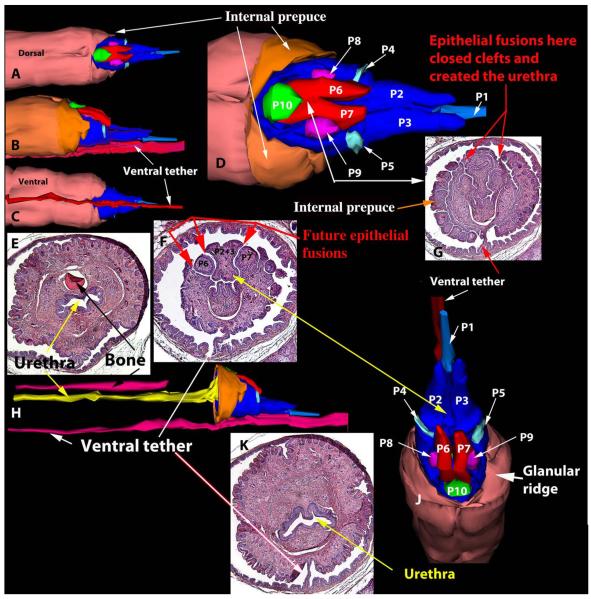
Given the profound phenotypic alterations seen in the glans penis of Arom+ mice, which have elevated serum estrogen and reduced androgen levels, the question arises as to whether the morphological alterations are due to elevated estrogen or reduced androgen. While the answer to this question is addressed to some extent in the literature as discussed below, an additional experiment sheds light on this subject. CD-1 mice treated from birth to day 10 with DES exhibited profound alterations in the organization of the MUMP and MUMP ridge in adulthood with several “unfused” processes. Figure 10 is a three-dimensional reconstruction of neonatally DES-treated male mice in which the MUMP is represented by three processes that remain separate over a considerable distance (processes P1, P2, and P3 in Fig. 10a,b,d,f). Two additional prominent processes (P6 and P7) lie lateral to the “MUMP” (Fig. 10d,f,j). Through fusion of processes P1–3, P6, P7 with the ventral-lateral portion of the MUMP ridge (Fig. 10f,g,k) a tubular urethra is formed. Because of the presence of a ventral tether (Fig. 10a,b,f,g,k), the ventral cleft in the MUMP ridge is absent. While the penile malformations seen in postnatally DES-treated mice are different in many respects from those seen in the Arom+ penis, both specimens show a profound disturbance in formation of the clefted MUMP ridge and thus both exhibit malformations of the urethral orifice. Other effects of neonatal DES treatment will be reported elsewhere. Penises of adult mice treated from birth to day 10 with oil (not illustrated) exhibited penile morphology identical to untreated wild-type mice described above (Figs. 5 and 6). Another malformation seen in neonatally DES-treated mice is a ventral deficiency in the internal prepuce (Fig. 10a). Typically the dorsal and ventral distal edges of the internal prepuce terminate distally at about the same level in untreated wild-type mice (Figs. 2 and 3), whereas in neonatally DES-treated mice the ventral edge of the internal prepuce is substantially shorter that the dorsal edge (Fig. 10a,d). This defect is likely due to the fact that the internal prepuce, like the MUMP ridge is formed thru the fusion of multiple elements to constitute the right and left halves of the internal prepuce which fuse in the midline to close the ventral cleft (Fig. 6c).
Fig. 10.
Three-dimensional reconstructions (a–d, h, j) of a penis of an adult wild-type mouse treated neonatally with DES as described above. The 3DRs are presented in a variety of orientations with accompanying tissue sections (e–g, k). The patterns of epithelial-surfaced mesenchymal processes denoted in light and dark blue, red, turquoise, magenta and green are vastly different from that seen in untreated wild-type mice (compare with SEMs in Figs. 1–3).
Immunohistochemical Analysis of Androgen Receptor and Estrogen Receptors Alpha and Beta in Wild-Type 10-day-Old Mice
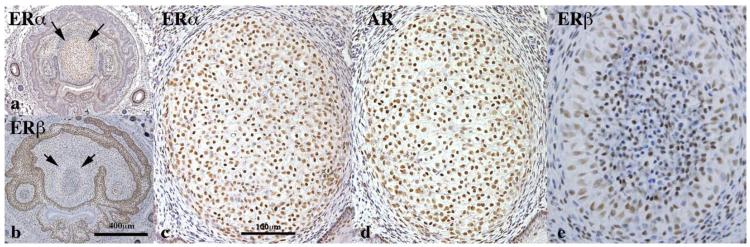
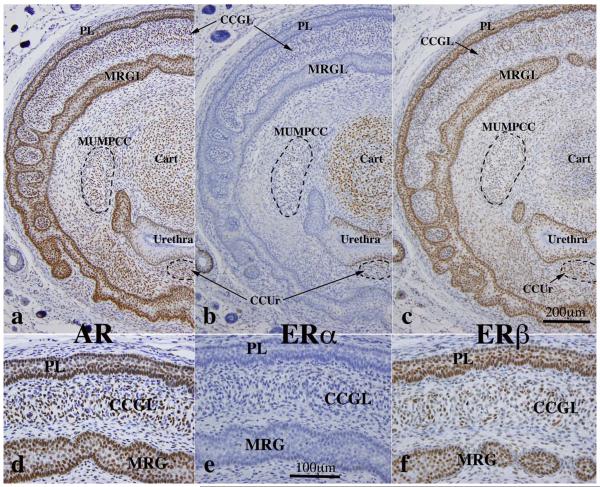
Given the profound alterations in penile morphology of adult untreated wild-type versus Arom+ and neonatally DES-treated mice, we examined the expression of AR, ERα and ERβ in wild-type mice at 10 days of age, a period when penile morphogenesis is particularly active (Rodriguez et al., 2012; Schlomer et al., 2013). Features enumerated in Table 1 were examined for AR, ERα, and ERβ. AR, ERα, and ERβ were detected in MUMP cartilage with AR and ERα being the predominant receptors expressed in virtually all chondrocytes (Fig. 11a,c,d). ERβ was detected in a subset of chondrocytes (Fig. 11e). AR and ERβ were detected in MUMP ridge groove epithelium (internal preputial lamina) and preputial epithelial lamina (Fig. 12a,c,d,f). ERα was not detected in the MUMP ridge groove epithelium and preputial epithelial lamina (Fig. 12b,e). AR, ERα and ERβ were detected in urethral epithelium (Fig. 10b and 12a–c). The expression of steroid receptors in erectile bodies (Fig. 12) was as follows: corpus cavernosum glandis = positive for AR, ERβ (but not ERα); MUMP corpora cavernosa = positive for AR and ERα and weakly positive if at all for ERβ; corpus cavernosum urethrae = positive for AR and ERα and negative for ERβ (Fig. 12). Table 2 summarizes the expression of steroid receptors in the developing murine glans penis.
Fig. 11.
Immunohistochemistry of developing penises of 10-day postnatal mice stained for androgen receptor (AR), estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) as indicated. This figure depicts staining of the MUMP cartilage (arrows in a and b), which expresses all three receptors. (c–e are higher magnifications of the MUMP cartilage. (a and b) are at the same magnification as are (c–e), see scale bars.
Fig. 12.
Immunohistochemistry of developing penises of 10-day postnatal mice stained for androgen receptor (AR), estrogen receptor alpha (ERα), and estrogen receptor beta (ERβ) as indicated. Abbreviations: PL=preputial lamina, MRGL=MUMP ridge groove lamina, MUMPCC=MUMP corpus cavernosum, CCUr=corpus cavernosum urethrae, CCG=corpus cavernosum glandis, Cart=MUMP cartilage. (a–c) are at the same magnification as are (d–f), see scale bars.
DISCUSSION
The penis is a particularly complex organ covered externally with an ectoderm-derived surface epithelium and containing an endoderm-derived tube, the urethra. The mesoderm associated with these epithelia forms a variety of structures and cell types: several erectile bodies, blood vessels, smooth muscle and (in mice) bone, and cartilage. These tissues are organized in the penis in precise patterns to form a uniquely shaped organ, whose external surface features can be optimally visualized with SEM.
The unique external shape of the adult mouse penis is the endpoint of a developmental process profoundly affected by hormones. According to current dogma, the ambisexual genital tubercle differentiates into a penis in the presence of androgens. More recently a role for estrogens in morphogenesis/differentiation of the ExG has been suggested based upon the presence of ERα, ERβ, and aromatase in the developing penis (Jesmin et al., 2002, 2004; Rodriguez et al., 2012), even though specific morphogenetic effects of estrogens in normal penile development have yet to be identified. To explore the role of the hormonal milieu on penile development, we examined the surface morphology and histology of the glans penis of wild-type, αERKO, βERKO, Arom-KO, and Arom+ adult mice and wild-type adult mice treated with DES during the first 10 days of life. The external morphology of the glans penises of αERKO, βERKO, and Arom-KO mice were strikingly similar to that of untreated wild-type mice as revealed by SEM, whereas the glans penises of AROM+ mice and neonatally DES-treated mice exhibited a variety of abnormal surface (as well as internal) features that illuminate both normal and abnormal morphogenetic processes.
A raphe is a seam or the line of union of two parts of an anatomical structure. Most raphes seen in adulthood (scrotal, lingual, penile, palatine, and others) are manifestations of embryonic processes in which an organ or structure is formed via the fusion of two or more parts. Developmentally a raphe is usually preceded by a groove or cleft where the two adjoining elements first make contact at initiation of the fusion process. Clefts or grooves are frequently seen during development, but are more rare in adulthood. Developmental clefts/grooves are frequently represented in adulthood as raphes. For example, the human philtrum is an adult midline groove on the upper lip and is formed as a result of the midline fusion of the medial nasal prominences during development (Moore and Persaud, 2003). Failure of this midline fusion results in midline cleft lip. Investigation of developmental processes as well as congenital malformations confirm the concept that development of the face occurs through the fusion of several individual elements (medial and lateral nasal prominences, maxillary, and mandibular processes). We propose that likewise the external urethral orifice of the penis and the mouse internal prepuce develops in similar fashion via the fusion of individual elements through a process that is sensitive to androgens and estrogens.
Our proposal is based upon four lines of evidence. (a) SEM analysis of the adult murine glans penis reveals a major ventral cleft that nearly bisects the MUMP ridge as well as several minor clefts that traverse the substance of the MUMP ridge. The presence of these clefts is confirmed by analysis of serial sections. We believe that these clefts are adult manifestations of fusion processes in which several epithelial-surfaced mesenchymal processes grow together and fuse to complete the outer contour of the urethral orifice. A similar process appears to occur in the development of the internal prepuce, which also manifests a prominent ventral cleft. (b) During murine penile development we have documented three paired mesenchymal columns covered by epithelium (Rodriguez et al., 2012; Schlomer et al., 2013). One pair of these epithelial-covered mesenchymal columns fuses in the midline to form the MUMP, which forms the dorsal aspect of the urethral orifice. (c) One of the “sex steroid” mutant mice exhibiting elevated serum estradiol, namely Arom+, exhibits truncation of the MUMP, and complete disturbance of the normal MUMP ridge clefting pattern. (d) In so far as Arom+ mice have elevated serum estrogens and reduced androgens, it is noteworthy that mice treated neonatally with DES, also have a constellation of epithelial-surfaced stromal processes that remain unfused around the urethral orifice of these neonatally DES-treated mice as well as an internal prepuce deficient ventrally. The malformations observed in neonatally DES-treated mice and in Arom+ mice are not exactly the same as would be expected given the differences in the timing and levels of elevated estrogen in these two models. However, in both cases the malformations are consistent with a profound disturbance of the assembly and fusion of mesenchymal processes constituting the internal prepuce and MUMP ridge, which surrounds and thus defines the urethral orifice. Thus, we propose that the murine urethral orifice forms via multiple fusion events in which the MUMP and several mesenchymal processes constituting the MUMP ridge fuse to form a circumferential urethral orifice with similar developmental events occurring during morphogenesis of the internal prepuce. The major and minor clefts seen in the adult MUMP ridge and internal prepuce are manifestations of earlier developmental fusions that appear to be profoundly influenced by signaling through ERα and/or ERβ. In a general sense the multiple fusion events inferred from examination of the adult mouse glans penis (the endpoint of development) are comparable to development of the human penile urethra in which fusion of the edges of the urethral groove results in formation of a tubular urethra.
The presence of steroid receptors in developing hormone target organs typically means that the receptor in question is involved in some way in the developmental process, but frequently also has pathogenic implications in so far as exposure to exogenous hormones at abnormally high levels or at inappropriate times may elicit congenital malformations. The function of a particular hormone receptor may be revealed by examination of the relevant knockout mouse, but due to possible redundancy of gene function, knockout mice may not be informative. With this caveat in mind, we speculate that disturbance in the balance between estrogen and androgen signaling may lead to abnormal development of penile architecture. This speculation, that penile development is dependent upon both estrogen and androgen signaling, is supported by the presence of AR, ERα, and ERβ and aromatase in the developing rodent penis (Jesmin et al., 2002, 2004; Yonezawa et al., 2011; Rodriguez et al., 2012). The expression of estrogen receptors (reported herein and by others [see Table 2 in (Rodriguez et al., 2012)]) in specific structures/tissues and especially ERβ in epithelium and stroma of the MUMP ridge and MUMP ridge groove (now called the internal preputial lamina) suggests an important role of ERβ in development of penile surface architecture. While this idea was not validated in the βERKO mouse (possibly due to compensation via ERα), it is important to recognize that the extensive malformations observed in Arom+ and neonatally DES-treated mice (both having elevated estrogen) were observed in tissues/structures expressing ERβ and/or ERα. Thus, both estrogen receptors may have important, possibly overlapping, roles.
The interpretation of our results is complicated because there are several endocrine parameters at play both in wild-type and mutant mice. In wild-type mice, tissues within the developing penis express AR, ERα, ERβ, and aromatase. Both serum testosterone and estradiol are present neonatally when penile morphogenesis/differentiation is occurring (Pang et al., 1979; Pang and Tang, 1984). Surprisingly, penile differentiation occurs in the neonatal period (Rodriguez et al., 2012; Schlomer et al., 2013) when serum androgen levels are particularly low. Thus, the penile phenotypes of the mutant mice must be interpreted carefully in regard to both local effects (presence or absence of receptors or aromatase) as well as possible systemic alteration in serum hormone levels.
Male αERKO mice have AR, aromatase, and ERβ but are devoid of ERα. Adult αERKO males have elevated testosterone levels (Eddy et al., 1996; McDevitt et al., 2008), even though testosterone levels are not elevated in female αERKO mice prior to puberty (Rosenfeld et al., 2000; Yang et al., 2010). Testosterone levels in prepubertal male αERKO mice have not been reported. The apparent normality of the surface features of the glans penis of αERKO mice observed in the present study may be interpreted as indicating that ERα is not involved in normal penile surface architecture and internal structure. Our SEM and histologic data attest to a relatively normal MUMP and MUMP ridge bounding the urethral orifice in αERKO mice. However, more precise morphometric analysis or use of other techniques may reveal other penile defects in αERKO mice.
Male βERKO mice have aromatase, AR, and ERα but lack ERβ. Despite the fact that ERβ is expressed in the developing wild-type penis (Rodriguez et al., 2012), SEM and histologic analysis of male βERKO mice reveals normal penile morphology/differentiation as well as normal internal accessory sexual organs (Krege et al., 1998). Again, more discriminating techniques may reveal more subtle phenotypic alterations in the penis of βERKO mice, even though penile surface morphology of these mice is remarkably normal.
Arom-KO male mice have AR, decreased ERα and ERβ, and lack aromatase but have a normal external phenotype (Honda et al., 1998). The key feature of Arom-KO male mice is elevated serum testosterone (Fisher et al., 1998; Honda et al., 1998) and low to undetectable estrogen levels (Murata et al., 2002; Jones et al., 2006). Penises of Arom-KO mice are also phenotypically normal. Given the profound reduction in serum estrogen in Arom-KO male mice, these mice are expected to have a phenotype similar to that of αERKO mice, since in both cases estrogen signaling is impaired. In the present study, penile surface morphology was normal and virtually identical in αERKO and Arom-KO male mice.
Male Arom+ mice have elevated aromatase, AR, ERα, and ERβ. They have increased serum estrogen and reduced testosterone levels (Li et al., 2001). We attribute the profound alterations in penile morphology/differentiation to the increased levels of estrogen since similar results were obtained in the neonatally DES-treated group as discussed above, although an effect of reduced testosterone cannot be excluded. Physiologic elevation in estrogen in Arom+ mice caused severe truncation of the MUMP, disrupted the pattern of MUMP ridge clefts, disrupted development of the internal prepuce, impaired cartilage differentiation, and inhibited or impaired development of erectile bodies. Likewise, in mice castrated at birth, treated with DES for 10 days and analyzed at 10 days postnatal, we similarly noted disruption of the MUMP cartilage, inhibition of erectile body formation, reduction in penile width, reduction in os penis length, and inhibition in penile surface epithelial differentiation (Rodriguez et al., 2012). Thus, physiologic or pharmacologic elevation of estrogen has effects on both the surface architecture as well as on differentiation of internal penile structures.
Androgens have two effects on penile development: (a) Prenatally androgens specify penile identity and (b) postnatally androgens elicit the actual morphogenesis and differentiation of penis-specific features from birth to puberty (Rodriguez et al., 2012). Curiously, masculine sex differentiation occurs in mice during the neonatal period when serum androgens are particularly low. The ExG of all of the mutant mice examined in this study were distinctly penile indicating an adequate androgen level for prenatal specification of penile identity (even in Arom+ mice known to have reduced serum androgen). Normal penile development occurred in all mice with normal or elevated serum testosterone (untreated wild-type, βERKO, αERKO, and Arom-KO). While reduced testosterone may have contributed to some of the phenotypic features exhibited by Arom+ mice, the DES-treated mice in this report and those presented earlier (Rodriguez et al., 2012) suggest that elevated estrogen during penile development is the key element for eliciting the observed malformations. The two models of estrogen excess in penile development are radically different. Neonatal exposure to DES provides an entirely different dosage and temporal exposure to estrogen levels than that occurs in Arom+ mice. The degree of estradiol elevation in Arom+ is much higher at 6 months than at 2 months of age (Li et al., 2003). It is unknown when serum estradiol levels become elevated in Arom+ mice, whether during pre- or neonatal periods. Likewise, any elevation in serum estradiol in perinatal Arom+ mice must be viewed in the context of the high affinity serum estrogen binder, alpha-fetal protein, whose levels are known to decay postnatally over an extended period. The one presumption to be made is that the penile phenotype seen in Arom+ mice and attributed to estrogen excess implies that elevated estrogen signaling through ERα and ERβ must be occurring during periods of developmental susceptibility to estrogen likely well before puberty when penile development is complete. However, it is possible that the penile phenotype of Arom+ mice could also be related to a reduction in testosterone levels.
This study emphasizes the complexity of endocrine effects on the developing penis. The penis is a hormone target organ for both androgens and estrogens containing cells/tissues expressing AR, ERα, and ERβ as well as aromatase. The penis contains epithelium derived from both ectoderm and endoderm associated with fibromuscular tissues derived from mesoderm that differentiates into multiple cell types. Epithelial-mesenchymal interactions (Murakami and Mizuno, 1986; Kurzrock et al., 1999) are known to play an important role in generating the exquisite epithelial and mesenchyme morphologies within ExG whose development are dependent upon the hormonal milieu as well as the steroid receptors resident within epithelial and mesenchymal cells of the developing penis. Based upon the major and minor clefting patterns in the MUMP ridge, which largely defines the penile urethral orifice (as well as in the internal prepuce), we suggest that morphogenesis of the penile urethral orifice and internal prepuce occurs through the growth and fusion of multiple epithelial-surfaced elements, a process sensitive to estrogen signaling.
ACKNOWLEDGEMENTS
Jennifer Boehner and Dennis Lubahn from the University of Missouri provided αERKO mice. Gail Risbridger from Monash University provided the Arom-KO and Arom+ mice. The authors appreciate the guidance from Gangway Min at the Electron Microscope Lab at University of California Berkeley in SEM sample preparation and use of the electron microscope.
Grant sponsor: NSF; Grant number: IOS-0920793; Grant sponsor: NIH; Grant number: RO1 DK0581050; Grant sponsor: NH&MRC; Grant number: APP1002733.
Abbreviations used
- αERKO
estrogen receptor-alpha knockout
- AR
androgen receptor
- Arom+
aromatase overexpression
- Arom-KO
aromatase knockout
- βERKO
estrogen receptor-beta knockout
- DES
diethylstilbestrol
- ERα
estrogen receptor-alpha
- ERβ
estrogen receptor-beta
- ExG
external genitalia
- MUMP
male urogenital mating protuberance
- SEM
scanning electron microscopy
LITERATURE CITED
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A. Androgen insensitivity syndrome: clinical features and molecular defects. Hormones (Athens) 2008;7:217–229. doi: 10.14310/horm.2002.1201. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Matsuda N, Salah-Eldin AE, Togashi H, Sakuma I, Hattori Y, Kitabatake A. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology. 2002;143:4764–4774. doi: 10.1210/en.2002-220628. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Sakuma I, Matsuda N, Togashi H, Yoshioka M, Hattori Y, Kitabatake A. Aromatase is abundantly expressed by neonatal rat penis but downregulated in adulthood. J Mol Endocrinol. 2004;33:343–359. doi: 10.1677/jme.1.01548. [DOI] [PubMed] [Google Scholar]
- Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17:55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Li Y, Cunha GR. Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs. 1999;164:125–130. doi: 10.1159/000016650. [DOI] [PubMed] [Google Scholar]
- Li X, Makela S, Streng T, Santti R, Poutanen M. Phenotype characteristics of transgenic male mice expressing human aromatase under ubiquitin C promoter. J Steroid Biochem Mol Biol. 2003;86:469–476. doi: 10.1016/s0960-0760(03)00376-5. [DOI] [PubMed] [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice over-expressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The developing human. 7th ed. Saunders; Philadelphia: 2003. [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat. 1987;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Mizuno T. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J Embryol Exp Morphol. 1986;92:133–143. [PubMed] [Google Scholar]
- Murata Y, Robertson KM, Jones ME, Simpson ER. Effect of estrogen deficiency in the male: the ArKO mouse model. Mol Cell Endocrinol. 2002;193:7–12. doi: 10.1016/s0303-7207(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Pang SF, Caggiula AR, Gay VL, Goodman RL, Pang CS. Serum concentrations of testosterone, oestrogens, luteinizing hormone and follicle-stimulating hormone in male and female rats during the critical period of neural sexual differentiation. J Endocrinol. 1979;80:103–110. doi: 10.1677/joe.0.0800103. [DOI] [PubMed] [Google Scholar]
- Pang SF, Tang F. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. J Endocrinol. 1984;100:7–11. doi: 10.1677/joe.0.1000007. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha GR, Baskin L. Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation. 2012;84:269–279. doi: 10.1016/j.diff.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, Jr, Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, Barcellos D, Chan LY, Risbridger G, Cunha GR, Baskin LS. New insights on the morphology of adult mouse penis. Biol Reprod. 2011;85:1216–11221. doi: 10.1095/biolreprod.111.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Cooke PS, Welsh TH, Jr, Simmer G, Hufford MG, Gustafsson JA, Hess RA, Lubahn DB. The differential fate of mesonephric tubular-derived efferent ductules in estrogen receptor-alpha knockout versus wild-type female mice. Endocrinology. 2000;141:3792–3798. doi: 10.1210/endo.141.10.7694. [DOI] [PubMed] [Google Scholar]
- Schlomer BJ, Feretti M, Rodriguez E, Blaschko S, Cunha GR, Baskin L. Sexual differentiation in the male and female mouse from day 0 to 21: a detailed and novel morphometric description. J Urol. doi: 10.1016/j.juro.2013.02.3198. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER. Models of aromatase insufficiency. Semin Reprod Med. 2004;22:25–30. doi: 10.1055/s-2004-823024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Q, Xu J, Liu Q, Wang W, Lin Y, Ma F, Chen T, Li S, Shen Y. Mutation analysis of five candidate genes in Chinese patients with hypospadias. Eur J Hum Genet. 2004;12:706–712. doi: 10.1038/sj.ejhg.5201232. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Griffin JE, George FW, Leshin M. The endocrine control of male phenotypic development. Aust J Biol Sci. 1983;36:101–128. doi: 10.1071/bi9830101. [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J Urol. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa T, Higashi M, Yoshioka K, Mutoh K. Distribution of aromatase and sex steroid receptors in the baculum during the rat life cycle: effects of estrogen during the early development of the baculum. Biol Reprod. 2011;85:105–112. doi: 10.1095/biolreprod.110.089508. [DOI] [PubMed] [Google Scholar]