Abstract
Purpose
The aim of the study is to dissect the cytotoxic mechanisms of 1-(4-hydroxy-3-methoxyphenyl)-7-(3,4-dihydroxyphenyl)-4E-en-3-heptanone (compound 1) in SH-SY5Y cells and therefore to provide new insight into neuroblastoma chemotherapy.
Methods
9 diarylheptanoids were isolated from Alpinia officinarum by chromatography and their cytotoxicity was evaluated by an MTS assay. Flow cytometry, Brdu incorporation assay and fluorescence staining were employed to investigate cytostatic and apoptotic effects induced by the compound 1. In addition, western blot, qPCR and siRNA techniques were used to elucidate the molecular mechanisms of the cytotoxicity.
Results
The study to elucidate the cytotoxic mechanisms of compound 1, the most potent diarylheptanoid showed that cell cycle related proteins cyclins, CDKs and CDKIs as well as two main apoptotic related families caspase and Bcl 2 were involved in S phase arrest and apoptosis in neuroblastoma cell line SH-SY5Y. Furthermore, following the drug treatment, the protein expression of p53, phospho-p53 (Ser20) as well as the p53 transcriptional activated genes ATF3, puma and Apaf-1 were increased dramatically; MDM2 and Aurora A, the two p53 negative regulators were decreased; the p53 protein stability was enhanced whereas the p53 mRNA expression level slightly decreased and ATF3 mRNA expression apparently increased. In addition, the knockdown of ATF3 gene by siRNA partially suppressed p53, caspase 3, S phase arrest and apoptosis triggered by compound 1.
Conclusion
These results suggest that compound 1 induces S phase arrest and apoptosis via up regulation of ATF3 and stabilization of p53 in SH-SY5Y cell line. Therefore, compound 1 might be a promising lead structure for neuroblastoma therapy.
Keywords: diarylheptanoid, cell cycle arrest, apoptosis, p53, ATF3
Introduction
Neuroblastoma, a tumor originating from the sympathetic nervous system, is the most frequently occurring extra cranial malignancy in childhood[23]. Despite many advances in diagnosis and standard interventions in the past three decades, neuroblastoma has remained a formidable challenge to clinic and basic scientists[3]. In search for novel therapeutic strategies for neuroblastoma, natural product has attracted interest to achieve new approach for chemotherapy.
Alpinia officinarum Hance (Zingiberaceae), a pungent and aromatic rhizome cultivated in southern China and Vietnam, is used as a spice ingredient for flavoring food throughout southeastern Asian countries [20, 21]. The dried rhizome of A. officinarum is a traditional Chinese medicine (TCM) with anti-inflammatory, antioxidant and analgesic activities and has been used for relieving stomachache, treating colds, invigorating the circulatory system, and reducing swelling for a long time[1]. Recent studies on A. officinarum showed that MeOH and CH2Cl2 extractable fractions possess significant cytotoxicity against COR L23 human large-cell carcinoma with IC50 values of 13.3 and 5.4 μg/ml respectively. A phenylpropanoid compound 1-acetoxychavicol acetate is one of the active constituents in the herb with IC50 values of 5.8 μM and 8.6 μM against COR L23 and MCF-7 cells[16]. Phytochemical studies showed that of the many chemical constituents isolated from this plant, diarylheptanoids are among the characteristic compounds [36]. Multiple lines of evidence showed that diarylheptanoids are cytotoxic agents against many cancer cell lines. Curcumin, a well-known diarylheptanoid has been postulated to be potential use not only in cancer chemoprevention but also in chemotherapy[30]. A numerous reports demonstrated that curcumin could inhibit chemical carcinogen or radiation-induced tumorigenesis and suppress the growth of mammary tumors via various pathways[2, 6].
Our previous screening study has shown that some diarylheptanoids possess good cytotoxicity in a series of cancer cell lines, including HepG2, MCF-7, SF-268 and SH-SY5Y with similar IC50, ranging from 6-10 μg/ml [1]. In addition, SH-SY5Y cells are more sensitive to the most potent diaryheptanoid named compound 1 in cell cycle analysis. Thus, it is of great interest to investigate the underlying mechanisms of compound 1 in the most potent cell line SH-SY5Y, and this will provide a new insight into neuroblastoma therapy.
Materials and Methods
Extraction and isolation
The dried rhizomes of A. officinarum (28 kg) were extracted with EtOH at room temperature. The extract yielded a residue of 2.2 kg, which was suspended in H2O and extracted with petrol ether, CHCl3, EtOAc and n-BuOH respectively. The dried CHCl3 part (150 g) was subjected to Si-gel, polyamide and Sephadex LH-20 chromatography to give 9 diarylheptanoids, which were identified as 1-(4-hydroxy-3-methoxyphenyl)-7-(3,4-dihydroxyphenyl)-4E-en-3-heptanone(1), 5-hydroxy-1-(3-methoxy4-hydroxyphenyl)-7-phenyl-4E,6E-dien-3-heptanone(2), (5S)-5-hydroxy-1-phenyl-7-(3,4-dihydroxyphenyl)- 3-heptanone(3), (5R)- 5-hydroxy-1-phenyl-7-(4,5-dihydroxy-3-methoxyphenyl)- 3-heptanone (4), (5R)-5-hydroxy-1-(3,4-dihydroxy phenyl)-7-(4-hydroxy -3-methoxyphenyl)- 3-heptanone (5), 1-(4-hydroxy-3-methoxyphenyl)-7-phenyl-3,5-heptandiol(6), (3R,5R)-1-(4-hydroxy-3-methoxyphenyl)-7-phenyl-3,5-heptanoidiol(7), (3R,5R)-1,7-bis(4-hydroxyphenyl)- 3,5-heptanoidiol(8), (3R,5R)-1-(4-hydroxy-3-methoxyphenyl)-7-(3,4-dihydroxyphenyl)-3,5-heptanoidiol (9) based on their IR, NMR and MS.
Cell culture
SH-SY5Y cells (ATCC, Manassas, VA) were grown in DMEM (Gibco, Grand Island, New York, USA) containing 10% FBS (Gibco), 2% L-Glutamine, 1% penicillin sodium salt and streptomycin sulfate at 37 °C under air plus 5% CO2 and passage regularly.
Cytotoxicity assay
SH-SY5Y cells were seeded in 96-well tissue culture plates and treated with the test compounds (100–3.125 μg/ml) at various concentrations or vehicle (0.1% DMSO). Cell viability in cell culture after compounds exposure was measured by a CellTiter 96® AQueous One Solution Cell proliferation assay (MTS) assay. The IC50 value was derived from the dose–response curve.
Cell cycle analysis
SH-SY5Y cells were treated with compound 1 at 2.5, 5 and 10 μg/ml for 48 h. At the end of treatment, cells were harvest and fixed in 70% cold ethanol (4 °C) overnight. After washing twice with PBS, cells were resuspended. RNase A (0.5 mg/ml) and PI (2.5 μg/ml) were added to the fixed cells and incubated at 37 °C for 30 min. The DNA content of cells was then analyzed with a FACSCalibur instrument (Becton Dickinson, San Jose, CA). The percentage of cells in different cell cycle phases was calculated by ModFit 3.0 (Verity Software House, Inc.).
BrdU incorporation assay
SH-SY5Y cells were seeded onto glass coverlips 24 h before drug treatment. After treatment with vehicle or compound 1 at 5 μg/ml for 24 h, cells were incubated with 20 mM Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) for another 1 h before fixation with acetone (−20 °C) for 10 min. After DNA denaturation in 2 N HCl at 37 °C for 30 min, the cells were blocking with PBSST for 20-30 min and followed by incubating with BrdU antibody (10 μl Brdu-Alexa 488/647 with DAPI 1 μl into 90 μl PBSST) for 1 h at room temperature. Pictures were taken under fluorescence microscopy.
Apoptotic morphology observation
Cells were plated in 6-well plate and treated with vehicle or compound 1 at 5 μg/ml for 48 h. After treatment, cells were fixed in 4% paraformaldehyde for 10 min at room temperature and then stained with Hochest33258 at 5 μg/ml for 10 min. Photographs were taken under an ECLIPSE TE2000-S fluorescence microscope (Nikon Instruments INC. Melville, NY).
Western blot
Following treatment, SH-SY5Y cells were washed three times with cold PBS and lysed with lysis buffer. Protein concentration was measured by the Bradford method. The lysates were subjected to electrophoresis on 10% polyacrylamide gels and then transferred onto a nitrocellulose sheet. The nitrocellulose membrane was then incubated with rabbit polycolonal anti-phospho-p53 (Ser20, #9287, Cell Signaling Technology, Danvers, MA); anti-Aurora A (07-648, Upstate, Billerica, MA); anti-p53, anti-MDM2, anti-cyclin A, anti-cyclin B, anti-cdc 2, anti-cdk4, anti-p21, anti-p27, anti-Bax, anti-PUMA a/b, anti-Apaf-1 anti-PARP antibodies; mouse monoclonal anti-cyclin E, anti-caspase-3, anti-Bcl-2, (sc-6243, sc-7918, sc-596, sc-25764, sc-954, sc-260, sc-397, sc-528, sc-493, sc-28226, sc-8339, sc-7150, sc-247, sc7272, sc-7382, Santa Cruz Biotechnology, Santa Cruz, CA); Mouse monoclonal GAPDH (MAB374, CHEMICON, Billerica, MA) was used as an internal control. Secondary antibody to IgG conjugated to horseradish peroxidase was used. Protein bands were visualized with the ECL Western blot detection system according to the manufacturer's instructions.
Caspase 9 activity assay
After drug treatment, SH-SY5Y cells were harvest and Caspase-9 activity was evaluated by using casepase 9 detection kit (QIA 115, Calbiochem, Darmstadt, Germany) as we described previously [31]. One-factor ANOVA was used and followed by Dunnett's test, p < 0.05 was considered significant.
RT-qPCR
Gene expression level of ATF3 and p53 were evaluated by using real-time PCR. Primers, designed using primer 3 were as follows: ATF3 forward primer: gccattggagagctgtctt, reverse primer: gggccatctggaacataag; p53 forward primer: gcccacttcaccgtactaa, reverse primer: tggtttcaaggccagatgt; GAPDH forward: gagtcaacggatttggtcgt, reverse: ttgattttggagggatctcg. Briefly, total RNA was prepared after drug treatment using an RNeasy® Mini Kit (Qiagen, Maryland, USA) according to the protocol. 1 μg RNA of each sample was used for cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) with RNAse H+ following instruction. Real Time PCR was performed on the iQ5 Real-Time PCR detection system with the iQ SYBR Green Supermix (Bio-RAD) and GAPDH was used as an internal control. The relative quantification of mRNA expression was calculated according to the literature[26].
Cycloheximide chase assay
Following treatment with vehicle or compound 1 at 5μg/ml for 48 h, we treated SH-SY5Y cells with 50 μg/ml cycloheximide, harvested the cells at indicated time points and subjected cell lysates to Western blotting.
Transient transfection
Silencer 1 Negative Control No. 1 siRNA (Cat No. 4635) and ATF3 siRNA (Cat No. AM16708A) were obtained from Ambion (Austin, TX). The sequence of siRNA duplex targeting ATF3 is as follows: #241437 sense, 5′-AAGUGCCGAAACAAGAAGAtt-3′; antisense, 5′-UCUUCUUGUUUCGGCACUUtg-3′; #115224 sense, 5′-CGAGAAGCAGCAUUUGAUAtt-3′; antisense, 5′-UAUCAAAUGCUGCUUCUCGtt-3′. SH-SY5Y cells were plated in 6-well plates in antibiotic-free medium for 24 h before transfection and transfected at 70% confluence. Transfection was done with 4 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) using 50 nmol/l of ATF3 siRNA mixed in serum-free OPTI-MEM (Invitrogen, Carlsbad, CA). 6 h post-transfection, the medium was changed to DMEM with 10% FBS without antibiotics. Forty-eight hour post-transfection, cells were treated with compound 1 for an additional 24 h and harvested for western blotting and cell cycle analysis.
Results
Cytotoxicity of diarylheptanoids in SH-SY5Y cells
The cytotoxicity of a series of diarylheptanoids was determined by MTS assay and curcumin, a well-known cytotoxic diarylheptanoid was used as positive control. The diarylheptanoids could inhibit the proliferation of SH-SY5Y cells in a dose-dependent manner (Figure. 1B). Among them, compound 1 and 2 are the most potent compounds, and even more efficacious than curcumin.
Fig. 1.
Cytotoxicity of the diarylheptanoids in SH-SY5Y cell line. A. structures of diarylheptanoids. B. Antiproliferation activity of diarylheptanoids in SH-SY5Y cells. Data shown represent the mean ± SD of four replicates.
Compound 1 induces S phase arrest in SH-SY5Y cells
We choose the most potent compound 1 to elucidate its cytotoxic mechanism in SH-SY5Y cells. Following treatment with compound 1 at various dosages for 48 h, consistent accumulations of S phase and apoptotic cells were observed in a dose-dependent manner (Figure. 2A). Since compound 1 can induce significant S phase arrest, we therefore perform BrdU incorporation assay to check the specific influence of compound 1 in DNA synthesis. After treatment with compound 1 at 5μg/ml for 24 h, the green signal from Brdu was less than that in control (Figure. 2B). This indicates that compound 1 inhibited DNA synthesis. Cell cycle is tightly governed by cyclins, cyclin-dependent kinases (CDKs) and cyclin-dependent kinase inhibitors (CDKIs). After drug treatment at different dosages for 48 h, cyclin A, cyclin B, cyclin E, CDK4 and Cdc2 were down regulated whereas p21 and p27 were up regulated (Figure. 2C).
Fig. 2.
Effects of compound 1 on cell cycle progression in SH-SY5Y cells. A. Compound 1 induces S phase cell cycle arrest in SH-SY5Y cells. 1, Control SH-SY5Y cells; 2-4, SH-SY5Y cells treatment with compound 1 at 2.5, 5 and 10 μg/ml for 48 h respectively. B. Compound 1 inhibits DNA synthesis. 1, Control SH-SY5Y cells; 2, SH-SY5Y cells treatment with compound 1 at 5 μg/ml for 24 h. C. Alteration of cell cycle related proteins following treatment with compound 1. Lane 1, Control SH-SY5Y cells; Lane 2-4, SH-SY5Y cells treatment with compound 1 at 2.5, 5 and 10 μg/ml for 48 h, respectively. The data shown were from one of the three independent experiments.
Compound 1 induces apoptosis in SH-SY5Y cells
Following treatment with compound 1 at 5 μg/ml for 48 h, chromatin aggregation, nuclear and cytoplasmic condensation and partition of cytoplasm and nucleus into membrane-bound vesicles were observed (Figure. 3A). Caspases and Bcl 2 are two main apoptosis related families. After treatment with compound 1 at different dosages for 48 h, procaspase 3 was down regulated, which meant that caspase 3 was up regulated and caspase 9 activity was significantly increased in a dose-dependent manner. PARP, the substrate of caspase 3, which serves as the hallmark of apoptosis, was cleaved into two bands. Meanwhile, Bcl 2 was decreased and bax was increased. In addition, the other two p53 transcriptional activated proteins, Apaf-1 and puma were up regulated (Figure 3B and 3C).
Fig. 3.
Compound 1 induces apoptosis in SH-SY5Y cells. A. Apoptotic morphology changes in SH-SY5Y cells. 1, Control SH-SY5Y cells; 2, SH-SY5Y cells treatment with compound 1 at 5 μg/ml for 48 h. B. Compound 1 induces caspase 9 activity in SH-SY5Y cells. C. Alteration of apoptotic related proteins. Lane 1, Control SH-SY5Y cells; Lane 2-4, SH-SY5Y cells treatment with compound 1 at 2.5, 5 and 10 μg/ml for 48 h, respectively.
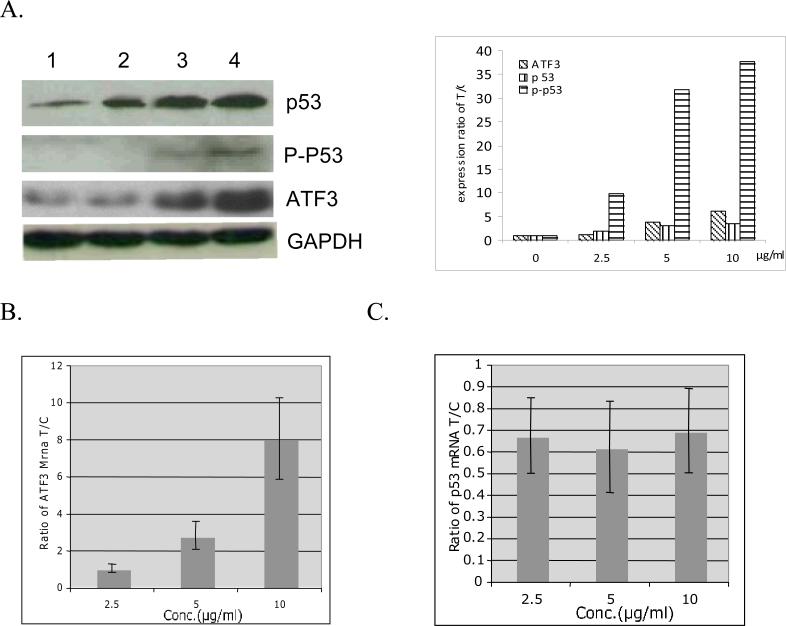
Influence of compound 1 on p53 and ATF3
P53 plays an important role in neuroblastoma cell death and ATF3 is always coincidently induced with p53 by many kinds of stimulations, we therefore checked the influence of compound 1 on ATF3 and p53. After treatment with compound 1 at different dosages for 48 h, the protein expression level of ATF3, p53 as well as p-p53 was up regulated. However, only ATF3 increased at the gene expression level whereas p53 gene expression level has no significant increase, in contrast, slightly decreased after treatment (Figure 4).
Fig. 4.
Influence of compound 1 on ATF3 and p53. A. Up regulation of protein expression of p53, p-p53 and ATF3 by compound 1. Lane 1, Control SH-SY5Y cells; Lane 2-4, SH-SY5Y cells treatment with compound 1 at 2.5, 5 and 10 μg/ml for 48 h, respectively. B. Stimulation mRNA expression of ATF3 by compound 1. Data show is mean ± SD of three independent experiments. C. Effect of compound 1 on mRNA expression of p53.
Compound 1 increases p53 stability
Since compound 1 could not increase the gene expression level of p53, the up regulation of p53 protein by compound 1 might be due to post-transcriptional regulation. Thereby, we conducted cycloheximide chase assay to investigate the influence of compound 1 on p53 protein stability. In our experiment, compound 1 can increase the stability of p53 protein. In addition, two p53 protein negative regulators MDM2 and Aurora A were down regulated upon compound 1 treatment (Figure 5).
Fig. 5.
Compound 1 increases p53 stability. A. Influence of compound 1 on p53 stability by using cycloheximide chase assay. Following treatment with vehicle or compound 1 at 5 μg/ml for 48 h, SH-SY5Y cells were then treated with cycloheximide at 50 μg/ml for the indicated times and harvested for western blotting. B. Down regulation of p53 negative regulators MDM2 and Aurora A by compound 1. Lane 1, Control SH-SY5Y cells; Lane 2-4, SH-SY5Y cells treatment with compound 1 at 2.5, 5 and 10 μg/ml for 48 h, respectively.
ATF3 knock down reduces apoptosis and cell cycle arrest triggered by compound 1
The role of ATF3 in tumorigenesis remains a controversy. In order to address the function of ATF3 in compound 1-triggered SH-SY5Y cell death, we knocked down ATF3 gene by ATF3 siRNA. ATF3 protein level was dramatically knocked down after transfection with ATF3 siRNA for 48 h. Following transfection, cells were treated with compound 1 at 5 μg/ml for an additional 24 h. Since we cannot completely knockdown a gene by 100%, ATF3 protein was partially stimulated by compound 1 post-transfection with ATF3 siRNA. However it was still less than ATF3 expression induced by compound 1 in control siRNA sample. In addition, knockdown of ATF3 gene partially decreased the caspase 3 (increased procaspase 3) and p53 expression induced by compound 1. As a result, cell cycle arrest and apoptosis induced by compound 1 were apparently inhibited by knockdown of ATF3 (Figure 6).
Fig. 6.
ATF3 knock down reduces apoptosis and cell cycle arrest triggered by compound 1. A. Knockdown of ATF3 gene partially decreased the caspase 3 and p53 expression induced by compound 1. Following 48 h transfection, SH-SY5Y cells were treated with vehicle or compound 1 for additional 24 h and harvested for western blotting. B. Knockdown of ATF3 genes partially reduced S phase arrest and apoptosis triggered by compound 1. 1, Control SH-SY5Y cells; 2, SH-SY5Y cells transfected with control siRNA for 48 h; 3, SH-SY5Y cells transfected with ATF3 siRNA for 48 h; 4, SH-SY5Y cells transfected with ATF3 siRNA for 48 h and treatment with compound 1 at 5 μg/ml for additional 24 h; 5, SH-SY5Y cells transfected with control siRNA for 48 h and treatment with compound 1 at 5 μg/ml for additional 24 h. C. S phase arrest and apoptosis induced by compound 1 were inhibited by knockdown of ATF3.
Discussion
Induction of cell cycle arrest and apoptosis has been implicated as an important mechanism underlying the cytotoxic activities of many cancer chemotherapeutic agents [9, 29, 32]. In our present study, we investigated the cytotoxicity and mechanisms of diarylheptanoids in SH-SY5Y cells. We found that compound 1, the most effective diarylheptanoid could inhibit the proliferation of SH-SY5Y cells via inducing S phase arrest and apoptosis. It is well known that the cell cycle is governed by cyclins, CDKs and CDKIs. Among them, Cyclin A is required for both the initiation and elongation of DNA in the late G1 and S phase as well as involving in the activation of cdc2-cyclin B complex[25, 34]. Meanwhile, p21 and p27 inhibit Cyclin A to block cell cycle progression from S phase to G2/M phase. In our experiments, following drug treatment, DNA synthesis was prominently inhibited; Cyclin A, Cyclin B, Cyclin E, cdc2, cdk4 were down regulated whereas p21 and p27 were up regulated. These suggest that suppression of Cyclin A and increase of p21 and p27 as well as inhibition of DNA synthesis may be implicated in the S phase arrest induced by compound 1.
Unlike the majority of other human cancers, which is defect in p53, neuroblastomas usually carry wild-type p53 [15, 27, 35]. Although cytoplasmic sequestration of p53 has been proposed as a mechanism for inactivation of the function of p53 in neuroblastoma cells, numerous studies reveal that the p53 signaling pathway is functional in various neuroblastoma cell lines examined[8]. P53 plays a key role in genotoxicity mediate apoptosis. In response to various of stimuli induced DNA damage, p53 protein accumulates rapidly through a post transcriptional mechanism(s) and is also activated as a transcription factor, which leads to cytostasis or apoptosis. Phosphorylation of p53 at Ser20 leads to reduced interaction of p53 with its negative regulator, MDM2 and enhances its tetramerization, stability and activity [4, 12, 28]. MDM2 inhibits the accumulation of p53 by targeting it for ubiquitination and proteasomal degradation [13, 33]. In addition, Aurora A, another p53 negative regulator could suppress p53 function via at least two mechanisms: first, Aurora A phosphorylates Ser315 of p53 leading to its MDM2 association and degradation[14]; second, Aurora A also phosphorylates p53 at Ser215 resulting in inactivation of its transcriptional activity[18, 22]. In the light of our study, following drug treatment, the protein expression of p53, phospho-p53 (Ser20) as well as the p53 transcriptional activated genes, Bax, puma, Apaf-1 and ATF3 were increased dramatically; MDM2 and Aurora A, the two p53 negative regulators were decreased; the p53 protein stability was enhanced whereas the p53 mRNA expression level slightly decreased. Our results suggest that compound 1 increase the p53 activity and stability via post-transcriptional regulation. The slightly down regulation of p53 mRNA expression might be due to the negative feedback of over expression of p53 protein.
ATF3 (activating transcription factor 3), transcription factor of ATF/CREB family plays an important role in carcinogenesis via influence of cell death and cell cycle progression [5, 7, 10, 17]. Several lines of evidence demonstrated that ATF3 directly binds p53 and is coincidently induced with p53 by a broad spectrum of stimulations [11, 38]. In addition, P53 transcriptional activates ATF3 and in turn, ATF3 increases p53 stability [39]. However, there is still a controversy about the function of ATF3 in apoptotic cell death. Most approaches both from gain-of-function and loss-of-function support a pro-apoptotic role of ATF3 [19]. In particular, ATF3 is a novel contributor to the proapoptotic effect of curcumin, which has similar structure as our tested compounds [37]. On the other hand, several reports suggest that ATF3 is anti-apoptotic [24]. In our study, compound 1 induced expression of ATF3 both on protein level and mRNA level. Furthermore, knockdown of ATF3 gene by siRNA partially suppressed p53, caspase 3(increased procaspase 3), S phase arrest and apoptosis induced by compound 1. These results indicate that ATF3 is functional as a pro-apoptotic protein in compound 1 induced cell death.
Overall, compound 1 induces S phase arrest and apoptosis via up regulation of ATF3 and stabilization of p53 in SH-SY5Y cells. It might be a prospective neuroblastoma therapeutics after structure modification to increase the cytotoxicity and improve the solubility.
Acknowledgements
The research was supported by National Institute of Health training grant # 2 T15 LM007092-16 and National Nature Science Foundation of China grant # 30470195. We wish to extend our thanks to Ronald E. Vincent (BIOCON SCIENTIFIC) for thoughtful reading of the manuscript.
References
- 1.An N, Zou ZM, Tian Z, Luo XZ, Yang SL, Xu LZ. Diarylheptanoids from the rhizomes of Alpinia officinarum and their anticancer activity. Fitoterapia. 2008;79:27–31. doi: 10.1016/j.fitote.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 3.Castel V, Grau E, Noguera R, Martinez F. Molecular biology of neuroblastoma. Clin Transl Oncol. 2007;9:478–83. doi: 10.1007/s12094-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 4.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci U S A. 1999;96:13777–82. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho RJ, Huang M, Campbell MJ, Dong H, Steinmetz L, Sapinoso L, Hampton G, Elledge SJ, Davis RW, Lockhart DJ. Transcriptional regulation and function during the human cell cycle. Nat Genet. 2001;27:48–54. doi: 10.1038/83751. [DOI] [PubMed] [Google Scholar]
- 6.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–24. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 7.Fan F, Jin S, Amundson SA, Tong T, Fan W, Zhao H, Zhu X, Mazzacurati L, Li X, Petrik KL, Fornace AJ, Jr., Rajasekaran B, Zhan Q. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21:7488–96. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 8.Goldman SC, Chen CY, Lansing TJ, Gilmer TM, Kastan MB. The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization. Am J Pathol. 1996;148:1381–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Goldsmith KC, Hogarty MD. Targeting programmed cell death pathways with experimental therapeutics: opportunities in high-risk neuroblastoma. Cancer Lett. 2005;228:133–41. doi: 10.1016/j.canlet.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 11.Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–7. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 13.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–7. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 14.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 15.Kurata K, Yanagisawa R, Ohira M, Kitagawa M, Nakagawara A, Kamijo T. Stress via p53 pathway causes apoptosis by mitochondrial Noxa upregulation in doxorubicin-treated neuroblastoma cells. Oncogene. 2007 doi: 10.1038/sj.onc.1210672. [DOI] [PubMed] [Google Scholar]
- 16.Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100:237–43. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 17.Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J Biol Chem. 1996;271:1695–701. doi: 10.1074/jbc.271.3.1695. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, Cheng JQ. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279:52175–82. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Wolfgang CD, Hai T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem. 2006;281:10473–81. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- 20.Ly TN, Shimoyamada M, Kato K, Yamauchi R. Isolation and characterization of some antioxidative compounds from the rhizomes of smaller galanga (Alpinia officinarum Hance). J Agric Food Chem. 2003;51:4924–9. doi: 10.1021/jf034295m. [DOI] [PubMed] [Google Scholar]
- 21.Ly TN, Yamauchi R, Shimoyamada M, Kato K. Isolation and structural elucidation of some glycosides from the rhizomes of smaller galanga (Alpinia officinarum Hance). J Agric Food Chem. 2002;50:4919–24. doi: 10.1021/jf025529p. [DOI] [PubMed] [Google Scholar]
- 22.Mao JH, Wu D, Perez-Losada J, Jiang T, Li Q, Neve RM, Gray JW, Cai WW, Balmain A. Crosstalk between Aurora-A and p53: frequent deletion or downregulation of Aurora-A in tumors from p53 null mice. Cancer Cell. 2007;11:161–73. doi: 10.1016/j.ccr.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 24.Nobori K, Ito H, Tamamori-Adachi M, Adachi S, Ono Y, Kawauchi J, Kitajima S, Marumo F, Isobe M. ATF3 inhibits doxorubicin-induced apoptosis in cardiac myocytes: a novel cardioprotective role of ATF3. J Mol Cell Cardiol. 2002;34:1387–97. doi: 10.1006/jmcc.2002.2091. [DOI] [PubMed] [Google Scholar]
- 25.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. Embo J. 1992;11:961–71. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronca F, Yee KS, Yu VC. Retinoic acid confers resistance to p53-dependent apoptosis in SH-SY5Y neuroblastoma cells by modulating nuclear import of p53. J Biol Chem. 1999;274:18128–34. doi: 10.1074/jbc.274.25.18128. [DOI] [PubMed] [Google Scholar]
- 28.Shieh SY, Taya Y, Prives C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. Embo J. 1999;18:1815–23. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–74. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 30.Steward WP, Gescher AJ. Curcumin in cancer management: Recent results of analogue design and clinical studies and desirable future research. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200700148. [DOI] [PubMed] [Google Scholar]
- 31.Tian Z, Shen J, Moseman AP, Yang Q, Yang J, Xiao P, Wu E, Kohane IS. Dulxanthone A induces cell cycle arrest and apoptosis via up-regulation of p53 through mitochondrial pathway in HepG2 cells. Int J Cancer. 2008;122:31–8. doi: 10.1002/ijc.23048. [DOI] [PubMed] [Google Scholar]
- 32.Tian Z, Yang M, Huang F, Li K, Si J, Shi L, Chen S, Xiao P. Cytotoxicity of three cycloartane triterpenoids from Cimicifuga dahurica. Cancer Lett. 2005;226:65–75. doi: 10.1016/j.canlet.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Van Maerken T, Speleman F, Vermeulen J, Lambertz I, De Clercq S, De Smet E, Yigit N, Coppens V, Philippe J, De Paepe A, Marine JC, Vandesompele J. Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma. Cancer Res. 2006;66:9646–55. doi: 10.1158/0008-5472.CAN-06-0792. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Xia D, Li N, Wang C, Chen T, Wan T, Chen G, Cao X. Bone marrow stromal cell-derived growth inhibitor inhibits growth and migration of breast cancer cells via induction of cell cycle arrest and apoptosis. J Biol Chem. 2005;280:4374–82. doi: 10.1074/jbc.M408708200. [DOI] [PubMed] [Google Scholar]
- 35.Xue C, Haber M, Flemming C, Marshall GM, Lock RB, MacKenzie KL, Gurova KV, Norris MD, Gudkov AV. p53 determines multidrug sensitivity of childhood neuroblastoma. Cancer Res. 2007;67:10351–60. doi: 10.1158/0008-5472.CAN-06-4345. [DOI] [PubMed] [Google Scholar]
- 36.Yadav PN, Liu Z, Rafi MM. A diarylheptanoid from lesser galangal (Alpinia officinarum) inhibits proinflammatory mediators via inhibition of mitogen-activated protein kinase, p44/42, and transcription factor nuclear factor-kappa B. J Pharmacol Exp Ther. 2003;305:925–31. doi: 10.1124/jpet.103.049171. [DOI] [PubMed] [Google Scholar]
- 37.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4:233–41. [PubMed] [Google Scholar]
- 38.Yan C, Lu D, Hai T, Boyd DD. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. Embo J. 2005;24:2425–35. doi: 10.1038/sj.emboj.7600712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Gao C, Kawauchi J, Hashimoto Y, Tsuchida N, Kitajima S. Transcriptional activation of the human stress-inducible transcriptional repressor ATF3 gene promoter by p53. Biochem Biophys Res Commun. 2002;297:1302–10. doi: 10.1016/s0006-291x(02)02382-3. [DOI] [PubMed] [Google Scholar]