Abstract
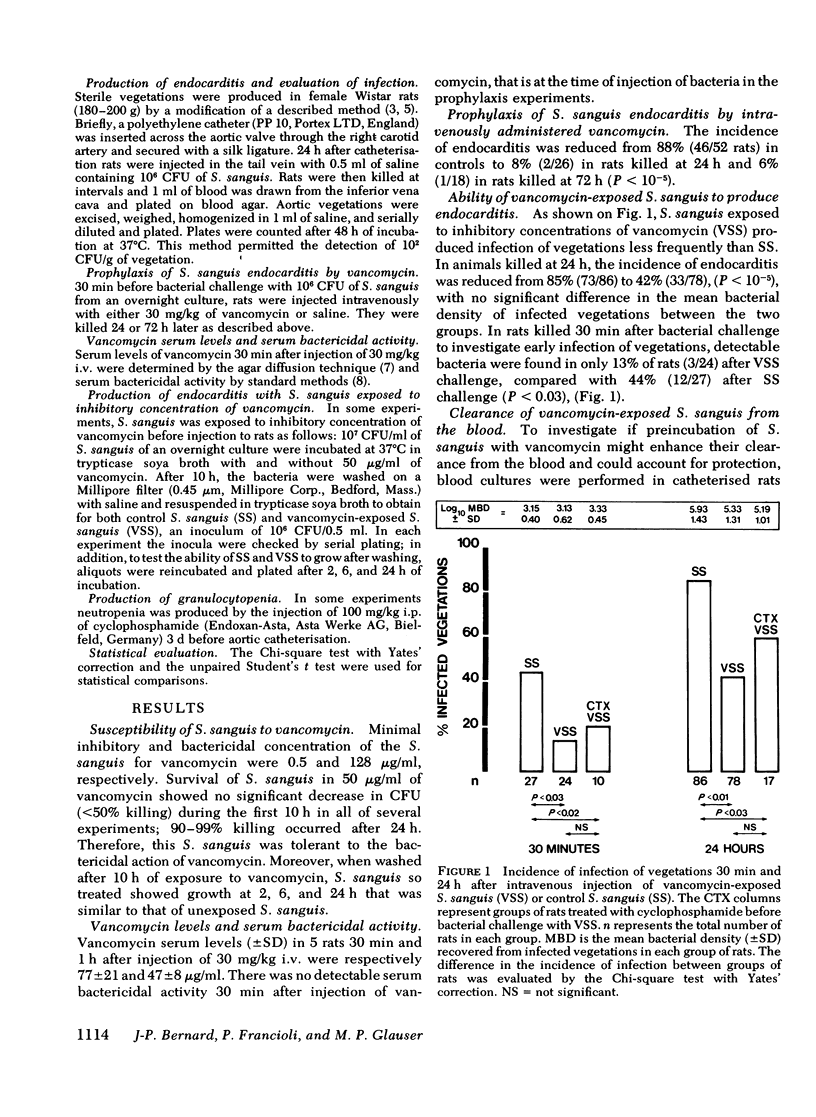
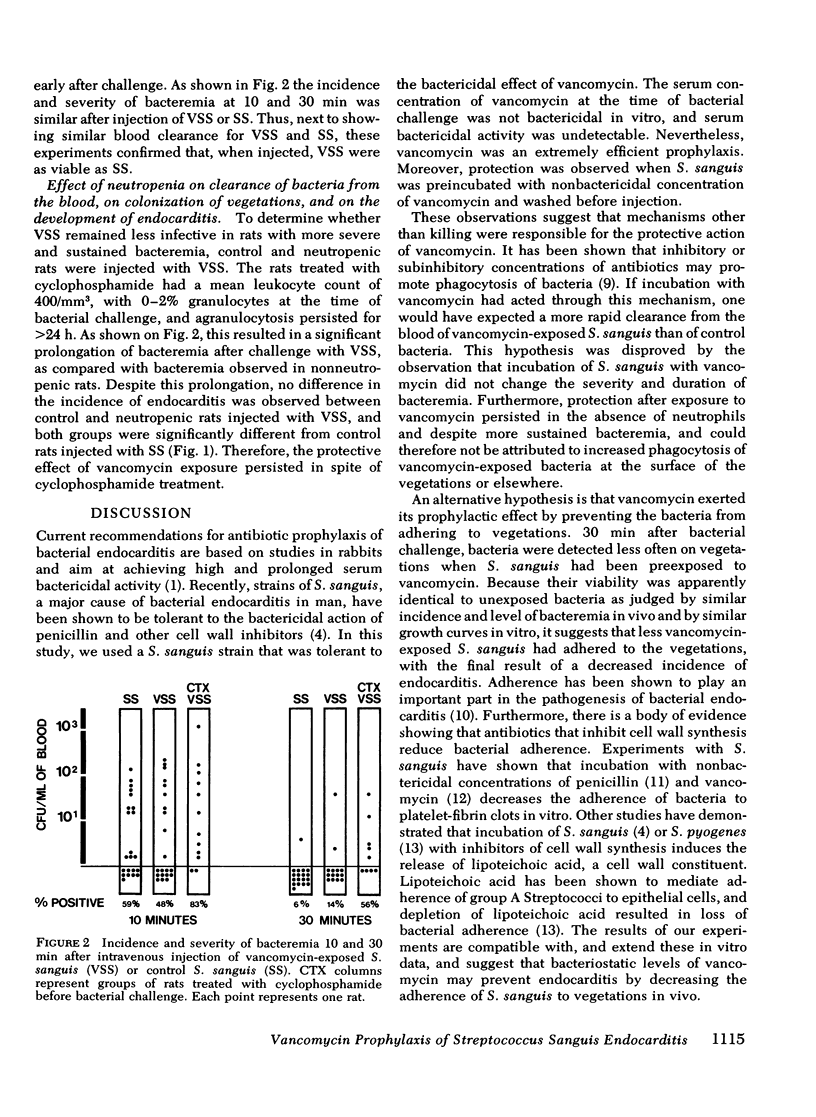
Using a strain of Streptococcus sanguis tolerant to vancomycin to infect aortic vegetations in rats, we found that prophylactic intravenous vancomycin given 30 min before bacterial challenge decreased the incidence of endocarditis from 88 to 8% (P less than 10(-5)). Because peak vancomycin serum levels were below the minimal bactericidal concentration, mechanisms of protection other than bacterial killing were investigated. S. sanguis were incubated with inhibitory concentration of vancomycin (50 microgram/ml) for 10 h and washed. 85% of rats (73/86) inoculated with control bacteria developed endocarditis, whereas only 42% (33/78) of those inoculated with vancomycin-exposed bacteria did so (P less than 10(-5)). When rats were killed 30 min after bacterial challenge, S. sanguis were detected by culture of the vegetations in 44% of rats injected with control bacteria, but in only 13% of those challenged with vancomycin-exposed bacteria (P less than 0.03). Enhanced clearance of vancomycin-exposed streptococci was not responsible for this protection because blood cultures showed no difference in the level and duration of bacteremia after injection of control or vancomycin-exposed S. sanguis. Moreover, this protection was not abolished in neutropenic rats injected with vancomycin-exposed bacteria, despite more prolonged bacteremia. These results suggest that vancomycin exerted its protection by lowering adherence of tolerant S. sanguis to vegetations rather than through bactericidal activity or enhanced clearance of bacteria by phagocytic cells. In the choice of antibiotics for prophylaxis of endocarditis, reduction of bacterial adhesion may be a criterion as important as bacterial killing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. B., Valone J. A., Askenase P. W., Kashgarian M., Freedman L. R. Diffuse glomerulonephritis in rabbits with Streptococcus viridans endocarditis. Lab Invest. 1975 Jun;32(6):681–689. [PubMed] [Google Scholar]
- Durack D. T., Petersdorf R. G. Chemotherapy of experimental streptococcal endocarditis. I. Comparison of commonly recommended prophylactic regimens. J Clin Invest. 1973 Mar;52(3):592–598. doi: 10.1172/JCI107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. J., Wetherall B. L., Pruul H. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981 Jan-Feb;3(1):38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick F. S., Durack D. T. Chemotherapy of experimental streptococcal endocarditis. 3. Failure of a bacteriostatic agent (tetracycline) in prophylaxis. J Clin Pathol. 1974 Apr;27(4):261–264. doi: 10.1136/jcp.27.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]



