Abstract
Pancreatic cancer significantly affects the quality of life due to the severe abdominal pain. However, the underlying mechanism is not clear. This study aimed to determine the relationship between substance P (SP) and pancreatic cancer perineural invasion (PNI) as well as mechanism of SP mediating pancreatic cancer PNI which cause pain in patients with pancreatic cancer. Human pancreatic cancer cells MIA PaCa-2, BxPC-3 and newborn dorsal root ganglions (DRGs) were used to determine the expression of SP or NK-1R in pancreatic cancer cells and DRGs cells by QT-PCR and Western blotting. The effects of SP on pancreatic cancer cell proliferation and invasion were analyzed using MTT assay and Transwell matrigel invasion assay, respectively. Alterations in the neurotropism of pancreatic cancer cells were assessed by co-culture system which mimics the interaction of tumor/neuron in vivo. SP is not only widely distributed in the neurite outgrowth from newborn DRGs but also expressed in MIA PaCa-2 and BxPC-3 cells. NK-1R is found to be overexpressed in the pancreatic cancer cell lines MIA PaCa-2 and BxPC-3. SP induces cancer cell proliferation and invasion and the expression of MMP-2 in pancreatic cancer cells; and NK-1R antagonists inhibit these effects. Furthermore, SP is also able to promote neurite outgrowth and the migration of pancreatic cancer cell cluster to the DRGs, which is blocked by NK-1R antagonists in the co-culture model. Our results suggest that SP plays an important role in the development of pancreatic cancer metastasis and PNI, and blocking the SP/NK-1R signaling system is a novel strategy for the treatment of pancreatic cancer.
Keywords: Substance P (SP), NK-1 receptor (NK-1R), pancreatic cancer, perineural invasion (PNI)
Introduction
Pancreatic cancer is a deadly disease with a mortality rate very near to the incidence rate. A lack of early symptoms, explosive outcomes, short survival, and resistance to therapy are hallmarks of this type of cancer (1). The treatment of pancreatic cancer has not improved substantially during the past 30 years. Perineural invasion (PNI) in pancreatic cancer is a common pathologic phenomenon. It has been reported that all of pancreatic tumors would reveal PNI if enough sections are evaluated (2), of which up to 90% of patients have intra-pancreatic nerves infiltrated by tumor cells and 69% have involvement of the extra-pancreatic nerve terminations. The presence of tumor cells in the perineurium space of local peripheral nerves in the pancreas may be associated with a higher risk of retropancreatic tumor extension, precluding curative resection and promoting local recurrence after tumor resection.
Pancreatic cancer significantly affects the quality of life due to the chronic symptoms and severe abdominal pain (3-4). Substance P (SP) is an undecapeptide, released from primary sensory nerve fibers, that belongs to the tachykinin family, which has been implicated in a myriad of physiological processes (5-6). SP is widely expressed in the central and peripheral nervous system as well as in peripheral tissues such as B and T cells in an autocrine or paracrine manner (7) and macrophages (8). NK-1R, a receptor of SP, is overexpressed in several normal (10-12) and neoplastic cell types (13-19). SP regulates many biological functions; and has also been implicated in neurogenic inflammation, pain, and depression (6,9). Up on the binding of SP to its high affinity receptor, NK-1R, SP initiates multiple biological functions including tumor cell proliferation, angiogenesis (10), and migration which are critical for tumor cells invasion and metastasis (7,11), These biological functions can reversed by the NK-1R antagonists. These reports suggest that SP/NK-1R signaling may play an important role in the cancer progression and metastasis, as SP may be a mitogen in NK-1R-expressing tumor cell types (11-12). Therefore, targeting the NK-1 receptor could be a promising approach for treating patients with cancer; and NK-1 receptor antagonists could improve cancer treatment .
Friess and co-workers (13), in their pioneer work on the expression of NK-1R, showed that the expression levels of NK-1R mRNA and protein in human pancreatic cancer samples were increased 36.7-fold and 26.0-fold, respectively, compared with normal controls. Enhanced NK-1R levels in the tumor tissues were associated with advanced tumor stage and poorer prognosis. SP analogs stimulate pancreatic cancer cell growth, depending on the NK-1R expression level, and this effect could be blocked by a selective NK-1R antagonist. These findings suggest that there may be a neuro-cancer cell interaction in vivo (13).
The pancreas is an organ with rich innervations that are associated with PNI in pancreatic cancer (14). We reason that SP stimulating NK-1R which is overexpressed in tumor cells and in the tumor and peritumoral tissue (7) may be a molecular mechanism for tumor cells to develop PNI.
To date, the relationship between SP and pancreatic cancer metastasis and PNI has not been reported. The purpose of the present study was to test whether SP/NK-1R signaling could influence the progression of pancreatic cancer. Our data suggest that SP plays an important role in the development of pancreatic cancer by inducing cell proliferation, metastasis, and PNI; and blocking the SP/NK-1R signaling may be a novel strategy for the treatment of pancreatic cancer.
Materials and methods
Cell lines, animals, and reagents
The human pancreatic tumor cell lines MIA PaCa-2, BxPC-3, CFPAC-1, HAPC, Panc-1, and SW1990 were obtained from ATCC (American Type Culture Collection) (15). Newborn rats were purchased from the laboratory animal center of the Xi'an Jiaotong University. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). Polyclonal anti-NK-1R and polyclonal anti-SP antibodies were bought from Sigma-Aldrich (USA). A polyclonal anti-human MMP-2 antibody was obtained from Santa Cruz Biotechnology (USA). SP acetate salt (Sigma-Aldrich, USA) was dissolved in distilled water, and different concentrations of SP (5, 10, 50, 100 and 120 nM) were evaluated. (2S, 3S) 3- ([3, 5-Bis(trifluoromethyl)phenyl] methoxy)-2-phenylpiperidine hydrochloride (L-733,060) was procured from Tocris Cookson (Bristol, UK). N-Acetyl-L-tryptophan 3, 5-bis (trifluoromethyl) benzyl ester (L-732,138) was purchased from Tocris Cookson (Bristol, UK). Unless otherwise specified, all other reagents were required from Invitrogen (Carlsbad, CA). All experiments were performed in triplicate.
Cell lines and rat dorsal root ganglions (DRGs)
Pancreatic cancer cells were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), 50 U/ml penicillin G, 50 μg/ml streptomycin sulfate, and 25 mM glucose at 37°C in a humidified 5% CO2, 95% air atmosphere. Newborn rats were euthanized with carbon dioxide and sterilized with 75% ethanol. DRGs from the lumbar areas were dissected, collected into medium (DMEM), stripped of meninges and nerve stumps, and plated into a drop of liquid Matrigel (BD Biosciences, Oxford, UK). After solidification, medium (DMEM containing 0 nM, 5 nM, 10 nM, 50 nM or 100 nM SP) was carefully added and renewed every two days (16-17).
Immunocytochemical staining
For fluorescence immunocytochemistry, pancreatic tumor cell lines and neurite outgrowths from newborn DRGs were fixed for 30 min in 4% paraformaldehyde in PBS and the endogenous peroxidase activity was quenched by 3% hydrogen peroxide. The specimens were pre-blocked for 30 min with bovine serum albumin (BSA) at 37°C and incubated with primary antibody against SP (1:100) or NK-1R (1:100) overnight at 4°C. Then staining was detected with FITC-conjugated goat anti-rabbit IgG antibody or CY3-conjugated goat anti-rabbit IgG antibody (Jackson Immuno Research). Slides were mounted and then examined by a Nikon Instruments confocal microscope (Nikon Instruments Inc, Shanghai, China).
Reverse transcription-PCR (RT-PCR) and real time quantitative PCR (QPCR)
Total RNA from PC cells or DRGs were extracted using a Fastgen200 Kit RNA isolation system (Fastgen, Shanghai, China) according to the manufacturer's protocol. Total RNA was reverse-transcribed into cDNA using the Fermentas RevertAidTM Kit (MBI Fermentas, Canada). The primer sequences were as follows:
SP -F: 5’-GACTCCTCTGACCGCTAC-3’.
SP -R: 5’- AGACCTGCTGGATGAACT-3’.
NK-1R -F: 5’-AGGTTCCGTCTGGGCTTCAA-3’.
NK-1R -R: 5’-TCCAGGCGGCTGACTTTGTA-3’.
MMP-2 -F: 5’-GATGATGCCTTTGCTCGTGC-3’.
MMP-2 -R: 5’-CAAAGGGGTATCCATCGCCA-3’.
β-ACTIN -F: 5’-GACTTAGTTGCGTTACACCCTTTCT-3’.
β-ACTIN -R: 5’-GAACGGTGAAGGTGACAGCAGT-3’.
RT-PCR products were resolved by 1.5% agarose gel electrophoresis. After each QT–PCR, a dissociation curve analysis was conducted. Relative gene expression was calculated using the 2−ΔΔCt method reported previously (18). Each measurement was performed in triplicate.
Western blotting
Proteins were electrophoretically resolved on a denaturing SDS polyacrylamide gel and electrotransferred onto nitrocellulose membranes. The membranes were initially blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) for 2 h and then probed with antibodies against SP, NK-1R, MMP-2 or β-ACTIN. After co-incubation with the primary antibodies at 4°C overnight, the membranes were hybridized with secondary goat anti-mouse antibody or goat anti-rabbit antibody (Sigma-Aldrich, USA) for 2 h at room temperature. Immunopositive bands were developed using an enhanced chemiluminescence (ECL) detection system (Amersham Bioscience, Piscataway, NJ, USA). All analyses were done in duplicate.
MTT assay
MIA PaCa-2 and BxPC-3 cells were seeded in 96-well tissue culture plates at a density of 5,000-10,000 cells per well 24 h prior to serum starvation. After serum starvation for 24 h, cells were cultured in DMEM with different concentrations of SP (0 nM, 5 nM, 10 nM, 50 nM, 100 nM, and 120 nM), L-733,060 (0 μM, 5 μM, 10 μM, 20 μM, 30 μM, and 40 μM) or L-732,138 (0 μM, 20 μM, 40 μM, 60 μM, 80 μM, and 100 μM) and incubated at 37°C. After 24, 48 or 72 h, the medium was removed, and MTT reagent (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide) was added to each well and incubated at 37°C for 4 hours. Optical densities (OD) at 490 nm were measured by a microplate reader (BIO-TEC Inc, VA). The proliferation rate was defined as OD (cells plate)/OD (control plate).
Transwell Matrigel invasion assay
An invasion assay was performed with a Millicell invasion chamber (Millipore, Billerica, MA, USA). The 8 μM pore inserts were coated with 25 μg of Matrigel (Becton Dickinson Labware, Bedford, MA, USA). After serum starvation for 24 h, MIA PaCa-2 and BxPC-3 cells (5 × 104) were seeded in the top chamber and medium with SP or NK-1R antagonists were also added to the bottom chamber to induce the cancer cell lines. The Matrigel invasion chamber was incubated for 48 h in a humidified tissue culture incubator. Non-invading cells were removed from the top of the Matrigel with a cotton-tipped swab. Invading cells on the bottom surface of filter were fixed in methanol and stained with Crystal Violet (Boster Biological Technology Ltd., Wuhan, China). Invasion ability was determined by counting the stained cells.
Co-culture assay
Co-culture experiments were performed using a modified method based on previously described methods (16-17). The DRGs were kept on ice after collection in DMEM medium (Invitrogen, Carlsbad, CA) and subsequently seeded on 24-well petri dishes in 25 μl of Matrigel gel as described above. BxPC-3 cells were suspended in 25 μl of solidified Matrigel and placed next to the DRG suspension. To exclude the possibility of a non-specific guided migration of BxPC-3 cells, an additional 25 μl of Matrigel containing no neural cells was positioned on the opposite side. The petri dishes were then placed for 20 min in an incubator at 37°C saturated with 5% CO2 in a humid atmosphere to allow polymerization of the Matrigel. After solidification, medium (DMEM containing SP or/ and NK-1R antagonists) was added and renewed every two days. Photographic documentation of the two adjacent sides of the cell suspensions was performed with an inverted light microscope imaging system (Ti-E; Nikon Instruments Inc, Shanghai, China) and analytical system (NIS BR3.0; Nikon Instruments Inc, Shanghai, China).
Statistical analysis
Statistical analysis was done with SPSS software (version 17.0, SPSS Inc., Chicago, USA). Data were presented as the means ± SEM of three replicate assays. Differences between the groups were analyzed by analysis of variance (ANOVA), followed by the Bonferroni's correction for multiple comparisons. P < 0.05 was considered statistically significant. All experiments were repeated independently at least three times.
Results
SP is mainly expressed in DRGs while NK-1R is expressed in pancreatic cancer cells
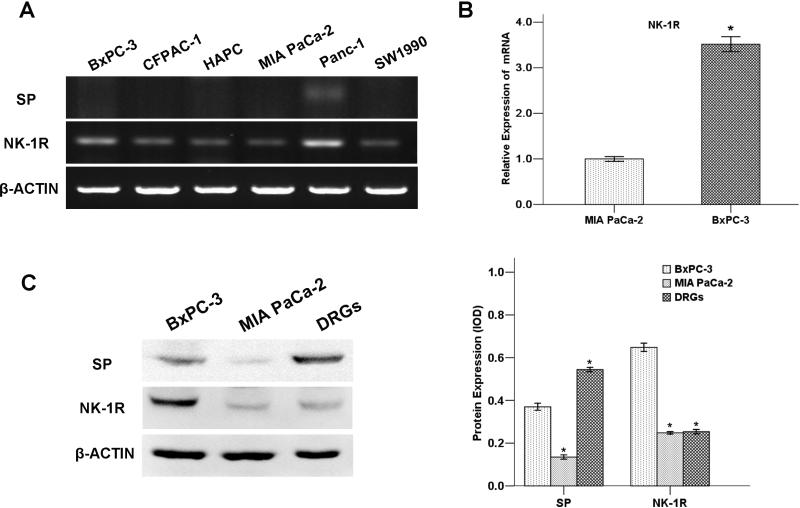
To determine whether SP or NK-1R is expressed in pancreatic cancer cells, we tested six pancreatic cancer cell lines: MIA PaCa-2, BxPC-3, CFPAC-1, HAPC, Panc-1 and SW1990. As shown in figure 1A, the expression of SP in pancreatic cancer cells at mRNA level was low. Among the six cell lines, the expression levels of NK-1R from high to low are in the following order: Panc-1> BxPC-3> CFPAC-1> SW1990> HAPC > MIA PaCa-2 (Figure 1B).
Fig. 1. Expression of SP and NK-1R in pancreatic cancer cells and DRGs.
(A) The mRNA level of SP and NK-1R in six pancreatic cancer cell lines: MIA PaCa-2, BxPC-3, CFPAC-1, HAPC, Panc-1, and SW1990. (B) Analysis of expression levels of NK-1R in BxPC-3 and MIA PaCa-2 by QT-PCR. (C) The protein level of SP and NK-1R in DRGs and pancreatic cancer cell lines BxPC-3 and MIA PaCa.
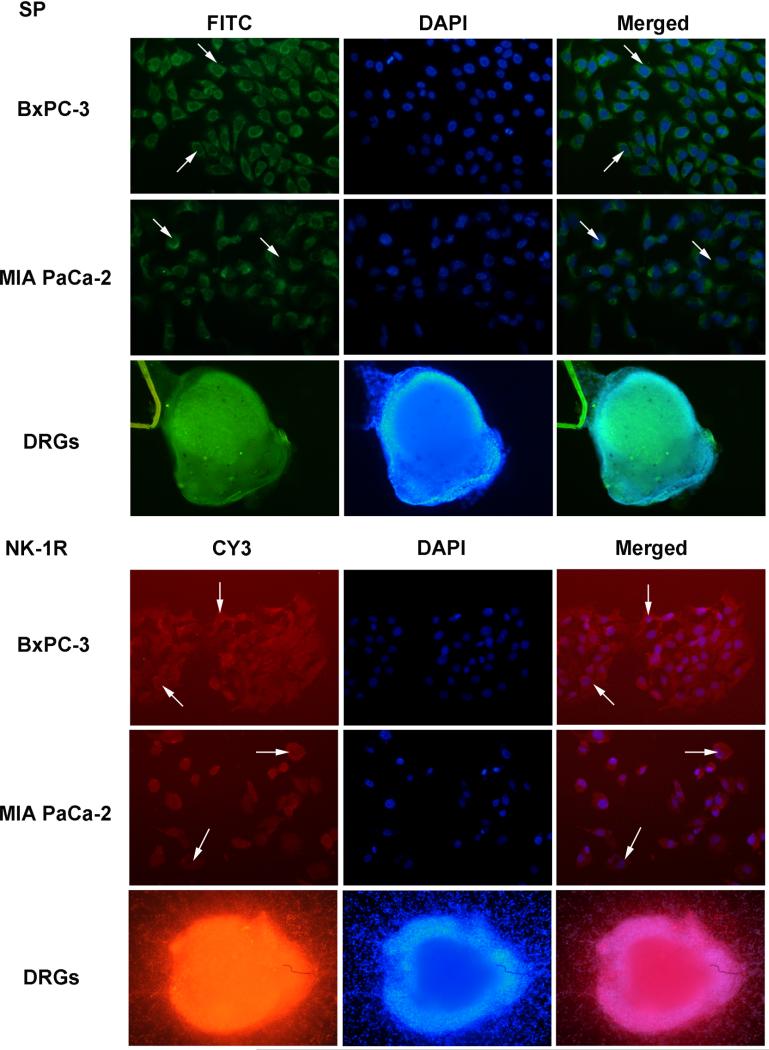
The expression of NK-1R and SP was also tested by Western blotting (Figure 1C) and immunofluorescence (Figure 2) in BxPC-3, MIA PaCa-2 and DRGs. In the two pancreatic cancer cell lines MIA PaCa-2 and BxPC-3, the NK-1R was visualized as a single band 45 kDa. A higher expression level of the NK-1 receptor was present in BxPC-3. SP was also detected in these two cell lines, whereas the expression of SP was much higher present in the neurite outgrowth from newborn DRGs.
Fig. 2. Expression of SP and NK-1R protein in BxPC-3, MIA PaCa-2 cells and DRG.
Cells were labeled with fluorescence-conjugated SP specific antibody (green) and fluorescence-conjugated NK-1R specific antibody (red) in both pancreatic cancer cells (200×) and DRG (100×). Nucleus was stained with DAPI. Arrows indicate that both SP and NK-1R were expressed in cancer cell plasma.
SP induces proliferation of pancreatic cancer cells
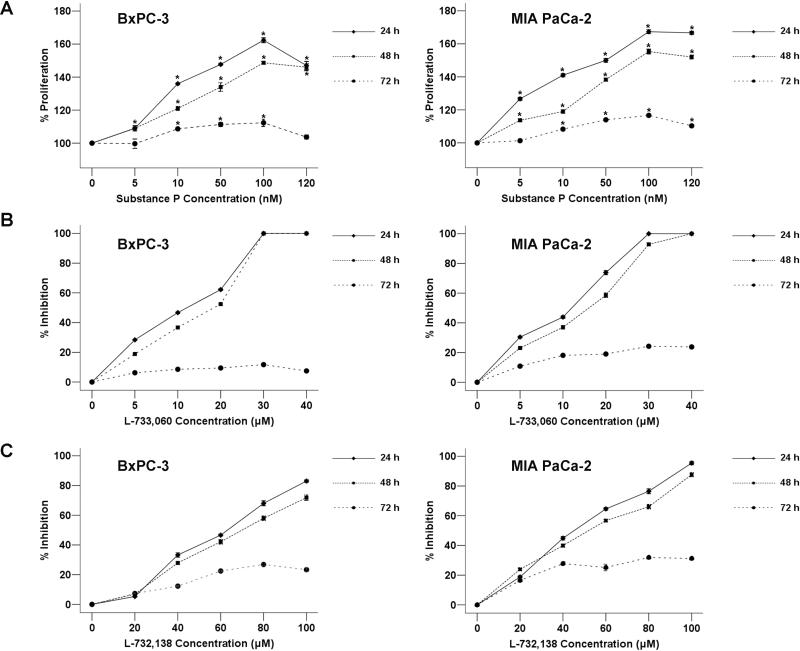
To determine the effects of SP on pancreatic cancer cell growth, we cultured BxPC-3 and MIA PaCa-2 cells in the media containing increasing concentrations (5 nM to 120 nM) of SP and the effects on cell proliferation were determined using MTT assay. The results showed that SP induced cell proliferation in BxPC-3 cells with statistical significance in a dose-dependent manner after incubation for 24, 48 or 72 h with 5 nM to 100 nM SP while proliferation rate in 120 nM begins to drop (Figure 3A). A similar effect was observed in MIA PaCa-2 cancer cells.
Fig. 3. The effect of SP and/or NK-1R antagonists on cancer cell proliferation.
(A) Induction of cell proliferation of human pancreatic tumor cell lines MIAPaCa-2 and BxPC-3 by SP at several nanomolar concentrations (0, 5, 10, 50, 100, and 120 nM). In both cases, SP induced cell proliferation. Using the ANOVA test, a significant difference between each group and the control group was found. *P < 0.05 as compared with control group. The percentage of growth inhibition of human pancreatic cancer BxPC-3 and MIA PaCa-2 cells treated by increasing concentrations (0–40 μM) of L-733,060 (B) or (0-100 μM) of L-732,138 (C) at 24 h, 48 h, and 72 h. The concentrations of NK-1R antagonists that less than IC50 were selected (L-733,060 [10 μM] and L-732,138 [60 μM in BxPC-3 and 40 μM in MIA PaCa-2]).
NK-1R antagonists inhibit pancreatic cancer growth
We next treated both BxPC-3 and MIA PaCa-2 cells with two different NK-1R antagonists: L-733,060 which showed high affinity for the human NK-1R in vitro and L-732,138 that showed a competitive antagonism for the receptor (19). Treatment of pancreatic cancer cells with the antagonists resulted in a concentration-dependent cytotoxicity in both cell lines. The 50% inhibitory concentration (IC50) of L-733,060 in both pancreatic cancer cell lines BxPC-3 and MIA PaCa-2 was approximately 10 μM. Furthermore, the IC50 of L-732,138 was approximately 60 μM in BxPC-3 while it is 40 μM in MIA PaCa-2 (Figure 3B, C).
SP/NK-1R signaling system promotes invasion of pancreatic cancer cells
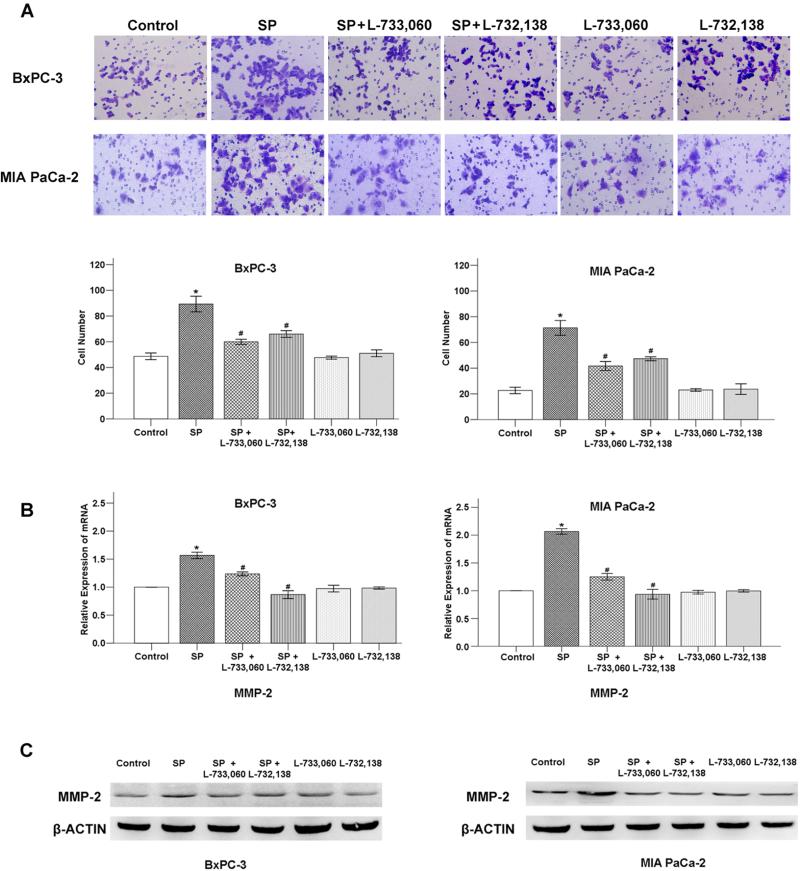
Next, we tested the effect of SP on pancreatic cancer cell invasion in vitro. The results show that the number of invasive BxPC-3 and MIA PaCa-2 cells increased with the addition of 100 nM SP compared to the control and this effect was significantly inhibited by NK-1R antagonists (Figure 4A).
Fig. 4. The effect of SP and/or NK-1R antagonists on cancer cell invasive ability.
(A) SP promotes the invasion of pancreatic cancer cells which can be inhibited by NK-1R antagonists: L733, 060 and L-732,138. *P < 0.05 as compared with control group, #P < 0.05 as compared with SP group. SP increases the expression of MMP-2 at mRNA level (B) and protein level (C) that can be counterbalanced by the NK-1R antagonists. *P < 0.05 as compared with control group, #P < 0.05 as compared with SP group.
We further determined the expression of metastatic-related factor MMP-2 by QT-PCR (Figure 4B) and western blotting (Figure 4C). Interestingly, SP was able to promote the expression of MMP-2 which also conterbalanced by the NK-1R antagonists. Taken together, these results suggest that SP/NK-1R signaling system promotes the invasion of pancreatic cancer cells which can be mediated by MMP-2.
SP promotes neurotropism in pancreatic cancer cells
To determine if SP contributes to the neurotropism of pancreatic cancer cell, BxPC-3 cancer cells, which express a higher level of NK-1R were used to co-culture with newborn rat DRGs in Matrigel in the addition of SP or/and NK-1R antagonists for 8 days. We first measured the effect of SP levels on neurite regeneration (Supplementary Figure 1A-E). Newborn rat DRGs were cultured in the medium containing various concentrations of SP (0 nM, 5 nM, 10 nM, 50 nM or 100 nM) for 8 days. The neurite regeneration exhibited a slow but significant increase when the concentration of SP increased from 5 nM to 100 nM (Supplementary Figure 1F). These results suggest that SP have a stimulating effect on neurites. We also observed that BxPC-3 cancer cells facing the DRGs formed peak-like clusters (Supplementary Figure 1G-I). The neurite outgrowth (Supplementary Figure 1J, K) extended to the clusters from the DRGs and provided an invasive pathway for the clusters.
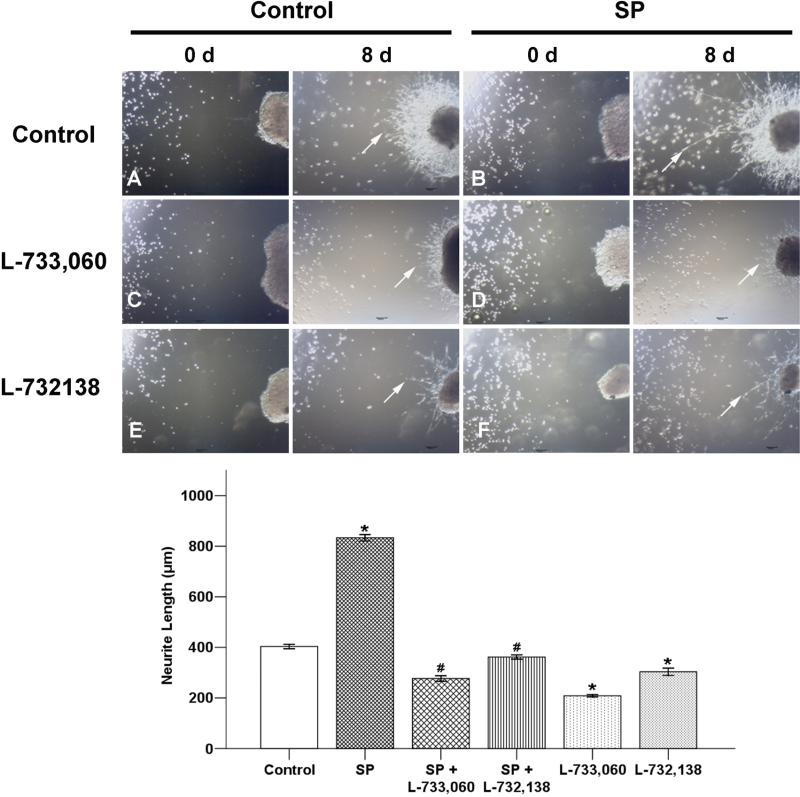
In addition, our results demonstrated that SP promotes pancreatic cancer cell clusters gradually migrating to the DRG and NK-1R antagonists could significantly reduce these effects (Figure 5). These interesting phenomena in the new co-culture environment indicate that the nerve might play an important role in the process of PNI.
Fig. 5. SP promotes neurotropism in BxPC-3 cells.
In the co-culture model, pancreatic cancer cell clusters gradually migrated to the DRG and the neurite outgrowth extended to the clusters from the DRGs that provided an invasive pathway for the clusters (A, original magnification ×40). This trend was enhanced by adding 100 nM SP significantly (B). L-733,060 and L-732,138 could impaire the promoting effect of SP on cancer cell clusters migration and neurite outgrowth (D, F). Adding NK-1R antagonists alone in the co-culture system could also reduce the number of migrated pancreatic cancer cell clusters to the DRG and the length and dencity of neurite outgrowth (C, E). Arrows indicate the outgrowth of neurites. *P < 0.05 as compared with control group, #P < 0.05 as compared with SP group.
Discussion
In the current study, we demonstrated that SP is highly expressed in the neurite outgrowth from newborn DRGs. We found that NK-1R is overexpressed in the pancreatic cancer cell lines. Furthermore, we showed that SP induces pancreatic cancer cell proliferation and invasion with statistically significant and these effects could be counterbalanced by NK-1R antagonists. We then demonstrated that the neurite regeneration slowly was increased in response to various SP concentrations from 5 nM to 100 nM in the co-culture system. Moreover, we showed that SP promotes pancreatic cancer cell clusters gradually migrating to the DRG and SP-induced neurite regeneration extended to the clusters from the DRGs which provides an invasive pathway for the clusters. Taken together, to our knowledge, it is the first time to demonstrate that SP, which mediates the interaction between cancer cells and nerves, may promote the proliferation, invasion, and neurotropism of pancreatic cancer cells. SP-promoted perineural invasion in pancreatic cancer may constitute a novel mechanism for how pain decreases the survival of pancreatic cancer patients. Blocking the SP/NK-1R signaling system may provide a novel strategy for the pancreatic cancer therapeutics.
Traditionally, SP has been classified as a neurotransmitter that exerts its effects on the periphery only by being released from nerve endings. However, SP has been recently shown to be expressed in non-neuronal cell types, including endothelial cells, macrophages and monocytes, eosinophils, lymphocytes, and Leydig cells (7,9). This suggests that it may not only act as a neurotransmitter but also as a functional regulator in an autocrine or paracrine manner.
It has been shown that SP exerts pro-migratory effects on many types of cancer cells such as colon carcinoma, small cell lung cancer (20), and breast carcinoma (21). In the U-373MG astrocytoma cell line, SP stimulates mitogenesis by activating the MAPK pathway through receptors of the NK-1 subtype, including extracellular signal-regulated kinases 1 and 2 (ERK1/2), which translocate to the nucleus to induce cell proliferation and protect the cell from apoptosis (7,22). SP also stimulates cell proliferation via transactivation of the epidermal growth factor receptor (EGFR) (23) and activation of Akt, resulting in the suppression of apoptosis (24).
Cancer patients usually do not succumb to the primary tumor but to the development of metastases. The active migration of tumor cells is a crucial requirement for metastasis and cancer progression. Entschladen et al. (25) suggested that neurotransmitters are key players in the regulation of tumor-cell migration. It has also been shown that SP induces the migration of tumor cells in breast and prostate cancer cells (26). Our results show that SP/NK-1R signaling system also induces pancreatic cancer cell line invasion. We demonstrated that the proliferative and invasive abilities of BxPC-3 and MIA PaCa-2 pancreatic cancer cells were increased with the addition of SP. NK-1R antagonist could inhibit these effect of SP. SP may induce the advancement of pancreatic cancer via a dual mechanism involving both proliferative and invasive properties.
Friess (13) showed new evidence on neuro-cancer cell interaction. Moreover, it has been demonstrated that tumor samples from patients with advanced stages exhibit significantly higher NK1-R levels and that such patients have poorer prognosis. We also showed that the pancreatic cancer cells BxPC-3 and MIA PaCa-2 express NK-1R. SP is higher expressed in the surrounding cells (normal and/or stromal tumors, etc.) or nerve endings, and then interacts with pancreatic cancer cells. In our experiments, we found that the proliferative and invasive ability of pancreatic cancer cells, especially in the SP nerve endings, may stimulate cancer cell migration. Our observations suggest that SP promotes the growth of neurite regeneration as well as pancreatic cancer cell invasion to the DRG and there is a SP-neuro-cancer cell interaction. Our co-culture study shows that the high-SP tumor microenvironment enhances PNI. Taken together, these data suggest that SP and NK-1R could play an important role in the development of metastasis.
The degree of pain control is an important end-point of therapy, along with clinical outcome following surgical and medical treatment for pancreatic cancer (27). Pain is a main complaint from patients with pancreatic cancer. SP is involved in peripheral pain generation, and our experimental results may in part explain the severe pain experienced by advanced patients with poor prognoses, which may involve SP. SP is associated with tumor formation, and specific SP antagonists (12,28) have shown clinical efficacy in patients, acting as the following: analgesics (29), antidepressants (30), anti-emetics [29], and neuroprotectors (31).
In conclusion, our results show that SP, which mediates the interaction between cancer cells and nerves, may promote the proliferation, invasion, and neurotropism of pancreatic cancer cells. These results suggest that SP promotes perineural invasion in pancreatic cancer, constituting a novel mechanism for how pain decreases the survival of pancreatic cancer patients. These findings suggest that blocking the SP/NK-1R signaling system may provide a novel strategy for the pancreatic cancer therapeutics.
Supplementary Material
Acknowledgements
The authors thank the staff of the Biology and Genetics Laboratory, Xi'an Jiaotong University for their technical assistance in these studies. This study was supported by 13115 Major Project (2010ZDKG-49), National Natural Science Foundation of China (81172360, 81201824), and project grants from the National Center for Research Resources (NCRR; P20 RR020151 and P20 RR015566) and the National Institute of General Medical Sciences (NIGMS; P20 GM103505 and P30 GM103332-01) from the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH, NCRR, or NIGMS.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–25. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: Challenge of the facts. World Journal of Surgery. 2003;27:1075–84. doi: 10.1007/s00268-003-7165-7. [DOI] [PubMed] [Google Scholar]
- 4.Zielinska D, Durlik M. Quality of life in patiens after pancreas resection - review paper. Przeglad Gastroenterologiczny. 2010;5:83–87. [Google Scholar]
- 5.di Mola FF, di Sebastiano P. Pain and pain generation in pancreatic cancer. Langenbecks Arch Surg. 2008;393:919–22. doi: 10.1007/s00423-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 6.Hokfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. Journal of Internal Medicine. 2001;249:27–40. doi: 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- 7.Esteban F, Munoz M, Gonzalez-Moles MA, Rosso M. A role for substance P in cancer promotion and progression: a mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer and Metastasis Reviews. 2006;25:137–45. doi: 10.1007/s10555-006-8161-9. [DOI] [PubMed] [Google Scholar]
- 8.Marriott I, Bost KL. IL-4 and IFN-gamma up-regulate substance P receptor expression in murine peritoneal macrophages. Journal of Immunology. 2000;165:182–91. doi: 10.4049/jimmunol.165.1.182. [DOI] [PubMed] [Google Scholar]
- 9.Harrison S, Geppetti P. Substance P. Int. J. Biochem. Cell Biol. 2001;33:555–76. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 10.Guha S, Eibl G, Kisfalvi K, Fan RS, Burdick M, Reber H, et al. Broad-spectrum G protein-coupled receptor antagonist, D-Arg(1) D-Trp(5,7,9),Leu(11) SP: A dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Research. 2005;65:2738–45. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 11.Munoz M, Rosso M, Covenas R. A New Frontier in the Treatment of Cancer: NK-1 Receptor Antagonists. Curr. Med. Chem. 2010;17:504–16. doi: 10.2174/092986710790416308. [DOI] [PubMed] [Google Scholar]
- 12.Munoz M, Rosso M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Invest. New Drugs. 2010;28:187–93. doi: 10.1007/s10637-009-9218-8. [DOI] [PubMed] [Google Scholar]
- 13.Friess H, Zhu ZW, Liard V, Shi X, Shrikhande SV, Wang L, et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Laboratory Investigation. 2003;83:731–42. doi: 10.1097/01.lab.0000067499.57309.f6. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Ma Q, Liu H, Guo K, Li F, Li W, et al. Relationship between Neural Alteration and Perineural Invasion in Pancreatic Cancer Patients with Hyperglycemia. PLoS One. 2011;6:e17385. doi: 10.1371/journal.pone.0017385. Article No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Molecular and Cellular Biochemistry. 2011;347:95–101. doi: 10.1007/s11010-010-0617-0. [DOI] [PubMed] [Google Scholar]
- 16.Li JH, Ma QY, Shen SG, Hu HT. Stimulation of dorsal root ganglion neurons activity by pancreatic cancer cell lines. Cell Biology International. 2008;32:1530–35. doi: 10.1016/j.cellbi.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Muller MW, et al. Neural invasion in pancreatic cancer: A mutual tropism between neurons and cancer cells. Biochemical and Biophysical Research Communications. 2008;374:442–47. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Munoz M, Gonzalez-Ortega A, Covenas R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Invest New Drugs. 2012;30:529–40. doi: 10.1007/s10637-010-9594-0. [DOI] [PubMed] [Google Scholar]
- 20.Seckl MJ, Higgins T, Widmer F, Rozengurt E. [D-Arg1,D-Trp5,7,9,Leu11]substance P: a novel potent inhibitor of signal transduction and growth in vitro and in vivo in small cell lung cancer cells. Cancer Res. 1997;57:51–4. [PubMed] [Google Scholar]
- 21.Bigioni M, Benzo A, Irrissuto C, Maggi CA, Goso C. Role of NK-1 and NK-2 tachykinin receptor antagonism on the growth of human breast carcinoma cell line MDA-MB-231. Anticancer Drugs. 2005;16:1083–9. doi: 10.1097/00001813-200511000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Luo W, Sharif TR, Sharif M. Substance P-induced mitogenesis in human astrocytoma cells correlates with activation of the mitogen-activated protein kinase signaling pathway. Cancer Research. 1996;56:4983–91. [PubMed] [Google Scholar]
- 23.Koon HW, Zhao DZ, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. Journal of Biological Chemistry. 2004;279:45519–27. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- 24.Akazawa T, Kwatra SG, Goldsmith LE, Richardson MD, Cox EA, Sampson JH, et al. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J Neurochem. 2009;109:1079–86. doi: 10.1111/j.1471-4159.2009.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entschladen F, Drell TL, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncology. 2004;5:254–58. doi: 10.1016/S1470-2045(04)01431-7. [DOI] [PubMed] [Google Scholar]
- 26.Lang K, Drell TL, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. International Journal of Cancer. 2004;112:231–38. doi: 10.1002/ijc.20410. [DOI] [PubMed] [Google Scholar]
- 27.di Mola FF, Di Sebastiano P. Pain and pain generation in pancreatic diseases. Am. J. Surg. 2007;194:S65–S70. [Google Scholar]
- 28.Munoz M, Rosso M, Gonzalez A, Saenz J, Covenas R. The broad-spectrum antitumor action of cyclosporin A is due to its tachykinin receptor antagonist pharmacological profile. Peptides. 2010 doi: 10.1016/j.peptides.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Rupniak NM, Carlson E, Boyce S, Webb JK, Hill RG. Enantioselective inhibition of the formalin paw late phase by the NK1 receptor antagonist L-733,060 in gerbils. Pain. 1996;67:189–95. doi: 10.1016/0304-3959(96)03109-0. [DOI] [PubMed] [Google Scholar]
- 30.Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–45. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Cadet JL, Angulo JA. Neurokinin-1 (NK-1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain. J Neurochem. 2002;83:613–22. doi: 10.1046/j.1471-4159.2002.01155.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.