Abstract
Purpose
To characterize the involvement of Semaphorin 7A (Sema7a) in corneal neovascularization (NV).
Methods
We generated anti-Sema7A antibodies to detect protein expression. Corneal fibroblast cells were cultured, stimulated with bFGF, immunostained with anti-Sema7A antibodies, and visualized by confocal microscopy. bFGF pellets were implanted in mouse corneal micropockets for 3–10 days, and corneal sections were immunostained with anti-Sema7A antibodies. Mouse corneas were injected with naked Sema7A DNA and vector control for 3, 7, and 10 days. Mouse corneas were imaged by slit lamp microscopy, and areas of corneal NV were calculated using the ImageJ program. Mouse corneal sections were also immunostained with anti-macrophage marker (F4/80) and anti-vascular endothelial growth factor (VEGF)-A antibodies.
Results
Our data showed enhanced Sema7A expression levels in bFGF-stimulated cultured corneal fibroblasts. bFGF corneal implantation also demonstrated enhanced Sema7A expression. Corneas injected with a Sema7A expression vector showed evidence of significant corneal NV compared to controls on day 10 (1.8 mm2 vs. 0.11 mm2; p<0.02). Additionally, immunolocalization of Sema7A DNA-injected corneas (at day 7) revealed macrophage recruitment and enhanced VEGF-A levels.
Conclusions
We demonstrated that Sema7A was expressed in vascularized corneas and showed pro-angiogenic properties in our corneal model. Understanding the mechanism of Sema7A in angiogenesis may provide a therapeutic target for the treatment of corneal angiogenesis-related disorders.
Keywords: Semaphorin 7A, Sema7A, corneal neovascularization, angiogenesis
INTRODUCTION
The semaphorin family of transmembrane and secreted proteins was initially described as composed of axon guidance factors involved in the development of the central nervous system 1–3. Recent studies have reported that semaphorins, including Semaphorin 3B, 3F, 3E, 4D, and 6A, can either promote or inhibit tumor angiogenesis and progression 4–7. We previously detected Semaphorin 7A (Sema7a) expression in mouse ocular tissues during the course of ocular angiogenesis analysis 8, 9.
Sema7A has been implicated in the regulation of the immune and nervous systems 1, 10, 11. Sema7A is associated with the cell surface through a glycophosphatidylinositol linkage 12 and exerts pronounced effects on axon outgrowth and nerve sprouting through its integrin-binding motif (Arg-Gly-Asp) and effects on the mitogen-activated protein kinase pathway 13. Emerging evidence suggests that axon growth cones and capillary tip cells use common signaling cues to regulate their guidance 14.
The normally avascular cornea is a good model for evaluating pro- and anti-angiogenic factors 8, 15. The cornea requires low levels of angiogenic factors and high levels of anti-angiogenic factors under basal conditions; shifting the balance towards higher levels of angiogenic factors is associated with pathological processes. Corneal neovascularization (NV) is induced by the upregulation of angiogenic factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and chemokines 15. Under corneal hypoxia and inflammatory conditions, VEGF is secreted by macrophages, T cells, astrocytes, and keratocytes, and binds to endothelial cell-specific receptor tyrosine kinases (i.e., VEGFR-1 and VEGFR-2) to mediate angiogenic responses.
In the present study, we demonstrate that Sema7A, previously known for its immunomodulatory and neuronal effects, mediates vascular growth. We observed an increase in the expression levels of corneal Sema7A after bFGF pellet implantation. Sema7A expressed in bFGF-stimulated corneal fibroblasts was visualized by confocal immunostaining. In addition, macrophage recruitment, VEGF-A expression, and NV were evident in corneas injected with Sema7A cDNA. These data suggest a pro-angiogenic role for Sema7A in corneal NV.
MATERIALS AND METHODS
Animal studies were conducted in accordance with the Animal Care and Use Committee Guidelines of the University of Illinois at Chicago and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 wild-type mice were used in this research.
Generation of anti-Sema7A antibody
A Sema7A peptide (REDNPDKNPEAPLNVSRVAQLC) was synthesized based on the mouse Sema7A amino acid sequence (242–264) and injected into a rabbit. Rabbit serum was collected, and anti-Sema7A antibodies were purified using a Sema7A peptide affinity column. The specificity of the anti-Sema7A antibody was confirmed by peptide competition assay.
bFGF pellet preparation and corneal micropocket assay
Pellets were made of the slow-release polymer Hydron (polyhydroxyethylmethacrylate), which contains sucralfate (45 ng/pellet; Sigma-Aldrich, St. Louis, MO) and human bFGF (120 ng/pellet; R&D Systems, Minneapolis, MN) as previously described 16. A suspension of recombinant bFGF and sucralfate was prepared in sterile saline and concentrated for 10 minutes, and then 12% Hydron in ethanol (10 μl) was added. The suspension was then deposited onto a sterilized nylon mesh (LAB Pak, Sefar America, Depew, NY) and embedded between the fibers. The resulting grid (10×10-mm squares) was allowed to dry on a sterile Petri dish for 1 hour. The fibers of the mesh were separated under a microscope, and among the approximately 100 pellets produced, 30–40 pellets of uniform size (0.4×0.4×0.2 mm) were selected for implantation.
Corneal micropockets were created with a modified von Graefe knife in adult mice, and the pellets were implanted into corneal pockets. On days 1, 3, and 10 after implantation, the eyes were harvested, embedded in optimal cutting temperature (OCT) compound (Miles, Elkhart, IN), and frozen in liquid nitrogen prior to immunolocalization assay.
Sema7A immunolocalization
Cryostat sections (8 μm) of the OCT-embedded corneas were fixed in 4% paraformaldehyde for 15 minutes. After blocking with 1% bovine serum albumin (Sigma-Aldrich) for 30 minutes, the sections were incubated for 1 hour with rabbit anti-mouse Sema7A polyclonal antibody, goat anti-mouse VEGF-A antibody (R&D Systems), and rat anti-mouse F4/80 antibody (ebiosciences, San Diego, CA), followed by Incubation with fluorescein-conjugated donkey anti-rabbit IgG, FITC-donkey anti-goat IgG, and Cy5 donkey anti-rat IgG secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Negative controls were prepared in the same manner, but without the primary antibody. Propidium iodide (PI; Vector Laboratories, Burlingame, CA) was used for nuclear staining. Immunostained sections were viewed by confocal microscopy (TCS 4D; Leica, Heidelberg, Germany).
Corneal injection of naked Sema7A cDNA
The expression vector containing mouse Sema7A cDNA (pCMV-Sport6-Sema7A) was purchased from ATCC (Manassas, VA). Mice received a 5-μl injection of pCMV-Sport6-Sema7A (400 ng/μl; n=9) into the central corneal stroma, or a 5-μl injection of empty vector (400 ng/μl pCMV-Sport6; n=9). An antibiotic ophthalmic ointment was used after injection.
Mouse corneas were evaluated on days 3, 7, and 10 after DNA injection. Corneal NV was digitally photographed using a slit lamp microscope (Nikon FS-2, Tokyo, Japan). The neovascular area was measured and analyzed with NIH ImageJ software (NIH, Bethesda, MD). Statistical analysis was performed with SPSS 14.0 software. p<0.05 was considered significant.
RESULTS
Sema7A expression in cultured corneal fibroblasts
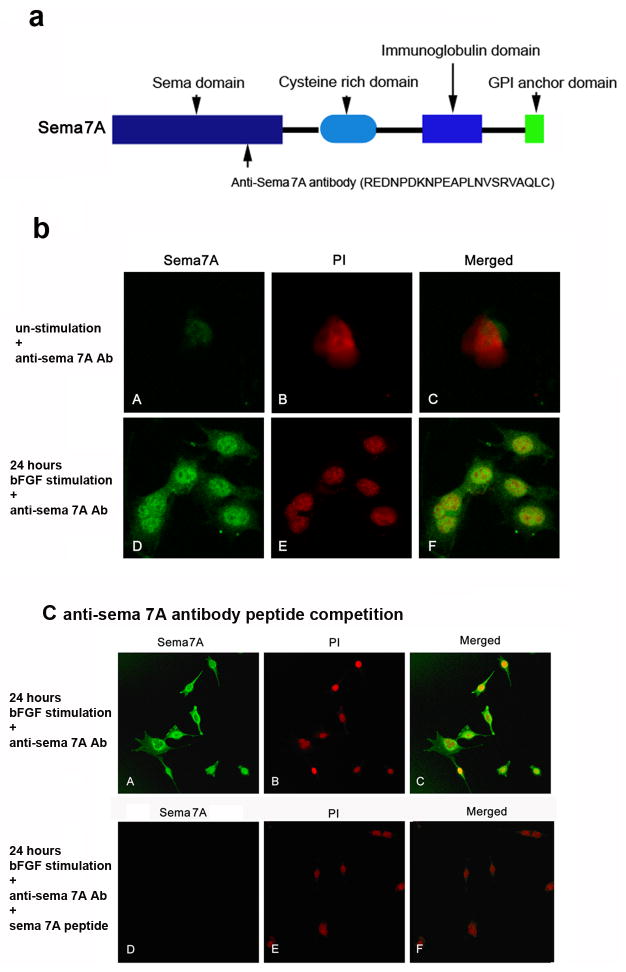
A 22-mer peptide (REDNPDKNPEAPLNVSRVAQLC) based on the mouse Sema7A sequence was used to generate an anti-Sema7A antibody (Fig. 1a). To determine whether Sema7A is expressed in mouse fibroblasts, cultured corneal fibroblasts were immunostained for Sema7A (Fig. 1b). Only low levels of Sema7A were detected in unstimulated fibroblasts (Fig. 1b, A–C); however, an increase in Sema7A expression was found in bFGF-stimulated fibroblasts (Fig. 1b, D–F). The specificity of the anti-Sema7A antibody was determined by the positive staining of Sema7A in bFGF-stimulated corneal fibroblasts (Fig. 1c, A–C). Negative immunofluorescence staining of Sema7A in bFGF-stimulated corneal fibroblasts after preincubation of the anti-Sema7A antibodies with cognate Sema7A peptides further confirmed the specificity of the anti-Sema7A antibodies (Fig. 1c, D–F).
Figure 1.
Sema7A expression is induced by bFGF in cultured corneal fibroblasts. (a) Schematic representation of Sema7A and the location of the amino acid sequence (REDNPDKNPEAPLNVSRVAQLC) used to generate the rabbit anti-Sema7A antibody. (b) Cultured corneal fibroblasts were unstimulated (panels A–C) or stimulated with bFGF (120 ng/pellet) for 24 hours (panels D–F) and immunostained for Sema7A. The nuclei were stained with PI. (c) The specificity of these antibodies was confirmed by binding to Sema7A in bFGF-stimulated corneal fibroblasts (panels A–C), which was blocked by pre-incubation with cognate peptides (panels D–F).
Increased Sema7A expression after bFGF-induced corneal neovascularization
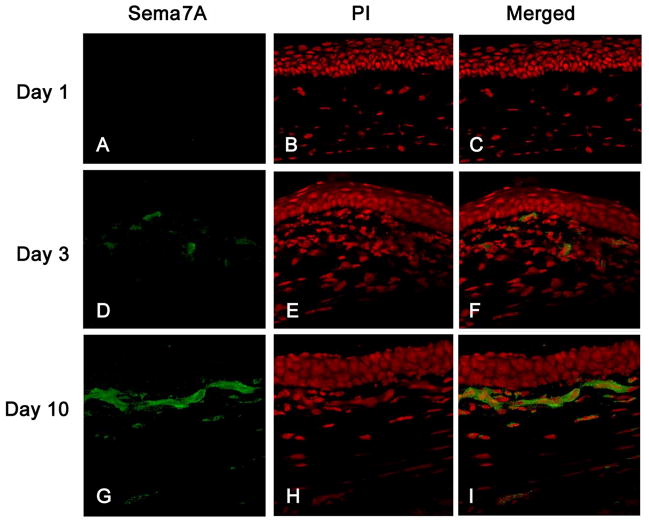
Mouse eyes implanted with a bFGF pellet were immunostained for Sema7A on days 1, 3, and 10 post-implantation. On day 1 after implantation, little to no Sema7A was detected in the cornea (Fig. 2A–C). However, on days 3 and 10, increased expression levels of Sema7A were observed in the stroma between the implanted pellet and the corneal limbus (Fig. 2D–I).
Figure 2.
Sema7A immunolocalization in bFGF-induced mouse corneas. After implantation of bFGF pellets, mouse eyes were enucleated and frozen in OCT compound. Corneal sections were stained with anti-Sema7A antibody and PI on day 1 (panels A–C), day 3 (panels D–F), and day 10 (panels G–I) after implantation.
Sema7A-induced corneal neovascularization
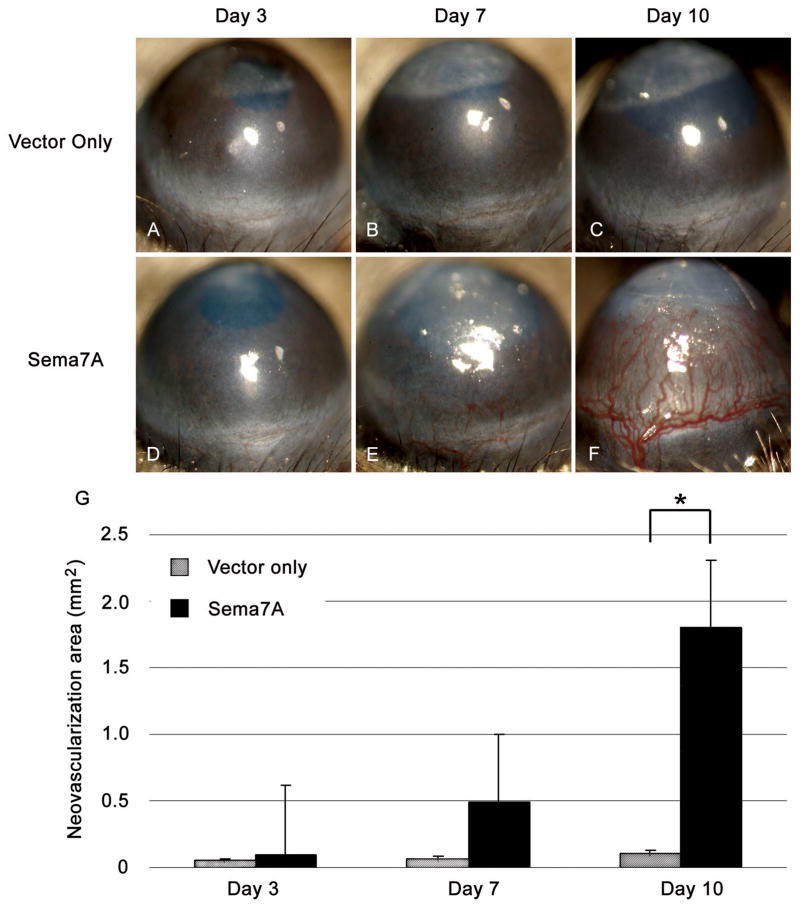
To determine whether Sema7A could induce NV, mouse corneas were injected with Sema7A cDNA or empty vector pCMV-Sport6 (Fig. 3). No corneal NV was detected in the control group (empty vector) at days 3, 7, and 10 (Fig. 3A–C). In contrast, corneal NV was evident on day 7 in corneas injected with Sema7A cDNA (Fig. 3E), and was considerable on day 10 (Fig. 3F). New vessels developed through the superficial cornea from the limbus towards the central area, where the Sema7A cDNA was injected. Compared with controls, the neovascular area appeared to increase in Sema7A cDNA-injected corneas on days 3 (0.1 mm2 vs. 0.05 mm2; p=0.39) and 7 (0.49 mm2 vs. 0.06 mm2; p=0.12), but the amount of corneal NV was not significantly higher until day 10 (1.8 mm2 vs. 0.11 mm2; p<0.02; Fig. 3G).
Figure 3.
Sema7A induces corneal neovascularization. Mouse corneas were injected with Sema7A cDNA or empty vector (pCMV-Sport6) as control. The control group (panels A–C) showed no evidence of corneal NV at days 3, 7, and 10 after injection of empty vector. In contrast, in corneas injected with the Sema7A cDNA (panels D–F), corneal NV began to develop in the periphery at day 7 and increased substantially by day 10. The neovascular area of Sema7A-injected corneas was calculated and compared to controls (G). The area of Sema7A-induced corneal NV at day 10 was significantly greater than that of the vector DNA-injected cornea (P<0.02). * indicates statistical significant.
Sema7A recruits macrophages and induces VEGF-A expression in the cornea
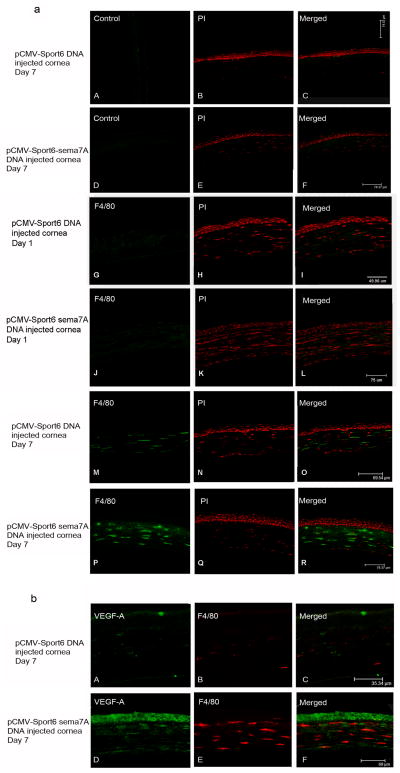
Sema7A has been shown to stimulate human monocytes during T-cell responses. To assay the effect of naked Sema7A DNA in injection-induced corneal NV, recruitment of inflammatory cells and VEGF-A expression levels in Sema7A cDNA- and empty vector-injected mouse corneas were determined. Both Sema7A cDNA- and empty vector-injected corneas were immunostained with antibodies against both the macrophage-specific marker F4/80 and VEGF-A. Sections from the Sema7A cDNA- and vector-injected corneas on day 1 showed neither F4/80 (Fig. 4a) nor VEGF-A (Fig. 4b) immunostaining. By day 7, vector-injected corneas showed low levels of F4/80 (Fig. 4a M) and VEGF-A (Fig. 4b A) immunostaining; furthermore, by day 7, Sema7A-injected corneas showed increases in F4/80 (Fig. 4a P) and VEGF-A (Fig. 4b D) immunostaining.
Figure 4.
Macrophage recruitment and VEGF-A expression in Sema7A-injected corneas. Corneas were injected with Sema7A (pCMV-Sport6-Sema7A) or empty vector (pCMV-Sport6) as control and then immunostained for F4/80, a macrophage marker, on day 1 (a; G and J) and day 7 (a; M and P) and VEGF-A on day 7 (b; A and D). Controls are images with primary antibody omission (a; A and D).
DISCUSSION
In the present study, we demonstrated that Sema7A is not constitutively expressed in mouse corneas; however, Sema7A expression was observed after bFGF stimulation. Furthermore, mouse corneas injected with Sema7A cDNA developed significant NV by day 10 compared to corneas injected with empty vector. These findings suggest a previously unknown pro-angiogenic role for Sema7A in corneal NV.
Semaphorins have been described as inhibitory signals because of their ability to prevent cell migration and axon outgrowth 17. However, semaphorins can, at times, promote cell chemotaxis and axon/dendrite outgrowth and attraction 13, 18, 19. Several reports have shown that Sema7A induces chemotaxis, inflammatory cytokine production [e.g., tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and IL-8], and superoxide release in monocytes 20. In this report, the increase in corneal NV in Sema7A-injected corneas coincided with the presence of F4/80, a macrophage marker, suggesting that Sema7A may mediate an inflammatory response and recruit infiltrating cells, including macrophages. Additionally, the presence of VEGF-A in Sema7A cDNA-injected vascularized corneas supports the hypothesis that Sema7A-induced NV is mediated through VEGF-A production.
Although the roles of semaphorins have not been characterized as extensively in the vascular system as in the nervous and immune systems, members of the semaphorin family appear to be important in vascular remodeling. Sema3A has been reported to play a crucial role in regulating endothelial cell migration and angiogenesis 21–23, and appears to mediate the topographic congruence of nerves and blood vessels 24. Sema3F inhibits angiogenesis in vitro and in vivo and is a potent inhibitor of tumor angiogenesis 25. The soluble extracellular domain of Sema6A-1 blocks VEGF-mediated endothelial cell migration in vitro, and its intraperitoneal administration inhibited both bFGF- and VEGF-induced NV in vivo 26. Other semaphorins may exert opposing effects. Sema4D was reported to potentially induce chemotaxis and tubulogenesis in endothelial cells in vitro and promote blood vessel formation in an in vivo mouse model; these functions are believed to be mediated by the plexin B1 receptor 27–29.
The characterization of Sema7A receptors remains controversial. Plexin C1 was identified first as a Sema7A receptor 30; further studies demonstrated a β1 subunit-containing integrin receptor as a Sema7A receptor 13. Further research is needed to characterize the mechanisms and receptors involved in the actions of Sema7A in corneal NV.
In the present report, we demonstrated that Sema7A was expressed in vascularized corneas and showed pro-angiogenic properties in our corneal model. These findings indicate a previously unknown role for Sema7A in corneal NV. Understanding the mechanism of Sema7A in angiogenesis may provide a therapeutic target for the treatment of corneal NV and other angiogenesis-related disorders, such as diabetic retinopathy, macular degeneration, and cancer.
Acknowledgments
Grant support: An unrestricted departmental grant from Research to Prevent Blindness New York (NY) and National Institutes of Health Grants EY10101 (DTA) and EY001792 (DTA).
Abbreviations
- bFGF
Basic fibroblast growth factor
- NV
neovascularization
- Sema7A
Semaphorin 7A
- VEGF
vascular endothelial growth factor
- OCT
optimal cutting temperature
- PI
propidium iodide
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Melani M, Weinstein BM. Common factors regulating patterning of the nervous and vascular systems. Annual review of cell and developmental biology. 2010;26:639–665. doi: 10.1146/annurev.cellbio.093008.093324. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nature reviews. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 3.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 4.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. Journal of cell science. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai A, Gavard J, Annas-Linhares Y, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadanandam A, Rosenbaugh EG, Singh S, Varney M, Singh RK. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvascular research. 2010;79:1–9. doi: 10.1016/j.mvr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klagsbrun M, Shimizu A. Semaphorin 3E, an exception to the rule. The Journal of clinical investigation. 2010;120:2658–2660. doi: 10.1172/JCI44110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 9.Albe E, Chang JH, Azar NF, Ivanov AR, Azar DT. Proteomic analysis of the hyaloid vascular system regression during ocular development. Journal of proteome research. 2008;7:4904–4913. doi: 10.1021/pr800551m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Juo ZS, Shim AH, et al. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Ng S, Wu ZL, et al. Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J Biol Chem. 1998;273:22428–22434. doi: 10.1074/jbc.273.35.22428. [DOI] [PubMed] [Google Scholar]
- 13.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 14.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Progress in retinal and eye research. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 17.He Z, Wang KC, Koprivica V, Ming G, Song HJ. Knowing how to navigate: mechanisms of semaphorin signaling in the nervous system. Sci STKE. 2002;2002:RE1. doi: 10.1126/stke.2002.119.re1. [DOI] [PubMed] [Google Scholar]
- 18.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 19.Giordano S, Corso S, Conrotto P, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 20.Holmes S, Downs AM, Fosberry A, et al. Sema7A is a potent monocyte stimulator. Scand J Immunol. 2002;56:270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 21.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serini G, Valdembri D, Zanivan S, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 23.Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130:3227–3236. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- 24.Bates D, Taylor GI, Minichiello J, et al. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 25.Kessler O, Shraga-Heled N, Lange T, et al. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 26.Dhanabal M, Wu F, Alvarez E, et al. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther. 2005;4:659–668. doi: 10.4161/cbt.4.6.1733. [DOI] [PubMed] [Google Scholar]
- 27.Conrotto P, Valdembri D, Corso S, et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 28.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 30.Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]