Abstract
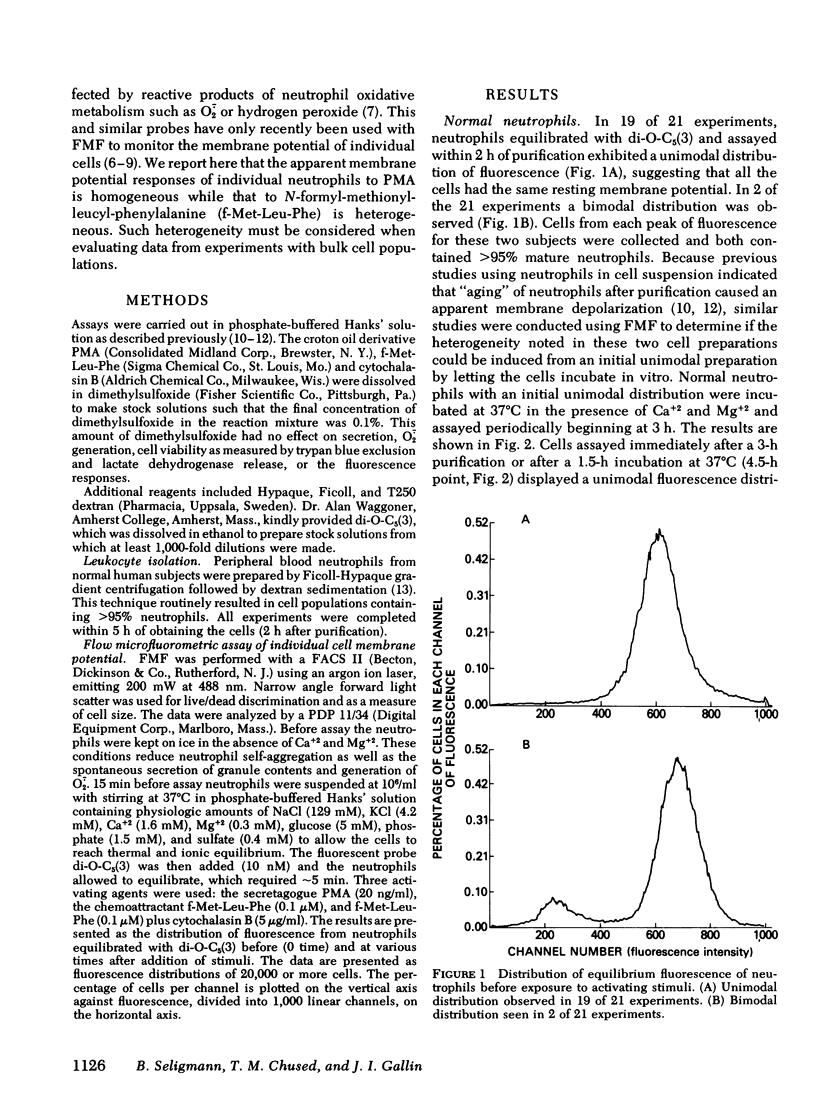
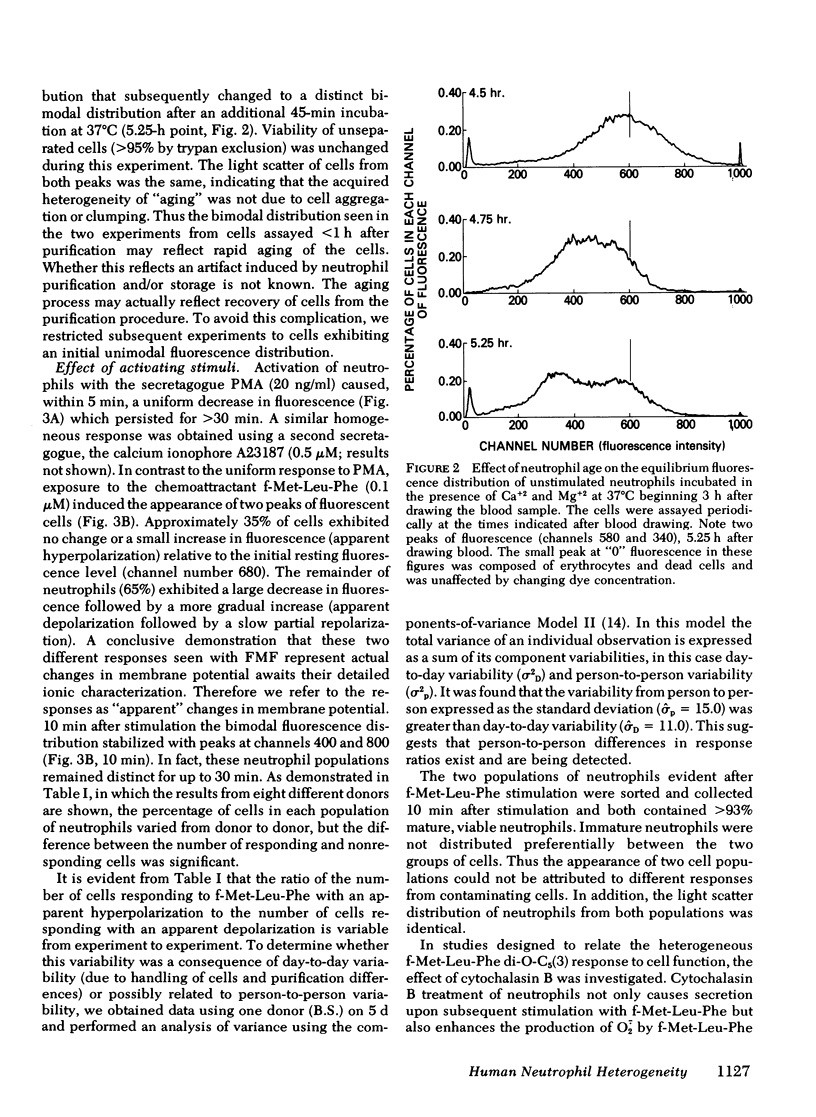
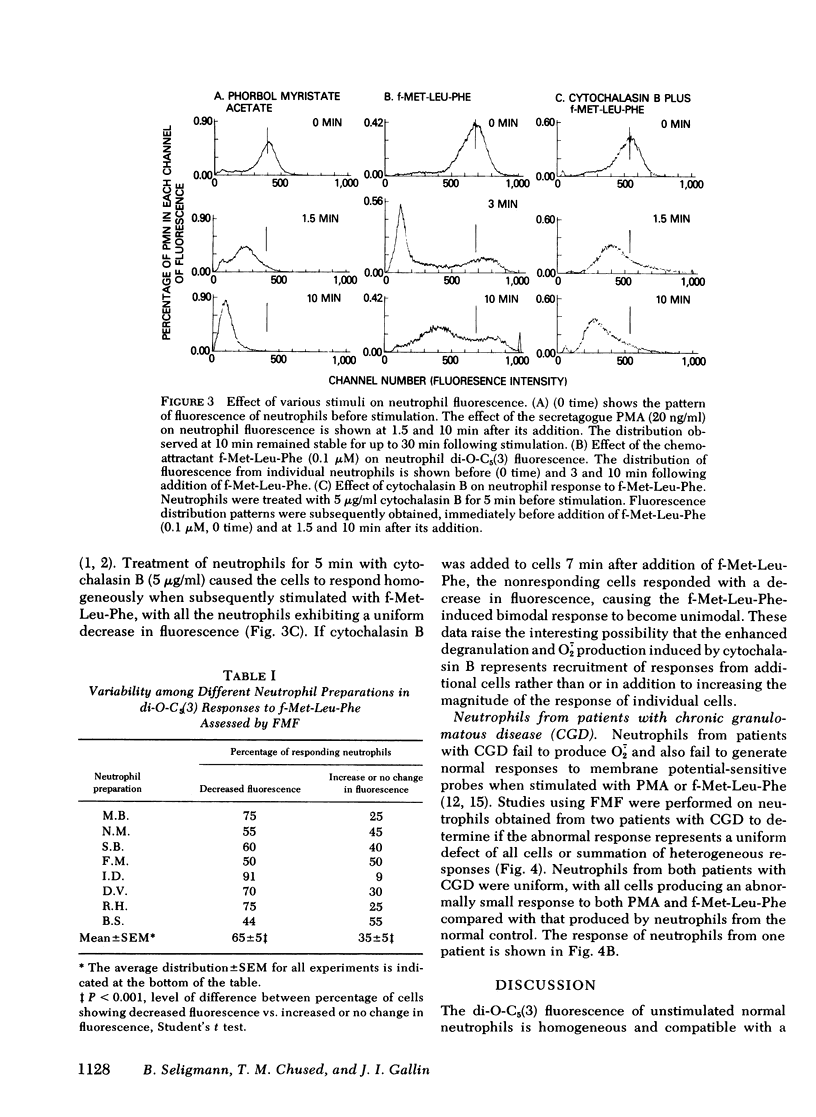
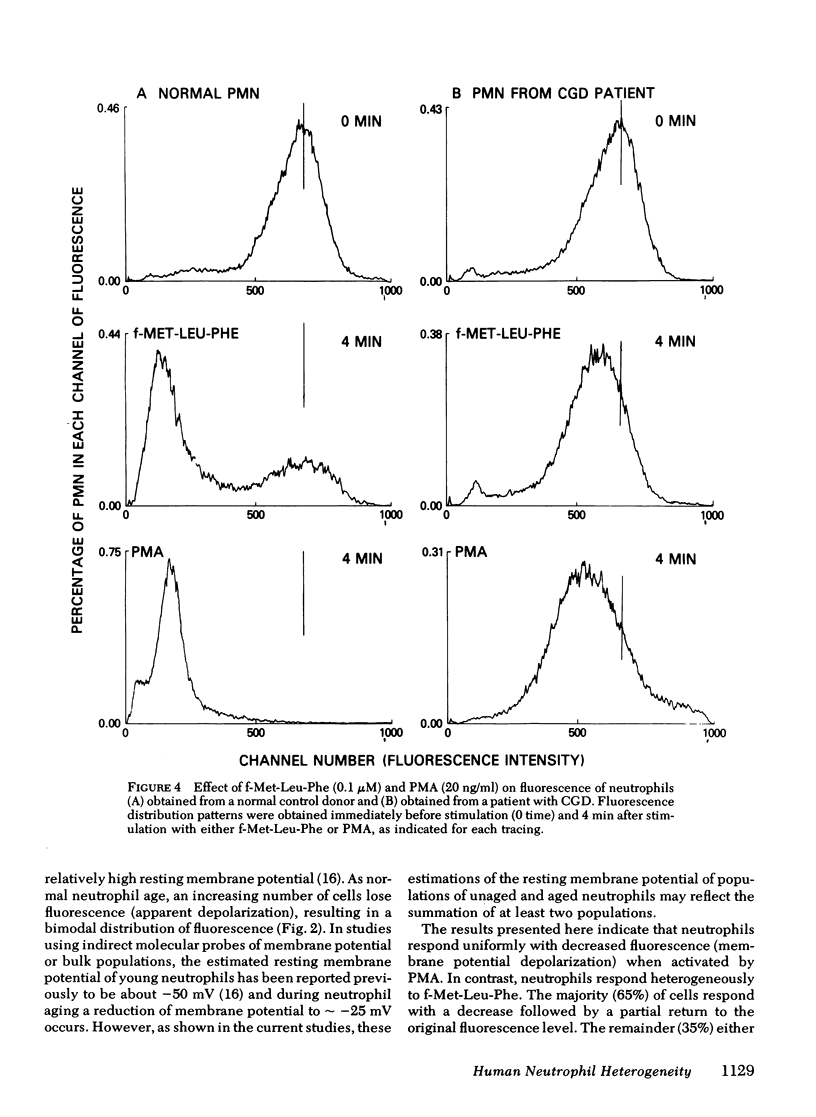
Previous studies of neutrophil nitroblue tetrazolium dye reduction in response to endotoxin and rosetting of IgG-coated erythryocytes have suggested functional heterogeneity of peripheral blood neutrophils. In the following study we utilized flow microfluorometry and the membrane potential-sensitive fluorescent dye 3-3′-dipentyloxacarbocyanine to assess the heterogeneity of neutrophils upon activation by a variety of stimuli. Unstimulated neutrophils from normal subjects exhibited a unimodal distribution of fluorescence, suggesting that all the cells possessed the same resting membrane potential. As neutrophils aged (>5 h), some cells lost fluorescence producing a bimodal distribution. In studies with fresh cells, the secretagogue phorbol myristate acetate (20 ng/ml) stimulated a uniform loss of fluorescence (apparent depolarization). The chemoattractant N-formyl-methionyl-leucyl-phenylalanine (f-Met-Leu-Phe) (0.1 μM) caused the neutrophils to assume and maintain (for > 30 min) a bimodal fluorescence distribution in which 65±5% of the neutrophils first decreased and then increased fluorescence (apparent depolarization/partial repolarization), and 35±5% of the cells exhibited either an increase in fluorescence (apparent hyperpolarization) or no change. Treatment of neutrophils with cytochalasin B before stimulation caused the cells to respond homogeneously to f-Met-Leu-Phe. Additional studies using neutrophils from patients with chronic granulomatous disease, which exhibit abnormal membrane potential responses, indicated that this defect affected all such neutrophils uniformly. These observations demonstrate the need to investigate the physiological significance of the heterogeneity of neutrophil function and indicate that the f-Met-Leu-Phe-induced changes in membrane potential observed in bulk population cell studies are the summation of two different responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broxmeyer H. E., Ralph P., Bognacki J., Kincade P. W., Desousa M. A subpopulation of human polymorphonuclear neutrophils contains an active form of lactoferrin capable of binding to human monocytes and inhibiting production of granulocyte-macrophage colony stimulatory activities. J Immunol. 1980 Aug;125(2):903–909. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Jones G. S., Van Dyke K., Castranova V. Purification of human granulocytes by centrifugal elutriation and measurement of transmembrane potential. J Cell Physiol. 1980 Sep;104(3):425–431. doi: 10.1002/jcp.1041040315. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Gallin J. I. Separation and functional characterization of human neutrophil subpopulations. Blood. 1978 Apr;51(4):659–669. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. The chemotactic factors induced movement of calcium and sodium across rabbit neutrophil membranes: effect of densensitization to cytochalasin B. J Cell Physiol. 1979 Aug;100(2):239–250. doi: 10.1002/jcp.1041000205. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Rasmussen B., White J. G. An improved nitroblue tetrazolium test using phorbol myristate acetate-coated coverslips. Am J Clin Pathol. 1979 May;71(5):582–585. doi: 10.1093/ajcp/71.5.582. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin E. K., Martin D. L., Shain W., Gallin J. I. Interaction of chemotactic factors with human polymorphonuclear leukocytes: studies using a membrane potential-sensitive cyanine dye. J Membr Biol. 1980;52(3):257–272. doi: 10.1007/BF01869194. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann B., Gallin J. I. Secretagogue modulation of the response of human neutrophils to chemoattractants: studies with a membrane potential sensitive cyanine dye. Mol Immunol. 1980 Feb;17(2):191–200. doi: 10.1016/0161-5890(80)90071-1. [DOI] [PubMed] [Google Scholar]
- Shapiro H. M., Natale P. J., Kamentsky L. A. Estimation of membrane potentials of individual lymphocytes by flow cytometry. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5728–5730. doi: 10.1073/pnas.76.11.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Atkinson J. P., Spilberg I. Stimulus-specific deactivation of chemotactic factor-induced cyclic AMP response and superoxide generation by human neutrophils. J Clin Invest. 1980 Oct;66(4):736–747. doi: 10.1172/JCI109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham P. E., Delves P. J., Shen L., Roitt I. M. Chemotactic factor-induced membrane potential changes in rabbit neutrophils monitored by the fluorescent dye 3,3'-dipropylthiadicarbocyanine iodide. Biochim Biophys Acta. 1980 Nov 4;602(2):285–298. doi: 10.1016/0005-2736(80)90312-0. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]



