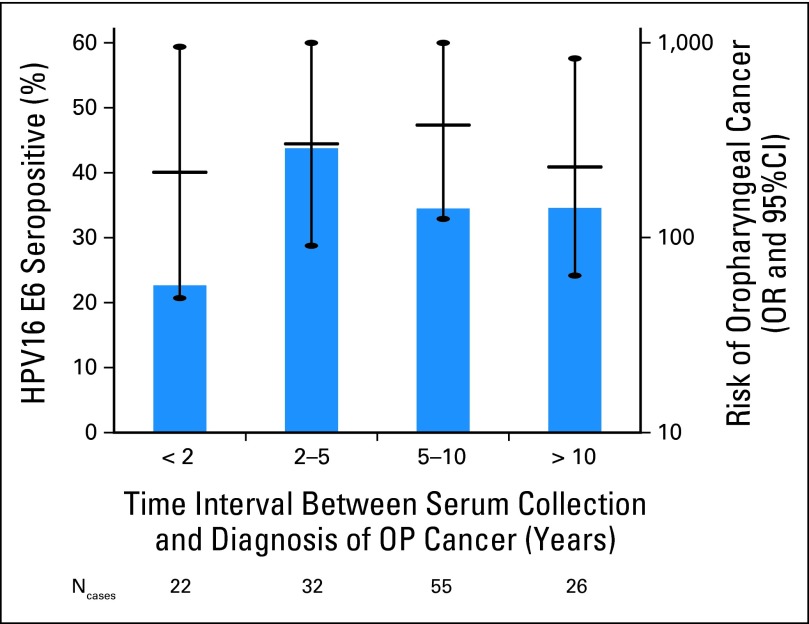
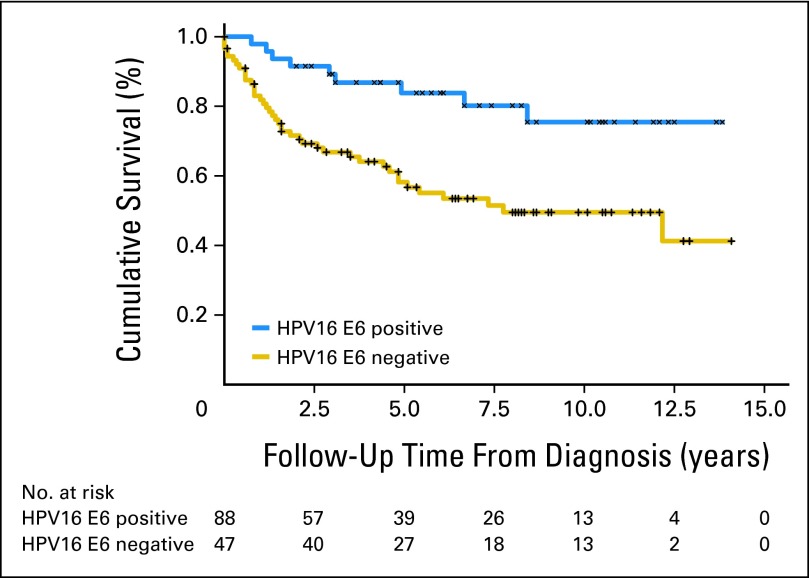
Aimée R Kreimer
Aimée R Kreimer
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Mattias Johansson
Mattias Johansson
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Tim Waterboer
Tim Waterboer
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Rudolf Kaaks
Rudolf Kaaks
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Jenny Chang-Claude
Jenny Chang-Claude
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Dagmar Drogen
Dagmar Drogen
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Anne Tjønneland
Anne Tjønneland
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Kim Overvad
Kim Overvad
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
J Ramón Quirós
J Ramón Quirós
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Carlos A González
Carlos A González
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Maria José Sánchez
Maria José Sánchez
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Nerea Larrañaga
Nerea Larrañaga
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Carmen Navarro
Carmen Navarro
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Aurelio Barricarte
Aurelio Barricarte
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Ruth C Travis
Ruth C Travis
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Kay-Tee Khaw
Kay-Tee Khaw
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Nick Wareham
Nick Wareham
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Antonia Trichopoulou
Antonia Trichopoulou
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Pagona Lagiou
Pagona Lagiou
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Dimitrios Trichopoulos
Dimitrios Trichopoulos
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Petra HM Peeters
Petra HM Peeters
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Salvatore Panico
Salvatore Panico
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Giovanna Masala
Giovanna Masala
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Sara Grioni
Sara Grioni
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Rosario Tumino
Rosario Tumino
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Paolo Vineis
Paolo Vineis
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
H Bas Bueno-de-Mesquita
H Bas Bueno-de-Mesquita
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Göran Laurell
Göran Laurell
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Göran Hallmans
Göran Hallmans
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Jonas Manjer
Jonas Manjer
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Johanna Ekström
Johanna Ekström
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Guri Skeie
Guri Skeie
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Eiliv Lund
Eiliv Lund
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Elisabete Weiderpass
Elisabete Weiderpass
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Pietro Ferrari
Pietro Ferrari
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Graham Byrnes
Graham Byrnes
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Isabelle Romieu
Isabelle Romieu
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Elio Riboli
Elio Riboli
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Allan Hildesheim
Allan Hildesheim
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Heiner Boeing
Heiner Boeing
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Michael Pawlita
Michael Pawlita
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,
Paul Brennan
Paul Brennan
1Aimée R. Kreimer, Allan Hildesheim, National Cancer Institute, National Institutes of Health, Rockville, MD; Pagona Lagiou, Dimitrios Trichopoulos, Harvard School of Public Health, Boston, MA; Mattias Johansson, Pietro Ferrari, Graham Byrnes, Isabelle Romieu, Paul Brennan, International Agency for Research on Cancer, Lyon, France; Tim Waterboer, Michael Pawlita, Rudolf Kaaks, Jenny Chang-Claude, German Cancer Research Center, Heidelberg; Dagmar Drogen, Heiner Boeing, German Institute of Human Nutrition Potsdam Rehbruecke, Nuthetal, Germany; Anne Tjønneland, The Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen; Kim Overvad, Institute of Public Health, Aarhus, Denmark; J. Ramón Quirós, Public Health Directorage, Asturias, Oviedo; Carlos A. González, Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona; Maria José Sánchez, Andalusian School of Public Health, Granada; Maria José Sánchez, Carmen Navarro, Nerea Larrañga, Aurelio Barricarte, Centros de Investigación Biomédica en Red (CIBER) de Epidemiología y Salud Pública, Madrid; Nerea Larrañga, Public Health Department of Gipuzkoa, Basque Government, San Sebastián; Carmen Navarro, Murcia Regional Health Council, Murcia; Aurelio Barricarte, Navarre Public Health Institute, Pamplona, Spain; Ruth C. Travis, University of Oxford, Oxford; Kay-Tee Khaw, University of Cambridge School of Clinical Medicine; Nick Wareham, Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge; Paolo Vineis, Elio Riboli, Imperial College, London, United Kingdom; Antonia Trichopoulou, Pagona Lagiou, WHO Collaborating Center for Food and Nutrition Policies, University of Athens Medical School; Antonia Trichopoulou, Dimitrios Trichopoulos, Hellenic Health Foundation; Pagona Lagiou, Dimitrios Trichopoulos, Bureau of Epidemiologic Research, Academy of Athens, Athens, Greece; Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, University Medical Center Utrecht, Utrecht; H. Bas Bueno de Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands; Salvatore Panico, Federico II University, Naples; Giovanna Masala, Cancer Prevention and Research Institute (ISPO), Florence; Sara Grioni, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale Tumori, Milan; Rosario Tumino, “Civile-M.P. Arezzo” Hospital, Ragusa; Paolo Vineis, HuGeF Foundation, Torino, Italy; Göran Laurell, Göran Hallmans, Umeå University, Umeå; Jonas Manjer, Johanna Ekström, Malmö University Hospital, Lund University, Malmö; Elisabete Weiderpass, Karolinska Institutet, Stockholm, Sweden; Guri Skeie, Eiliv Lund, Elisabete Weiderpass, University of Tromsø, Tromsø; Elisabete Weiderpass, Cancer Registry of Norway, Oslo, Norway; Elisabete Weiderpass, Samfundet Folkhälsan, Helsinki, Finland.
1,✉