Abstract
Purpose
Prior studies report that half of patients with lung cancer do not receive guideline-concordant care. With data from a national Veterans Health Administration (VHA) study on quality of care, we sought to determine what proportion of patients refused or had a contraindication to recommended lung cancer therapy.
Patients and Methods
Through medical record abstraction, we evaluated adherence to six quality indicators addressing lung cancer–directed therapy for patients diagnosed within the VHA during 2007 and calculated the proportion of patients receiving, refusing, or having contraindications to recommended treatment.
Results
Mean age of the predominantly male population was 67.7 years (standard deviation, 9.4 years), and 15% were black. Adherence to quality indicators ranged from 81% for adjuvant chemotherapy to 98% for curative resection; however, many patients met quality indicator criteria without actually receiving recommended therapy by having a refusal (0% to 14%) or contraindication (1% to 30%) documented. Less than 1% of patients refused palliative chemotherapy. Black patients were more likely to refuse or bear a contraindication to surgery even when controlling for comorbidity; race was not associated with refusals or contraindications to other treatments.
Conclusion
Refusals and contraindications are common and may account for previously demonstrated low rates of recommended lung cancer therapy performance at the VHA. Racial disparities in treatment may be explained, in part, by such factors. These results sound a cautionary note for quality measurement that depends on data that do not reflect patient preference or contraindications in conditions where such considerations are important.
INTRODUCTION
Improving health care quality is a national priority. The Institute of Medicine has called for a comprehensive, longitudinal, national quality measurement system that not only allows for care assessment at multiple levels (ie, patient, population, structure), but also facilitates shared accountability and public reporting.1,2 In addition, recognition is increasing that quality measures should be patient centered.2,3
There is an inherent tension to achieving these important goals. To be sustainable, a quality measurement system must have minimal burden of data collection. Current efforts generally rely on administrative data with occasional supplementation by limited review of medical records. However, a number of studies have shown that administrative data can fail to identify all eligible patients, may miss care delivered, and may overlook justifications for care deferral.4–8 In a recent study, researchers identified documented reasons for not pursuing recommended therapy in charts of 43% of randomly selected patients with coronary artery disease whose care did not meet a quality measure.8 Although concerns are often levied that administrative data may underestimate health care quality,9–11 there is little information on the extent to which patient preferences or medical contraindications affect variation in reported performance.
Lung cancer care provides an opportunity to understand the impact of refusals and contraindications on quality measurement, as population-based and largely administrative data-driven investigations have consistently revealed that half of patients do not receive care conforming to national guidelines,11–13 with disparities in care and outcomes noted among older,14–17 impoverished,18–20 less educated,14,19 and minority race individuals,16,17,19–28 even when controlling for access to care.21,26 It has been postulated that prevalent comorbid illness in these populations may represent contraindications,29 and several studies have suggested that patient refusals may explain observed disparities in receipt of surgery.15,30,31 Nevertheless, studies based on administrative data continue to question the quality of care delivered to patients with cancer. Using cancer registry data supplemented with administrative data, Wang et al29 recently reported that less than half of patients treated in the Veterans Health Administration (VHA) received guideline-concordant therapy. If the results from this study accurately represent VHA quality of care, this would be cause for great concern.
With manually abstracted data from a national study on lung cancer quality care conducted by the VHA, we sought to determine what proportion of patients who did not receive evidence-based treatment refused or bore a contraindication to it documented in the medical record—information not necessarily captured by administrative data sets. We also explored patient characteristics associated with refusal or contraindications to recommended therapy.
PATIENTS AND METHODS
The data for this study were obtained as part of a national evaluation of lung cancer quality of care conducted by the VHA in 2010. This study was approved by the Veterans' Administration Greater Los Angeles Healthcare System Institutional Review Board.
Quality Indicators
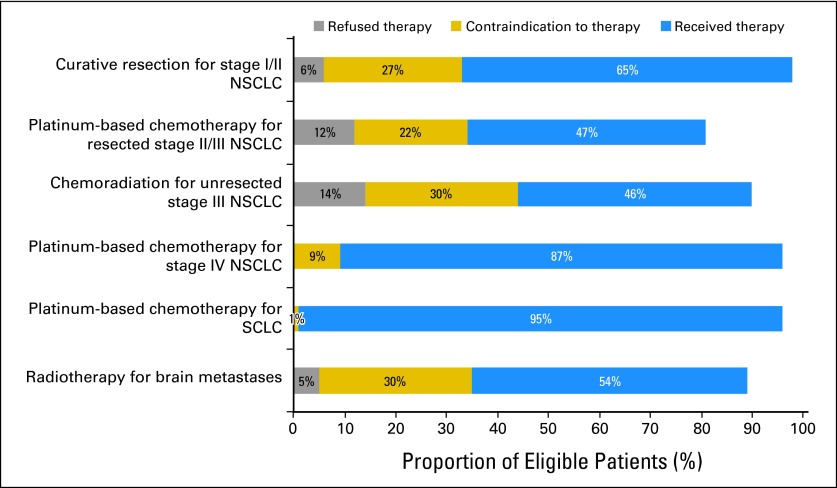
After systematic review of existing quality measures and guidelines for lung cancer management, proposed quality indicators were rated for feasibility, validity, and importance to the VHA by an expert panel using the RAND/University of California, Los Angeles modified Delphi method.32 The following six indicators specifically addressed cancer-directed therapy, with the last four indicators identifying therapy for advanced disease (Fig 1): curative surgery for stage I or II non–small-cell lung cancer (NSCLC); adjuvant, platinum-based chemotherapy for resected stage II or III NSCLC; chemoradiotherapy for unresected stage III NSCLC; platinum-based chemotherapy for stage IV NSCLC; platinum-based chemotherapy for small-cell lung cancer (SCLC); and radiotherapy for brain metastases. The criterion for fulfilling each indicator was receipt of recommended therapy or documentation that the patient either refused or had a clinical reason why care was contraindicated (eg, tumor unresectable; poor pulmonary function, surgery not recommended). Explicit documentation of poor performance status also qualified as a contraindication to cancer-directed therapy. Clinical reasons for care deferral were not appraised on their merits, only counted on their documented presence in the record, because determination of clinical propriety in judgments and recommendations was beyond the study scope.
Fig 1.
Quality indicator fulfillment by criterion: proportion of eligible patients refusing, having a contraindication to, or receiving recommended therapy. NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Study Population
Incident lung cancers diagnosed across 131 medical centers in the VHA during 2007 (N = 7,816) were identified through the VHA Central Cancer Registry (VACCR). Patients were excluded if the pathologic diagnosis was not identified in the electronic health record at the index facility that reported the patient to the registry (n = 1,297), because abstractors did not have access to records at other facilities or scanned documents (eg, pathology reports from other or non-VHA facilities). Patients without documentation confirming a diagnosis of lung cancer, compared with patients with documentation, were older (mean age, 71 v 68 years, respectively) and less likely to have SCLC (6% v 13%, respectively), but there were no statistically significant differences in sex, race, marital status, or stage of disease. Patients were also excluded if they had a second malignancy (n = 540); were diagnosed postmortem, died, or enrolled in hospice ≤ 30 days after diagnosis (n = 947); had documented comfort measures only ≤ 30 days after diagnosis (n = 91); had life expectancy ≤ 6 months in their Problem List (n = 39); or enrolled onto a clinical trial (n = 57) because documentation of care outside the VHA may not be complete in the VHA medical record. Among 4,863 patients included in the national VHA study, 3,927 patients were eligible for at least one of the six indicators pertaining to cancer-directed therapy.
Data Collection
Electronic health records of the year before diagnosis through 2009 were reviewed by VHA-contracted abstractors specifically trained for this study. Each facility was provided case-level quality indicator results and given the opportunity to identify documentation missed or inaccessible remotely; if verified by the abstractor, data were updated. Information on comorbid conditions was collected for patients with stage I or II NSCLC using the Adult Comorbidity Evaluation-27.33 Stage was determined through the VACCR or, if not available, by chart abstraction. The VACCR provided sociodemographic data.
Dependent Variable
For each quality indicator, patients were categorized as receiving specified care, refusing it, having a contraindication to it, or not receiving it in the absence of documented reasons.
Independent Variables
Patient characteristics included age, sex, race (white, black, or other/unknown), marital status (married/living with partner or other), urban or rural residence, NSCLC stage (I, II, III, or IV), SCLC extent of disease (limited or extensive), and medical oncology consultation within 9 months of diagnosis. For patients with stage I or II NSCLC, data on Adult Comorbidity Evaluation-27 comorbidity level (dichotomized to severe v none/mild/moderate), presence of severe pulmonary disease, and surgical consultation within 9 months of diagnosis also were collected.
Statistical Analyses
Univariate analyses were performed by all available patient characteristics based on a polytomous dependent variable (therapy refusal, contraindication, or lack of receipt without documented reason, with therapy receipt as reference) using clustering at the facility level; clustering did not assume a specific covariance structure, allowing robust, model-free SEs that are consistent even when assumptions are violated. Multinomial regression was used to model outcomes for curative surgery, adjuvant therapy, and therapy for advanced disease, again including clustering by medical center to account for within-facility correlation (provider-level data were unavailable). Independent variables significantly associated with outcome in univariate analyses or deemed requisite for adjustment from clinical or conceptual standpoints were included in multivariate models. Given potential endogeneity of specialist consultations with outcome, models were developed with and without these covariates; because results were unaffected, final models presented do not include specialty consultation. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Cary, NC), and Stata version 11 (Stata, College Station, TX).
RESULTS
In this cohort of predominantly male veterans newly diagnosed with lung cancer, mean age was 67.7 years (standard deviation, 9.4 years; Table 1). Black patients composed 15% of the sample. Among patients with stage I or II NSCLC, more than 86% had coexisting illness.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients (N = 3,927) | % |
|---|---|---|
| Age, years | ||
| < 55 | 287 | 7.3 |
| 55-64 | 1,476 | 37.6 |
| 65-80 | 1,725 | 43.9 |
| > 80 | 439 | 11.2 |
| Male | 3,842 | 97.8 |
| Race | ||
| White | 3,255 | 82.9 |
| Black | 595 | 15.2 |
| Other/unknown | 77 | 2.0 |
| Married/living with partner | 1,874 | 47.7 |
| Rural residence | 1,137 | 29.0 |
| NSCLC stage | ||
| I | 768 | 22.0 |
| II | 679 | 19.4 |
| III | 993 | 28.4 |
| IV | 1,053 | 30.1 |
| SCLC | ||
| Limited disease | 157 | 36.2 |
| Extensive disease | 239 | 55.1 |
| Unknown | 38 | 8.8 |
| Poor performance status | 553 | 14.1 |
| Medical oncology consultation | 1,933 | 49.2 |
| Death during study period | 2,573 | 65.5 |
| Comorbidity level* | ||
| None | 200 | 13.8 |
| Mild | 509 | 35.2 |
| Moderate | 352 | 24.3 |
| Severe | 386 | 26.7 |
| Severe pulmonary disease* | 285 | 19.7 |
| Surgery consultation* | 1,192 | 82.4 |
Abbreviations: NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Data collected only for patients with stage I and II NSCLC.
Quality Indicator Rates
Adherence to quality indicators ranged from 81% for adjuvant chemotherapy in resected stage II or III NSCLC to 98% for curative resection of stage I or II NSCLC (Fig 1). However, many patients met indicator criteria by having a refusal or contraindication documented and not actually receiving recommended therapy. Less than half of eligible patients received definitive chemoradiotherapy and adjuvant chemotherapy, whereas only 54% with brain metastases received radiation and 65% received curative resection.
The proportion of patients who refused recommended care varied considerably (Fig 1). Less than 1% of patients with SCLC and advanced NSCLC refused palliative chemotherapy, 5% with brain metastases refused radiotherapy, 6% refused curative surgery, 12% refused chemoradiotherapy for unresected stage III NSCLC, and 14% refused adjuvant chemotherapy for resected stage II or III NSCLC.
Proportions of eligible patients with contraindications to care were even higher (Fig 1). Although as few as 1% of patients with SCLC had a contraindication to platinum-based chemotherapy documented, as many as 30% of patients with brain metastases and 30% with unresected stage III NSCLC had contraindications to recommended treatment.
Refusal of Cancer-Directed Treatment
In univariate analyses, increasing age was associated with refusal of curative surgery, adjuvant therapy, and therapy for advanced disease (Table 2). Black patients were more likely to refuse surgery (P ≤ .001), but variation by race was not noted in refusals of adjuvant therapy or treatment for advanced disease.
Table 2.
Characteristics of Patients With Documented Refusal of or Contraindication to Curative Surgery, Adjuvant Therapy, or Therapy for Advanced Disease
| Characteristic | Curative Surgery |
Adjuvant Therapy |
Therapy for Advanced Disease |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Eligible Patients | Refusal |
Contraindication |
No. of Eligible Patients | Refusal |
Contraindication |
No. of Eligible Patients | Refusal |
Contraindication |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Overall | 1,447 | 90 | 6.2 | 397 | 27.4 | 273 | 33 | 12.1 | 60 | 22.0 | 2,329 | 140 | 6.0 | 434 | 18.6 |
| Age, years | |||||||||||||||
| < 55 | 93 | 2 | 2.2 | 13 | 14.0 | 24 | 2 | 8.3 | 5 | 20.8 | 181 | 4 | 2.2 | 31 | 17.1 |
| 55-64 | 467 | 18 | 3.9 | 105 | 22.5* | 112 | 10 | 8.9 | 23 | 20.5 | 948 | 35 | 3.7 | 126 | 13.3 |
| 65-79 | 702 | 42 | 6.0 | 197 | 28.1† | 124 | 17 | 13.7 | 28 | 22.6 | 956 | 73 | 7.6‡ | 191 | 20.0 |
| ≥ 80 | 185 | 28 | 15.1† | 82 | 44.3† | 13 | 4 | 30.8* | 4 | 30.8 | 244 | 28 | 11.5† | 86 | 35.3† |
| Sex | |||||||||||||||
| Male | 1,413 | 88 | 6.2 | 393 | 27.8 | 266 | 33 | 12.4 | 60 | 22.6 | 2,283 | 140 | 6.1 | 425 | 18.6 |
| Female | 34 | 2 | 5.9 | 4 | 11.8 | 7 | 0 | 0.0 | 0 | 0.0 | 46 | 0 | 0 | 9 | 19.6 |
| Race | |||||||||||||||
| White | 1,224 | 68 | 5.6 | 327 | 26.7 | 224 | 26 | 11.6 | 50 | 22.3 | 1,909 | 110 | 5.8 | 354 | 18.5 |
| Black | 196 | 21 | 10.7† | 64 | 32.7* | 37 | 4 | 10.8 | 8 | 21.6 | 378 | 26 | 6.9 | 73 | 19.1 |
| Other/unknown | 27 | 1 | 3.7 | 6 | 22.2 | 12 | 3 | 25.0 | 2 | 16.7 | 42 | 4 | 9.5 | 7 | 16.7 |
| Marital status | |||||||||||||||
| Married/living with partner | 696 | 35 | 5.0 | 182 | 26.2 | 134 | 14 | 10.5 | 35 | 26.1 | 1,105 | 62 | 5.6 | 191 | 17.3 |
| Other | 751 | 55 | 7.3 | 215 | 28.6 | 139 | 19 | 13.7 | 25 | 18.0 | 1,224 | 78 | 6.4 | 243 | 19.9 |
| Residence | |||||||||||||||
| Urban | 979 | 64 | 6.1 | 286 | 27.4 | 180 | 25 | 12.2 | 45 | 22.0 | 1,628 | 97 | 6.0 | 319 | 19.6 |
| Rural | 404 | 26 | 6.4 | 111 | 27.5 | 68 | 8 | 11.8 | 15 | 22.1 | 701 | 43 | 6.1 | 115 | 16.4 |
| NSCLC stage | |||||||||||||||
| I | 768 | 71 | 9.2† | 241 | 31.4† | — | — | — | — | — | — | — | — | — | — |
| II | 679 | 19 | 2.8 | 156 | 23.0 | 152 | 23 | 15.1 | 31 | 20.4 | — | — | — | — | — |
| III | — | — | — | — | — | 121 | 10 | 8.3 | 29 | 24.0 | 842 | 121 | 14.4 | 249 | 29.6 |
| IV | — | — | — | — | — | — | — | — | — | — | 1,053 | 15 | 1.4 | 162 | 15.4 |
| SCLC | |||||||||||||||
| Limited disease | — | — | — | — | — | — | — | — | — | — | 157 | 0 | 0 | 3 | 1.9 |
| Extensive disease | — | — | — | — | — | — | — | — | — | — | 239 | 4 | 1.7 | 19 | 8.0 |
| Unknown | — | — | — | — | — | — | — | — | — | — | 38 | 0 | 0 | 1 | 2.6 |
| Comorbidity level§ | |||||||||||||||
| None/mild/moderate | 1,061 | 64 | 6.0 | 251 | 23.7 | — | — | — | — | — | — | — | — | — | — |
| Severe | 386 | 26 | 6.7 | 146 | 37.8† | — | — | — | — | — | — | — | — | — | — |
| Pulmonary disease§ | |||||||||||||||
| None/mild/moderate | 1,162 | 72 | 6.2 | 282 | 24.3 | — | — | — | — | — | — | — | — | — | — |
| Severe | 285 | 18 | 6.3 | 115 | 40.4 | — | — | — | — | — | — | — | — | — | — |
| Surgery consultation§ | |||||||||||||||
| Yes | 1,192 | 56 | 4.7 | 199 | 16.7 | — | — | — | — | — | — | — | — | — | — |
| No | 255 | 34 | 13.3† | 198 | 77.7† | — | — | — | — | — | — | — | — | — | — |
| Medical oncology consultation | |||||||||||||||
| Yes | 1,010 | 68 | 6.7 | 345 | 34.2 | 245 | 30 | 12.2 | 49 | 20.0 | 789 | 93 | 11.8 | 237 | 30.0 |
| No | 437 | 22 | 5.0* | 52 | 11.9† | 28 | 3 | 10.7 | 11 | 39.3 | 1,540 | 47 | 3.1 | 197 | 12.8 |
Abbreviations: NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
P < .05; multinomial univariate regression, with clustering by facility.
P ≤ .001; multinomial univariate regression, with clustering by facility.
P < .01; multinomial univariate regression, with clustering by facility.
Data collected for stage I and II patients only.
Age and race remained significant factors associated with surgery refusal in multivariate analyses controlling for comorbidity and the interaction between race and comorbidity (Table 3). Compared with patients younger than age 55 years, those ≥ 80 years old had 16-fold increased risk of refusal (95% CI, 3.64 to 73.83). Compared with white patients without and with severe comorbidity, black patients had double and nearly five-fold increased risk of refusal, respectively (none/mild/moderate comorbidity: 95% CI, 1.25 to 5.64; severe comorbidity: 95% CI, 2.29 to 10.12). No other significant interactions were observed between age and race or age and comorbidity. Patients married or living with a partner were less likely to refuse surgery compared with those who were not, as were those with stage II as opposed to stage I disease.
Table 3.
Adjusted RRRs of Refusal or Contraindication
| Treatment and Characteristic | Refusal |
Contraindication |
||
|---|---|---|---|---|
| RRR | 95% CI | RRR | 95% CI | |
| Curative surgery for stage I/II NSCLC* | ||||
| Age, years | ||||
| < 55 | Reference | Reference | ||
| 55-64 | 1.99 | 0.44 to 9.07 | 1.71 | 0.95 to 3.10 |
| 65-79 | 3.85 | 0.95 to 14.54 | 2.50 | 1.38 to 4.54 |
| ≥ 80 | 16.39 | 3.64 to 73.83 | 6.62 | 3.37 to 13.02 |
| Race | ||||
| White | ||||
| None/mild/moderate comorbidity | Reference | Reference | ||
| Severe comorbidity | 1.18 | 0.60 to 6.01 | 2.12 | 1.54 to 2.91 |
| Black | ||||
| None/mild/moderate comorbidity | 2.40 | 1.25 to 4.64 | 1.78 | 1.20 to 2.65 |
| Severe comorbidity | 4.74 | 2.29 to 10.12 | 4.56 | 3.06 to 6.92 |
| Other | ||||
| None/mild/moderate comorbidity | 0.99 | 0.11 to 9.02 | 1.37 | 0.54 to 3.49 |
| Severe comorbidity | 2.18 | 0.75 to 11.20 | 3.49 | 2.08 to 6.40 |
| Marital status | ||||
| Not married/living with partner | Reference | Reference | ||
| Married/living with partner | 0.53 | 0.31 to 0.90 | 0.78 | 0.60 to 1.02 |
| Residence | ||||
| Urban | Reference | Reference | ||
| Rural | 1.22 | 0.70 to 2.10 | 1.04 | 0.78 to 1.40 |
| Stage | ||||
| I | Reference | Reference | ||
| II | 0.25 | 0.14 to 0.46 | 0.61 | 0.46 to 0.81 |
| Adjuvant chemotherapy for resected stage II/IIIA NSCLC | ||||
| Age, years | ||||
| < 55 | Reference | Reference | ||
| 55-64 | 0.79 | 0.15 to 4.19 | 0.63 | 0.22 to 1.81 |
| 65-79 | 1.43 | 0.27 to 7.52 | 0.80 | 0.25 to 2.57 |
| ≥ 80 | 19.54 | 1.34 to 284.41 | 6.48 | 0.58 to 71.82 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.24 | 0.39 to 3.89 | 1.21 | 0.46 to 3.16 |
| Other | 1.66 | 0.29 to 9.47 | 0.62 | 0.07 to 5.35 |
| Marital status | ||||
| Not married/living with partner | Reference | Reference | ||
| Married/living with partner | 0.71 | 0.29 to 1.71 | 1.56 | 0.82 to 2.96 |
| Residence | ||||
| Urban | Reference | Reference | ||
| Rural | 1.07 | 0.43 to 2.64 | 0.94 | 0.49 to 1.82 |
| Therapy for advanced disease | ||||
| Age, years | ||||
| < 55 | Reference | Reference | ||
| 55-64 | 1.82 | 0.72 to 4.63 | 0.82 | 0.52 to 1.27 |
| 65-79 | 4.49 | 1.72 to 11.70 | 1.44 | 0.92 to 2.26 |
| ≥ 80 | 10.42 | 3.67 to 29.60 | 3.88 | 2.29 to 6.57 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.19 | 0.74 to 1.92 | 0.96 | 0.75 to 1.23 |
| Other | 1.70 | 0.43 to 6.64 | 0.88 | 0.33 to 2.31 |
| Marital status | ||||
| Not married/living with partner | Reference | Reference | ||
| Married/living with partner | 0.74 | 0.54 to 1.01 | 0.76 | 0.62 to 0.94 |
| Residence | ||||
| Urban | Reference | Reference | ||
| Rural | 1.02 | 0.66 to 1.59 | 0.83 | 0.66 to 1.06 |
| Cancer type | ||||
| SCLC | Reference | Reference | ||
| NSCLC | 11.28 | 4.30 to 29.57 | 5.94 | 3.69 to 9.55 |
NOTE. Results not shown for no treatment without documented reason.
Abbreviations: NSCLC, non–small-cell lung cancer; RRR, relative risk ratio; SCLC, small-cell lung cancer
Model adjusted for interaction between race and comorbidity.
Increasing age was associated with refusal of adjuvant therapy for resected NSCLC and therapy for advanced disease, with patients age ≥ 80 years bearing the highest risk compared with patients younger than age 55 years (adjuvant therapy: relative risk ratio [RRR], 19.54; 95% CI, 1.34 to 284.41; therapy for advanced disease: RRR, 10.42; 95% CI, 3.67 to 29.60; Table 3). Patients with NSCLC were more likely to refuse treatment for advanced disease than patients with SCLC (RRR, 11.28; 95% CI, 4.30 to 29.57; Table 3).
Contraindication to Cancer-Directed Treatment
Increasing age was associated in univariate analyses with having a contraindication to curative surgery and treatment for advanced disease, but not adjuvant therapy (Table 2). Contraindication to surgery was more frequent among patients with greater comorbidity (P ≤ .001; Table 2).
The adjusted risk of contraindication to surgery remained higher for patients age ≥ 80 years (v < 55 years; RRR, 3.26; 95% CI, 1.46 to 7.27) and black patients, even when adjusting for comorbidity and the interaction between race and comorbidity. Compared with white patients without severe comorbidity, black patients without severe comorbidity bore a 1.78-fold increased risk of a contraindication to surgery (95% CI, 1.20 to 2.65), whereas black patients with severe comorbidity had a 4.56-fold increased risk (95% CI, 3.06 to 6.92; Table 3).
Older veterans were significantly more likely to have a contraindication to therapy for advanced disease (v < 55 years; RRR, 3.88; 95% CI, 2.29 to 6.57), as were those with NSCLC (v SCLC; RRR, 5.94; 95% CI, 3.69 to 9.55). Patients who were married or living with a partner were less likely to bear a contraindication compared with single patients (RRR, 0.76; 95% CI, 0.62 to 0.94; Table 3).
DISCUSSION
As national attention turns to assessing and monitoring quality of care electronically, this study highlights the importance of capturing patient preferences and contraindications to therapy. When accounting for refusals and contraindications, we found that quality of care for lung cancer was markedly higher than previous studies have suggested.11–13 Specifically, our results stand in stark contrast to the recent study of administrative data by Wang et al29 in which more than half of VHA patients received inadequate care. In another recent report, Keating et al34 used cancer registry data linked with administrative data from VHA and Medicare claims to compare quality of lung cancer care received by older patients treated within and without the VHA. Quality indicator rates ranged from 50% to 86%, with no differences observed between the two populations. Among patients with stage I or II NSCLC, 58% of VHA and 61% of Medicare patients underwent curative resection. Similarly, we found that 65% of VHA patients received surgery; however, medical record review identified an additional 6% of eligible patients refusing surgery and another 27% with a documented contraindication, underscoring the importance of evaluating reasons against recommended therapy if quality assessments are to accurately reflect the clinical context. Of note, in a related publication of the study by Keating et al,34 Landrum et al35 reported the number of refusals in the subsample of patients who did not receive surgery, translating to a 6% refusal rate in their cohort and providing further corroboration for our estimate.
Our study supports and extends previous findings that black patients are more likely to refuse or have a contraindication to surgery for early-stage lung cancer even when controlling for comorbidity. These findings may explain some of the disparities in surgery receipt reported elsewhere.15,21,26–28 Recent contributions elucidating reasons for higher surgery refusal and decreased treatment rates among black patients suggest not only continued poor access to care,21,24,26,31 but also particular beliefs regarding cancer (eg, what symptoms trigger care seeking,36 how disease is acquired and spread37), issues of trust,38 and negative perceptions of communication with the provider.31 As might be anticipated in an integrated health care system, specialist consultation did not seem to influence risk of refusal or contraindication in our study, yet persistently increased rates of both were observed among black patients with the same level of comorbidity as their white counterparts. Although it is impossible to discern from our data the extent of provider or patient aggressiveness regarding treatment or whether patients received adequate counseling for informed decision making, 63% of those declining surgery did consult with a surgeon; these patients had a mean of three visits with a surgeon, intimating that substantive discussions may have taken place.
Of note, virtually no patients felt to be candidates for treatment refused palliative chemotherapy for lung cancer. It is unclear whether this extremely low rate of refusal reflects poor communication by physicians regarding the marginal advantage of palliative chemotherapy, patient desire for life prolongation even when treatment is of limited benefit, or both. Surveys of patients with advanced lung cancer have found inconsistencies in acceptable level of survival benefit associated with palliative chemotherapy,39 overestimation of prognosis,40 or misunderstanding of possibility for cure41 leading to more aggressive therapy, suggesting other possible explanations for high rates of treatment among patients with advanced disease in our cohort.42 That said, if chemotherapy within 14 days of death is considered an indicator of poor-quality care, one study does suggest that VHA patients with lung and colon cancer are less likely than Medicare counterparts to receive such therapy.43 Deeper investigation of chemotherapy timing as well as patient-provider discussions nearing the end of life is warranted, especially because early intervention with palliative care consultation for late-stage lung cancer has been shown not only to help patients gain more accurate perceptions of their prognosis, but also limit chemotherapy received toward their final days.44
Given the frequency of both patient refusals and medical contraindications, our results underscore the need for performance measurement systems that capture this level of clinical detail or allow clinicians to identify administratively those patients who have reasons for not receiving recommended care. We would urge researchers to consider these limitations in registry and administrative data sets when seeking to report on care quality. Studies such as that by Wang et al29 that conclude that half of patients receive recommended therapy risk undermining confidence in institutions such as the VHA, which in the last two decades has been shown to provide better quality care than the medical system at large.34,43,45–50 Furthermore, acting on such data could potentially result in resource misallocation to quality improvement where it is not indicated. When studies have policy implications, researchers must ensure that the data used are adequate to support decision making based on the study's conclusions.51
Appropriately configured electronic health records have been successful in producing metrics that incorporate medical contraindications and patient abstentions to chronic illness and preventive care.8,52 To our knowledge, this approach has not yet been evaluated in conditions such as lung cancer, in which care is episodic and involves multiple providers from different specialties. As efforts and incentives converge toward assessing quality of care as a matter of national policy, including the use of e-measures by the Centers for Medicare and Medicaid Services,53 the ability to efficiently and accurately ascertain critical exceptions across a variety of settings will be pivotal for credible large-scale measurement and reporting as well as local use of results in quality improvement.
Our results must be considered in light of several limitations. Male veterans receiving care within the VHA carry a risk of lung cancer two times higher than those not within the VHA,54 highlighting significant demographic and clinical differences observed between VHA and non-VHA care recipients. Among these differences are higher prevalence of lung cancer risk exposures, older age, lower socioeconomic status, more comorbid disease, and lesser social support of the veteran population,55–58 which may have resulted in higher rates of refusals and contraindications in our study than might be observed in other populations. Although medical record abstraction provides access to the greatest amount of clinical detail of any retrospective data source, abstractors may have missed important information, providers may not have documented known preferences or contraindications, and data from care received outside VHA medical centers may not be completely captured; these limitations, however, would likely result in underestimation of quality care. Finally, our study was not designed to glean the specific reasons why patients refused care or why care was judged to be contraindicated, only whether a refusal or contraindication of any kind was documented.
In conclusion, refusals and contraindications were documented as reasons for not receiving recommended therapy in a substantial proportion of VHA patients with lung cancer and markedly impacted quality measure results. This may partially explain the racial disparities seen in receipt of curative resection. Electronic systems are needed that facilitate routine documentation of patient preference and medical considerations that affect decision making. Further studies should evaluate whether clinical judgments of contraindications are evidence based and whether patient refusals are ethically mutable.
Acknowledgment
Presented, in part, at the 28th Annual Veterans Affairs Health Services Research and Development Meeting, February 16-18, 2011, National Harbor, MD, and the 47th Annual Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL.
Jennifer L. Malin had full access to all of the data in the study and takes responsibility for the integrity of the data as well as the accuracy of the data analysis. All human investigations were performed following approval by a local human investigations committee at each participating site and in accordance with an assurance filed with and approved by the Department of Health and Human Services, where appropriate. The investigators obtained informed consent from each participant or each participant's guardian.
Footnotes
Supported in part by the Robert Wood Johnson Clinical Scholars Program (J.J.R.) and the University of California, Los Angeles Career Development Program in Cancer Prevention and Control Research National Cancer Institute R25 Grant (J.J.R.).
The authors are solely responsible for the design of the study, analysis or interpretation of the results, writing of the article, and decision to submit the article for publication. The views and opinions of authors expressed herein do not necessarily state or reflect those of the Department of Veterans Affairs, the US Government, Kaiser Permanente, or the University of California, Los Angeles.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jennifer L. Malin, WellPoint (C) Consultant or Advisory Role: None Stock Ownership: Jennifer L. Malin, WellPoint Honoraria: None Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Joan J. Ryoo, Diana L. Ordin, Michael K. Gould, Jennifer L. Malin
Collection and assembly of data: Joan J. Ryoo, Diana L. Ordin, Jennifer L. Malin
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Institute of Medicine. Washington, DC: National Academies Press; 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [PubMed] [Google Scholar]
- 2.Institute of Medicine. Washington, DC: National Academies Press; 2006. Performance Measurement: Accelerated Improvement. [Google Scholar]
- 3.National Quality Forum. Washington, DC: National Quality Forum; 2009. Measurement Framework: Evaluating Efficiency Across Patient-Focused Episodes of Care. [Google Scholar]
- 4.Persell SD, Wright JM, Thompson JA, et al. Assessing the validity of national quality measures for coronary artery disease using an electronic health record. Arch Intern Med. 2006;166:2272–2277. doi: 10.1001/archinte.166.20.2272. [DOI] [PubMed] [Google Scholar]
- 5.Pawlson LG, Scholle SH, Powers A. Comparison of administrative-only versus administrative plus chart review data for reporting HEDIS hybrid measures. Am J Manag Care. 2007;13:553–558. [PubMed] [Google Scholar]
- 6.Schneider EC, Nadel MR, Zaslavsky AM, et al. Assessment of the scientific soundness of clinical performance measures: A field test of the National Committee for Quality Assurance's colorectal cancer screening measure. Arch Intern Med. 2008;168:876–882. doi: 10.1001/archinte.168.8.876. [DOI] [PubMed] [Google Scholar]
- 7.Quach S, Blais C, Quan H. Administrative data have high variation in validity for recording heart failure. Can J Cardiol. 2010;26:306–312. doi: 10.1016/s0828-282x(10)70438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kmetik KS, O'Toole MF, Bossley H, et al. Exceptions to outpatient quality measures for coronary artery disease in electronic health records. Ann Intern Med. 2011;154:227–234. doi: 10.7326/0003-4819-154-4-201102150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kerr EA, Smith DM, Hogan MM, et al. Comparing clinical automated, medical record, and hybrid data sources for diabetes quality measures. Jt Comm J Qual Improv. 2002;28:555–565. doi: 10.1016/s1070-3241(02)28059-1. [DOI] [PubMed] [Google Scholar]
- 10.Pronovost PJ, Lilford R. A road map for improving the performance of performance measures. Health Aff. 2011;30:569–573. doi: 10.1377/hlthaff.2011.0049. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Saxman S, Wallace RB, et al. Population variations in the initial treatment of non-small-cell lung cancer. J Clin Oncol. 2004;22:3261–3368. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 12.Shugarman LR, Mack K, Sorbero ME, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–781. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 13.Langer CJ, Moughan J, Movsas B, et al. Patterns of care survey (PCS) in lung cancer: How well does current U.S. practice with chemotherapy in the non-metastatic setting follow the literature? Lung Cancer. 2005;48:93–102. doi: 10.1016/j.lungcan.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 15.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 16.Bradley CJ, Dahman B, Given CW. Treatment and survival differences in older Medicare patients with lung cancer as compared with those who are dually eligible for Medicare and Medicaid. J Clin Oncol. 2008;26:5067–5073. doi: 10.1200/JCO.2008.16.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy D, Xia R, Liu CC, et al. Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients. Cancer. 2009;115:4807–4818. doi: 10.1002/cncr.24521. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald HP, Polissar NL, Borgatta EF, et al. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88:1681–1684. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Cheung MC, Byrne MM, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116:2437–2447. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 21.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 22.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 23.Wisnivesky JP, McGinn T, Henschke C, et al. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med. 2005;171:1158–1163. doi: 10.1164/rccm.200411-1475OC. [DOI] [PubMed] [Google Scholar]
- 24.Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86:220–227. doi: 10.1016/j.athoracsur.2008.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang ET, Shema SJ, Wakelee HA, et al. Uncovering disparities in survival after non-small-cell lung cancer among Asian/Pacific Islander ethnic populations in California. Cancer Epidemiol Biomarkers Prev. 2009;18:2248–2255. doi: 10.1158/1055-9965.EPI-09-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley CJ, Dahman B, Given CW. Inadequate access to surgeons: Reason for disparate cancer care? Med Care. 2009;47:758–764. doi: 10.1097/MLR.0b013e31819e1f17. [DOI] [PubMed] [Google Scholar]
- 27.Blackstock AW, Herndon JE, 2nd, Paskett ED, et al. Outcomes among African-American/Non-African-American patients with advanced non-small-cell lung carcinoma: Report from the Cancer and Leukemia Group B. J Natl Cancer Inst. 2002;94:284–290. doi: 10.1093/jnci/94.4.284. [DOI] [PubMed] [Google Scholar]
- 28.Berger M, Lund MJ, Brawley OW. Racial disparities in lung cancer. Curr Probl Cancer. 2007;31:202–210. doi: 10.1016/j.currproblcancer.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012;30:1447–1455. doi: 10.1200/JCO.2011.39.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCann J, Artinian V, Duhaime L, et al. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–3446. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- 31.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–2376. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook RH. The RAND/UCLA appropriateness method. In: McCormick KA, Moore SR, Siegel RA, editors. Clinical Practice Guidelines Development: Methodology Perspectives. Rockville, MD: Agency for Healthcare Research and Quality; 1994. [Google Scholar]
- 33.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 34.Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: A cohort study. Ann Intern Med. 2011;154:727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 35.Landrum MB, Keating NL, Lamont EB, et al. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118:3345–3355. doi: 10.1002/cncr.26628. [DOI] [PubMed] [Google Scholar]
- 36.Lathan CS, Okechukwu C, Drake BF, et al. Racial differences in the perception of lung cancer: The 2005 Health Information National Trends Survey. Cancer. 2010;116:1981–1986. doi: 10.1002/cncr.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margolis ML, Christie JD, Silvestri GA, et al. Racial differences pertaining to a belief about lung cancer surgery: Results of a multicenter survey. Ann Intern Med. 2003;139:558–563. doi: 10.7326/0003-4819-139-7-200310070-00007. [DOI] [PubMed] [Google Scholar]
- 38.Gordon HS, Street RL, Jr, Sharf BF, et al. Racial differences in trust and lung cancer patients' perceptions of physician communication. J Clin Oncol. 2006;24:904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: Descriptive study based on scripted interviews. BMJ. 1998;317:771–775. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 41.Weeks JC, Catalano PJ, Cronin A, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin MY, Pisu M, Oster RA, et al. Racial variation in willingness to trade financial resources for life-prolonging cancer treatment. Cancer. 2011;117:3476–3484. doi: 10.1002/cncr.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keating NL, Landrum MB, Lamont EB, et al. End-of-life care for older cancer patients in the Veterans Health Administration versus the private sector. Cancer. 2010;116:3732–3739. doi: 10.1002/cncr.25077. [DOI] [PubMed] [Google Scholar]
- 44.Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol. 2011;29:2319–2326. doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 45.Petersen LA, Normand SL, Daley J, et al. Outcome of myocardial infarction in Veterans Health Administration patients as compared with Medicare patients. N Engl J Med. 2000;343:1934–1941. doi: 10.1056/NEJM200012283432606. [DOI] [PubMed] [Google Scholar]
- 46.Rosenthal GE, Sarrazin MV, Harper DL, et al. Mortality and length of stay in a Veterans Affairs hospital and private sector hospitals serving a common market. J Gen Intern Med. 2003;18:601–608. doi: 10.1046/j.1525-1497.2003.11209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashton CM, Souchek J, Petersen NJ, et al. Hospital use and survival among Veterans Affairs beneficiaries. N Engl J Med. 2003;349:1637–1646. doi: 10.1056/NEJMsa003299. [DOI] [PubMed] [Google Scholar]
- 48.Kerr EA, Gerzoff RB, Krein SL, et al. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: The TRIAD study. Ann Intern Med. 2004;141:272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 49.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141:938–945. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 50.Selim AJ, Berlowitz D, Kazis LE, et al. Comparison of health outcomes for male seniors in the Veterans Health Administration and Medicare Advantage plans. Health Serv Res. 2010;45:376–396. doi: 10.1111/j.1475-6773.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malin JL, Keating NL. The cost-quality trade-off: Need for data quality standards for studies that impact clinical practice and health policy. J Clin Oncol. 2005;23:4581–4584. doi: 10.1200/JCO.2005.01.912. [DOI] [PubMed] [Google Scholar]
- 52.Persell SD, Kaiser D, Dolan NC, et al. Changes in performance after implementation of a multifaceted electronic-health-record-based quality improvement system. Med Care. 2011;49:117–125. doi: 10.1097/MLR.0b013e318202913d. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Medicare and Medicaid Services, Department of Health and Human Services. Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75:44314–44588. [PubMed] [Google Scholar]
- 54.Harris RE, Hebert JR, Wynder EL. Cancer risk in male veterans utilizing the Veterans Administration medical system. Cancer. 1989;64:1160–1168. doi: 10.1002/1097-0142(19890901)64:5<1160::aid-cncr2820640533>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 55.Campling BG, Hwang WT, Zhang J. A population-based study of lung carcinoma in Pennsylvania: Comparison of Veterans Administration and civilian populations. Cancer. 2005;104:833–840. doi: 10.1002/cncr.21228. [DOI] [PubMed] [Google Scholar]
- 56.McKinney WP, McIntire DD, Carmody TJ, et al. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112:212–217. [PMC free article] [PubMed] [Google Scholar]
- 57.Kizer KW, Demakis JG, Feussner JR. Reinventing VA health care: Systematizing quality improvement and quality innovation. Med Care. 2000;38(suppl):I7–I16. [PubMed] [Google Scholar]
- 58.Miller DR, Kalman D, Ren XS, et al. Washington, DC: Veterans Health Administration Office of Quality and Performance, Department of Veterans Affairs; 2001. Health Behaviors of Veterans in the VHA: Tobacco Abuse: 1999 Large Health Survey of VHA Enrollees. [Google Scholar]